Abstract
We found that a ZnO film of 2 μm thickness which was laser-deposited at room temperature onto a plain soda lime glass substrate, exhibits notable antibacterial activity against a biofilm of Staphylococcus epidermidis when back-illuminated by a UVA light source with a peak emission wavelength of about 365 nm. X-ray diffraction (XRD), scanning electron microscopy (SEM), atomic force microscopy (AFM), UV-visible absorption spectroscopy, Raman spectroscopy and x-ray photoemission spectroscopy (XPS) were used to characterize the ZnO films before and after the interactions with the biofilm and the ultraviolet light, respectively. The as-deposited film was highly textured with the wurtzite (0002) in-plane orientation (c-axis perpendicular to ZnO surface) and had a surface rms roughness of 49.7 nm. In the as-deposited film, the Zn to O ratio was 1 to 0.95. After the UV and biofilm treatments, the ZnO film surface had become rougher (rms roughness 68.1 nm) and presented uniform micron-sized pitting randomly distributed, while the zinc to oxygen ratio had become 1 to 2.2. In this case, both the UV-visible and Raman spectra pointed to degradation of the structural quality of the material. On the strength of these data, we propose a model for the mediation of the bactericidal activity in which the photogeneration of highly oxidizing species and the presence of active surface defect sites both play an important role. This study is of particular interest for the acute problem of disinfection of pathogenic biofilms which form on medical device/implant surfaces.
Introduction
The resistance of certain biofilms to all known antibiotics and all forms of chemical treatment is currently driving substantial research into alternative disinfectant technologies. In this regard, Coagulase-negative Staphylococci, in particular Staphylococcus epidermidis which was once regarded as a harmless part of the skin flora, are now accepted as major nosocomial pathogens. To prevent the formation of S. epidermidis biofilms, new techniques using silver-doped phenyltriethoxysilane sol–gel coatings [Citation1] or antibiotic-loaded titania nanotubes [Citation2], for example, have been successfully tested.
One powerful disinfectant technology uses photocatalytic chemistry with the semiconductor titanium dioxide (TiO2) with anatase or rutile crystalline structures [Citation3]. The basic principle of this technique consists of illuminating the semiconductor surface with band gap light to create valence band holes which have a sufficiently positive potential to oxidize organic matter directly or to oxidize water yielding hydroxyl (OH•) and/or other radicals (O2−•, HO2•) with known bactericidal properties, see e.g. [Citation4–9]. Anatase/rutile TiO2 remains the most widely used and studied photocatalyst material and recent fundamental studies have demonstrated that surface defects such as oxygen vacancies mediate the dissociation of water on its surface [Citation10]. However, the most recent works point to the use of doped TiO2 for optimum performance and this requires the implementation of comparatively more complex deposition methods [Citation11]. Furthermore, titanium dioxide has just been classified as possibly carcinogenic to humans [Citation12]. Thus, research in the use of alternative oxide semiconductors for photocatalytic applications is important.
One such material is the n-type, wide bandgap, semiconductor zinc oxide (ZnO). It is a highly versatile chemical compound possessing numerous attractive physical and chemical properties that find applications in many domains of science and technology [Citation13] such as catalysis, fabrication of pigments, solar cells, electronics, dentistry, sensors, fabrication of sunscreens, etc. We mention here some structural and electronic properties of ZnO that are relevant to the present work. ZnO usually crystallizes with the (hexagonal) wurtzite structure and tends to grow and form needles along the [0001] direction (c-axis). The (0001) face is Zn-terminated (positive face) while the face is O-terminated (negative face), and, unlike titania, ZnO is a polar crystal. Thus, a change of the surface charge via removal of surface atoms, for example, is needed to lift the built-in field and obtain energetic stability of the lattice. The resulting nonstoichiometry is known to strongly influence the electronic properties through upward band-bending effects and the creation of active surface sites [Citation14, Citation15]. It is also relevant to mention here the amphoteric properties of ZnO which influence both its chemical behavior [Citation16] and its electronic properties upon doping [Citation17]. Furthermore, ZnO has interesting optical properties, due to its direct band-gap of 3.3 eV and a large binding exciton binding energy of 60 meV. Such a combination of an active surface and strong photoeffects make ZnO a prime candidate for the type of application considered in the present work.
ZnO has long been used as a mild antiseptic in the form of ointments. The antibacterial properties of ZnO in powder or nanoparticle forms have also been studied [Citation18–22] and can be further enhanced by ultraviolet light activation [Citation23]. Nanostructured ZnO has interesting properties towards the photocatalytic decomposition of organic pollutants [Citation24] and dyes [Citation25], while La-doped and silane-coated ZnO nanoparticles have recently shown remarkable photocatalytic action against monocrotophos [Citation26] and tetrachloroethylene [Citation27], respectively. Several studies have compared the effectiveness of the photocatalytic properties of ZnO for the inactivation of several pathogens with that of various metallic oxides and demonstrated that ZnO is highly effective in this context [Citation23, Citation28, Citation29]. These works suggest the use of zinc oxide coatings for the inactivation of biofilms, a subject which has remained hitherto unexplored.
In the present study, we show that an optically thick ZnO film deposited at room temperature by the pulsed laser deposition (PLD), onto a soda lime glass substrate, can be used to inactivate a biofilm of S. epidermidis grown directly on the ZnO surface, under rear-face illumination with above band-gap UV radiation (around 376 nm). This technique has potential for the disinfection of contaminated surfaces and inner-surfaces; a topic of great practical importance [Citation1]. In the following, we first present our experimental set-up and the conditions used for the bacterial inactivation. We then present data on the characterization of the ZnO films both before and after UV illumination with a view to identify some relevant physical/chemical mechanisms. The characterization techniques used were: (i) scanning electron microscopy (SEM) and atomic force microscopy (AFM) to visualize the effect(s) of the surface interactions; (ii) UV-visible absorption spectrometry to characterize the transmission of the exciting beam through the depth of the glass substrate/ZnO/biofilm multilayer; (iii) x-ray diffraction (XRD) to establish the crystalline properties and (iv) x-ray photoemission spectroscopy (XPS) to establish the stoichiometry and the chemical environment of the ZnO surface/subsurface. Finally, we propose a model to explain the bacterial inactivation that accounts for all the experimental observations.
Materials and methods
ZnO films preparation
The ZnO films were grown on soda lime glass substrates (plain microscope slides) using the PLD. Detailed descriptions of the PLD apparatus used by the authors can be found elsewhere [Citation30, Citation31]. Briefly, a high power, short pulse (6 ns) of UV laser light (266 nm) is focused onto a sintered disc of ZnO in a low-pressure oxygen ambient (0.35 mbar). The laser was operated at a repetition rate of 10 Hz and the fluence on target was of the order of 1.3 J cm−2. The resulting low-temperature plasma plume expands over a distance of about 5 cm and is captured by the glass substrate where it recondenses and forms a film. In the present work, the depositions were carried out at room temperature and 36 000 laser shots were needed to grow films of ∼2 μm thickness. The substrate holder was suitably translated during growth so as to uniformly cover the full length (75 mm) of the glass substrate, however, only the lower half of its height (12.5 mm) due to the presence of a tantalum foil mask. The non-coated glass surface of each slide is thus used as a control against which the anti-bacterial efficiency of the ZnO-coated part can be compared. The ZnO films thus produced were found to adhere strongly to the glass substrate and this must be regarded as an advantage of the PLD technique over thermal deposition techniques, as the materials in a PLD plume are deposited congruently at near supersonic velocity [Citation32]. Indeed, no visible degradation of the films in the form of cracks, tear-offs, delamination or otherwise was recorded after the various processing steps of the experiment that included autoclaving, prolonged immersion in aqueous media, biofilm growth and removal (see below for details). The as-deposited films have the characteristic whitish appearance of ZnO, while the processed films have a more yellowish tint.
Biofilms preparation and irradiation
The reference strain S. epidermidis RP62A (ATCC 35984) was chosen for the present study owing to its potent capability to form biofilms. The organism was stored at −80 °C and was resuscitated using BHI agar (CM0225, Oxoid Ltd, UK) incubated overnight at 37 °C. Bacteria were also grown on Congo red agar plates, which are composed of BHI agar supplemented with 5% sucrose (Sigma Dorset, UK) and 0.8 mg ml−1 Congo red (Sigma, Dorset, UK) to identify biofilm-positive (black, irregular-shaped, dry colonies) and biofilm-negative (red, smooth colonies) phenotypes. A single colony of the black phenotype was then inoculated into 5 ml of BHI broth which was incubated at 37 °C with shaking overnight (200 rpm). The ZnO coated slides were pre-sterilized by exposure to UVC light and then placed in a sterile Petri dish. A volume of 10 ml of BHI broth was added to the latter and then inoculated with an overnight culture to a final concentration of 106 CFU ml−1. The samples were then incubated for 18 h, without shaking, at 37 °C and, finally, removed from the broth and washed three times with sterile deionized water to remove nonadherent cells. At this point, all the slide samples have been prepared identically: half of their surface having first received a ZnO coating and then the entire surface being coated by a bio-layer of S. epidermidis. The samples can now be individually tested for the effect(s) of ZnO on the biofilm.
The samples were irradiated in a custom made cell, constructed out of Perspex, and specifically designed to hold a pair of slides immersed in a fluid (distilled water), so that all the experiments were performed in duplicate. The cell had a window made of fused quartz. A pair of low-pressure mercury vapor lamps (UVA lamp PL-S 9W/10) (Philips, Aylesbury, UK) was used as the source of ultraviolet light. Each lamp was positioned in front of one of the two slides, both biofilm and coating faced away from the irradiating source so that the UV light activates the coating without passing through the biofilm. These lamps emit long wave UVA radiation in the 350–400 nm region and peaks at around 365 nm (100% intensity) with a FWHM (50% intensity) of about 20 nm. They have a UVA/UVB ratio of less than 0.1%, the UV intensity used was 14 W m−2. The irradiations lasted for 2 h with the control samples being kept in the same fluid without illumination.
In situ viability staining and imaging
To view the direct action of the ZnO coatings on the biofilms in situ, we used a Live-Dead staining and confocal laser scanning microscopy (CLSM) assay that allows individual cells to be imaged. Other enumeration techniques were explored, such as sonication of the biofilm followed by cell culture, but were found to be unsatisfactory owing to an underestimation of the viable population resulting from clumping together of cells within the extracellular matrix of the biofilm.
The viability indicator used in this work was a measure of cell membrane integrity. Bacteria with intact membranes are assumed to be alive, with functional metabolic and respiratory activities. In contrast, bacteria with membrane damage (and without intact membrane) are assumed to be dead because they are unable to metabolize stored energy when their membrane is broken. In order to assess the bacteria membrane integrity, the LIVE/DEAD BacLight Bacterial Viability kit L-13152 (Molecular probes, Leiden, Netherlands) was used. The viability kit consists of two nucleic acid stains: (i) SYTO 9 (excitation maximum, 508 nm; emission maximum, 527 nm), a lipophilic membrane permeable cationic stain that labels live bacteria with green fluorescence and (ii) propidium iodide (excitation maximum, 536 nm emission maximum, 620 nm) a membrane impermeable anionic stain that labels membrane-compromised bacteria with red fluorescence. When used alone SYTO 9 labels both live and dead bacteria green, in contrast propidium iodide penetrates those cells with compromised cell membranes. The staining method has been used extensively as a viability indicator [Citation33–40]. The stain mixture ratio used in the majority of experiments was 75:25, SYTO 9: propidium iodide rather than the recommended 1:1 ratio, this was due to the red fluorescence being too prominent. The final concentration was 9 μm SYTO 9 to 15 μm PI. Control slides were used to negate non-specific staining signal due to auto-fluorescence of the bacterial cells and signal crossover between channels. These controls included live untreated biofilms as well as inactivated biofilms which were stained with SYTO 9 and propidium iodide separately. A freshly cultured biofilm was used as a live control.
The confocal microscopy work was performed using an LSM510 META Axoplan upright confocal scanning laser microscope (Carl Zeiss Ltd, Welwyn Garden City, Herts., UK) equipped with a ×63 magnification objective with a 1.4 numerical aperture. Confocal illumination was provided by either an argon-ion laser (excitation wavelength of 488 nm) fitted with a 505–550 nm band-pass emission filter for the detection of green fluorescence or a He–Ne laser (excitation wavelength of 543 nm) fitted with a 585–615 nm band-pass emission filter for the detection of red fluorescence. The viability analyses were carried out on CLSM images, each representing an area of 70×70 μm2, using the LSM 5 image browser software. The images of the stained bacteria were segmented using color thresholding to separate the green and red fluorescence signals. Two quantities were measured: (i) the green fluorescence as a percentage of the total fluorescence (green plus red) and (ii) the number of individual green fluorescing bacteria. Images were obtained at random from both coated and uncoated samples. In general, images were of the bacteria closest to the surface (and thus furthest from the UV excitation transmitted through the glass). The red cells and green cells from each image were then counted and percentage viability calculated as an average over at least three images taken from separate areas. On average there were between 800 and 1000 cells per image.
Statistics were carried out using Instat version 3 (Graphpad Software Inc). Experiments were carried out in triplicate and data analyzed by One-way ANOVA. P values of less than 0.05, 0.01 or 0.001 were considered to be respectively significant, highly significant or extremely significant. ANOVA cannot be carried out if there are less than two sets of data. Thus, when only two sets were to be compared, a pair two-tailed Student’s t test was carried out. Repeated ANOVA measures were carried out when the data was paired.
For the purpose of the characterization of the material properties of the ZnO coatings carried out in this study and presented in the following sections, the biofilms were treated with the disinfectant Virkon (Dupont, Herts, UK) then thoroughly rinsed with sterile water. Bacterial cell removal was subsequently checked using optical microscopy.
Characterization of the ZnO films
A double-beam UV-visible spectrophotometer (model Cary 50 from Varian) was used to measure the ZnO film samples transmission in the 300–700 nm wavelength range. The glass face was presented to the beam first so as to reproduce the experimental conditions of irradiation of the biofilms described above. A Leo 440 Stereoscan SEM was used to image the sample surfaces with typical magnification of ×30 000. Three-dimensional information about the sample surfaces was obtained with a Veeco dimension 3100 AFM operated in tapping mode using etched silicon cantilevers of nominal tip radius equal to 7 nm. The micro-Raman spectroscopy measurements were performed using a Jobin Yvon LabRaman HR800μRS system in backscattering geometry using a 488 nm argon-ion laser excitation at room temperature. A 100× objective was used to focus the laser source to a 1 μm diameter spot which also served to collect the Raman signal which was detected by a charge-coupled device (CCD) cooled at 77 K. A Bruker D8 texture diffractometer was used at the x-ray wavelength of 0.154 nm in θ–2θ mode to determine the crystalline state of the samples. The XPS measurements were made using a VG Clam 100 electron spectrometer with a standard Al Kα and Mg Kα twin anode x-ray source. The Mg Kα (1253.6 eV) was used to excite photoelectrons from the samples in these experiments. The analyzer pass energy was set at 20 eV with a step size of 0.1 eV and the dwell time was 0.2 s.
Results
Results of irradiation
A selection of typical CLSM images used for the present study are shown in figure and depict the effect of the ZnO coating on the activity of S. epidermidis using the viability staining technique both with and without exposure (2 h duration) to UVA radiation, respectively, figures (a) and (c) and the corresponding controls, respectively, figures (b) and (d). The complete cell viability and one-way ANOVA results are given in table (see section 2.3 for detailed procedures).
Figure 1 Fluorescence images of stained S. epidermidis bacteria grown on ZnO coated glass slides and recorded using confocal laser scanning microscopy (CLSM). The green and red fluorescence, respectively, indicate live and membrane compromised bacteria: (a) ZnO coated control slide, (b) uncoated control slide, (c) UVA exposed ZnO coated slide and (d) UVA exposed uncoated slide (see text of paper for details about UV irradiation conditions). The white length scale bars represent 10 μm for images (a) and (b) and 5 μm for images (c) and (d), respectively.
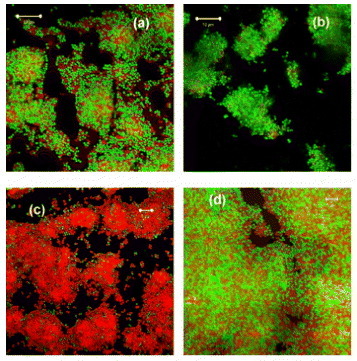
Table 1 Comparison of cell viability (%) of S. epidermidis biofilms grown on ZnO coatings with or without exposure to UVA lamp radiation and P-values from analysis of variance.
From table , we see that after 2 h exposure to UVA, 83 ±4% of the cells were viable on the uncoated portion as opposed to 30 ±12% on the ZnO coated one and the coating had a highly significant effect on viability (P <0.01). Notably, it is seen that the unexposed controls were 87 ±6% and 70 ±13% viable for the uncoated and coated samples, respectively, with a visible but insignificant effect of the coating on viability (P >0.05). From these data, we observe a slight inactivation consistent with the previously reported antibacterial properties of ZnO (see [Citation18–22] and introduction section). A highly significant inactivation is apparent for the UV activated ZnO coating where only 30 ±12% of the cells remains viable after 2 h UVA illumination compared to the 83 ±4% viability observed for the uncoated but identically illuminated surface. Since the staining method used to assess viability is indicative of loss of cell membrane integrity, then our results clearly show that UVA exposure initiates the release of active species on the ZnO surface that are responsible for this membrane degradation.
SEM and AFM analyses
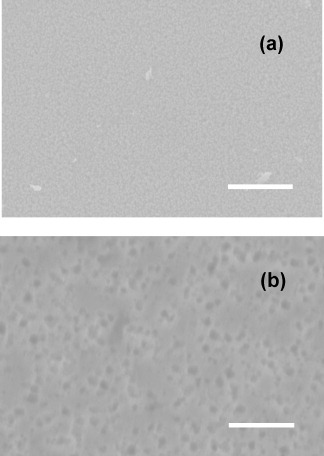
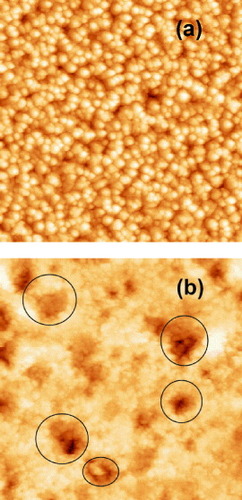
SEM and AFM images of the as-deposited ZnO film are presented in figures (a) and (a), respectively, while those corresponding to the cleaned (see details at the end of section 2.3) ZnO surface after exposure to UV radiation of the biofilm are presented on figures (b) and (b), respectively. Note that no evidence of residual bacterial cell ‘debris’ could be seen in the SEM or AFM scans of the latter. The as-deposited films present a uniform grain-like microstructure with a typical grain size of the order of 250 nm and an rms roughness of 49.7 nm with an average height of the features of 231.7 nm. The UV treated ZnO films present a pattern of randomly distributed micropits, indicated by the open circles on figure (b), with typical diameters in the 0.5–1 μm range and depth (estimated from the AFM images) of the order of 0.4 μm. The rms roughness has now increased to 68 nm with an average height of the features of 401.5 nm. As this pattern was only found for the UV back-illuminated films, these observations suggest that the irradiation induces the decomposition of ZnO material at the location of active sites.
XRD analysis
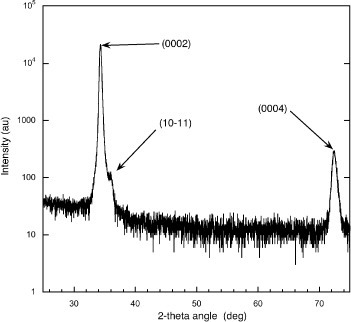
The crystalline texture of the as-deposited ZnO films was determined by XRD analysis using θ–2θ scans as shown in figure . The observed diffraction peaks are readily identified as due to the (0002), and (0004) reticular planes of wurtzite ZnO; the
peak having a considerably lower intensity than the (0002) peak. Thus, for the deposition conditions used here, all the films were found to be crystalline and very strongly c-axis oriented, i.e. the wurtzite (000 l) planes lies in the plane of the glass substrate surface. We point out that the successful room temperature growth of hard-wearing ZnO crystalline films on an amorphous substrate, such as glass, is enabled by the use of the PLD growth technique (see e.g. [Citation41]). The XRD data for the UV treated samples did not present any significant new features that could be attributed to known crystalline compounds other than ZnO.
UV-visible absorption spectroscopy
In figure , the UV-visible transmission spectra of ZnO-coated glass substrates before (solid line) and after (dashed line) the microbial and UV light interactions are presented. The spectral range of the UV lamp used for the bacterial inactivation is also reproduced on this graph. The solid-line spectrum shows the characteristic ultraviolet ‘cut-off’ behavior of ZnO below the band-gap and a higher transmission in the visible part of the spectrum. From figure , it is seen that the unprocessed glass/ZnO sample transmits about 0.2% below 384 nm and roughly 85% above 400 nm. For the processed sample, one observes a quite different, more gradual, non step-like, behavior in the region 400–700 nm. The visible transmission is lower also as expected from the yellowish-milky appearance of the sample described above.
Figure 5 Optical transmission (300–700 nm) spectra of (a) as-deposited and (b) UV treated and cleaned ZnO films.
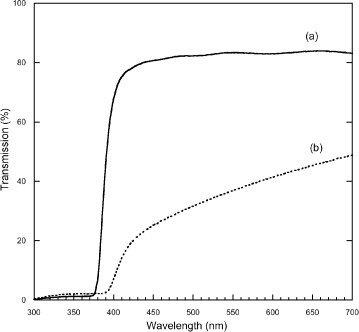
From these observations, we can draw two conclusions regarding the effect of the ultraviolet irradiation of the samples for biofilm inactivation: (i) band gap photons, i.e. with energies large enough to generate electron-hole pairs, are produced in the UVA source and they can penetrate and traverse the entire 2 μm thickness of the ZnO film and (ii) the processing steps may have lead to chemical/structural changes of the ZnO.
The values of the optical gap Eopt for the ZnO materials of figures (a) and (b) were estimated from a Tauc plot generated from the transmission data [Citation42], whereby an extrapolation of the linear part of the [α(hν)⋅hν]n versus (hν−Eopt) graph provides Eopt, where α(hν) is the linear absorption coefficient and hν is the photon energy. The linear absorption coefficients for the ZnO thin films were calculated from the absorption spectra assuming negligible reflectivity and scattering losses. Values of Eopt≈3.22 and 3.05 eV were found, respectively, for the best linear fit in the region of the band gap that corresponded to n=2 in both cases. The value of 3.22 eV found for the as-grown ZnO film is typical of the Eopt values for laser grown ZnO films that can be found in the current literature, e.g. [Citation13]. The small change in the Eopt value for the processed sample appears more difficult to interpret and may indicate that the UV exposure of the ZnO material has produced chemical and/or structural changes, these could lead to increased scattering losses for example.
It is interesting to remark that the hole diffusion length in ZnO is reported to be in the range 0.5–2 μm [Citation43] which is comparable to the thickness of the ZnO film used here. Thus, holes generated at the glass/ZnO interface can migrate to the ZnO/biofilm interface. This fact will be used as the basis of our model to explain the bactericidal properties of the ZnO film surface in section 4.
Raman spectroscopy
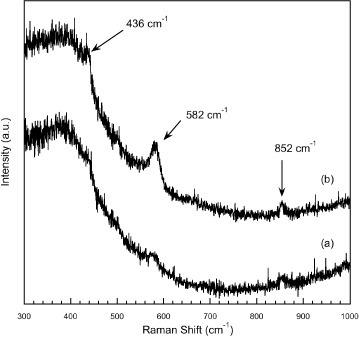
In figure , the Raman spectra of ZnO-coated glass substrates before and after the microbial and UV light interactions are presented as the traces labeled (a) and (b), respectively. Each spectrum is the result of the averaging of 3 scans each accumulated over a 300 s period. Such a procedure was necessary to obtain acceptable Raman signals. As the excitation laser wavelength of 488 nm will penetrate through the bulk of the thin film/glass assembly, the three weak Raman bands measured above the noise at 436, 582 and 852 cm−1 should correspond to Raman modes of ZnO or glass. The low-frequency band centered around 400 cm−1 in both spectra is a feature of the glass substrate. The band at 436 cm−1 can be readily attributed to the allowed E2(high) phonon mode of ZnO and has been extensively discussed, see [Citation44, Citation45], including in Raman studies of laser-deposited ZnO [Citation46]. The bands at 582 and 852 cm−1 are described as anomalous Raman modes variously attributed (see the discussion in [Citation44] and also [Citation45, Citation46]) to local vibrational modes of defects or impurities or silent ZnO modes allowed by the breakdown of crystal symmetry induced by defects or impurities. Although one has to keep in mind sample to sample variations and local inhomogeneities, the observed Raman spectra are indicative of poor structural quality. The enhancement of the 582 cm−1 mode and, to a lesser extent, that of the 852 cm−1 mode, in the spectrum of figure (b) indicate a further degradation of the structural quality of the ZnO material upon the UV treatment, which could be attributed to an increased defect concentration.
XPS analysis
The x-ray photoemission spectra of two characteristic samples are presented in figure . The O 1s core-level is shown with the binding energy scale in eV referenced to the surface C 1s binding energy at 285.0 eV. The reference ZnO sample of figure (a) was obtained from a sample having undergone the complete process of biofilm growth and its subsequent removal but no UV treatment while the sample of figure (b) was identically processed and cleaned (see procedure described at the end of section 2.3) as well as being UV-treated. The XPS data of figure should thus represent the sole effect of the ultraviolet back-illumination on the surface/subsurface ZnO films, i.e. at the ZnO/biofilm interface. We note that in both cases the spectra showed virtually no angular dependence indicating the absence of strongly surface-localized components. The XPS data were analyzed with the help of a peak fitting computer program that assumes a nonlinear background and a combination of Lorentzian and Gaussian line shapes (Voigt profiles) for the fitting peaks. The data of figure (a) is the sum of two contributions peaking at 530.8 and 532.5 eV with relative intensities of 77 and 23%, respectively, while a single peak fit with a maximum at 531.7 eV provided the best fit for the data of figure (b). Electron spectra were also measured in the region of the Zn 2p3/2 binding energy and showed identical pure Lorentzian line shapes with maxima at 1022.4 and 1022.8 eV for the reference and UV treated samples.
Figure 7 XPS of (a) ZnO reference film and (b) UV exposed ZnO film in the region of the O 1s binding energy (in eV). The spectra are referenced to the C 1s binding energy taken at 285.0 eV.
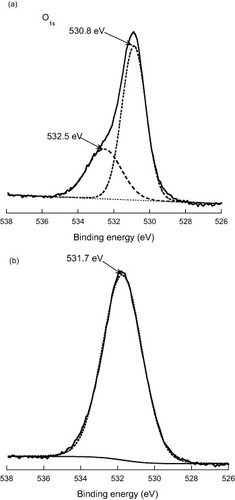
The relative Zn:O concentrations of the films were estimated from the relative intensity ratios of the Zn and O electron peaks and yielded values of 1:0.95 and 1:2.2 for the reference and UV treated samples, respectively. Analyses of the relative intensity of the C 1s peak also showed that the total amount of carbon has not changed significantly between the two samples. From these data, it is apparent that the UV back-illumination of the ZnO film has had a marked effect on the chemical environment of the oxygen in the film subsurface. The corresponding changes provide clues to elucidate the bactericidal processes.
The XPS spectrum of ZnO has been measured and interpreted by a number of authors, see for example [Citation47–49], while Dupin et al [Citation50] have carried out systematic XPS studies of metal oxides (including ZnO), hydroxides and peroxides in highly controlled experimental conditions. We interpret our O 1s XPS data following the general picture provided by these works. For the reference sample, the strong low-energy component at 530.8 eV corresponds to the O2− of the wurtzite structure, while the 532.5 eV component corresponds to oxygen in a lower ionization, e.g. O− species, that is supposed to result from the compensation of charge deficiencies in the subsurface of the metal oxide [Citation50]. The component at 531.7 eV, characteristic of the UV treated ZnO film, corresponds to oxygen integrated in the material with ionization characteristic of OH− (hydroxide) species or O22− (peroxide) species. Such species could result from the incorporation of water and the products of its oxidation in the ZnO surface/subsurface. Furthermore, we note that the 0.4 eV shift observed for the Zn 2p3/2 peak is similar to the core level chemical shift reported by Dake et al [Citation51] for the ZnO and Zn(OH)2 systems, respectively.
The picture obtained from the XPS analysis of the reference film is compatible with the known structure of ZnO possessing active surface defect sites, such as oxygen vacancies Vo, which confer on the material its potent catalytic properties and strong photoeffects [Citation14]. These were revealed in the present experiments carried out in an aqueous environment, in the form of the net oxidizing effect of the UV irradiation of ZnO films.
Discussion
In this study a bacterial biofilm of S. epidermidis RP62A was grown over a ZnO coating on a glass microscope slide and the antimicrobial activity of this coating was evaluated in vitro. After back-illumination using a UVA light source it was found that the ZnO coating had a significant effect on the viability of the adherent biofilm. We have mentioned above that ZnO films have been previously used for their photocatalytic properties. Also, photocatalytic disinfection/inactivation at the surface of the catalyst film is generally thought to result from either the direct oxidation of organic matter by positive photogenerated holes or the attack of radicals, like O2−•, HO2• and OH•, produced following water oxidation by the photo-holes (see section 1).
We now propose a model based on these mechanisms for the bactericidal activity to interpret our experimental results and show that it is supported by various aspects of the film characterizations that were detailed above. We recall first that the ZnO films are back-illuminated from the substrate face. In ZnO, it is known [Citation14] that oxygen and other adsorbed species can capture a conduction band electron thus creating a negatively charged surface. This leaves behind a depletion layer that can be up to 100 nm thick and produces pronounced upward band bending. Upon back-irradiation by band gap photons, electron-hole pairs are generated throughout the entire thickness of the film. We have already mentioned that the hole diffusion length in ZnO is in the range 0.5–2 μm, thus it is reasonable to assume that some photogenerated holes will be driven towards the surface and reach the ZnO/biofilm interface. The photo-holes can then directly oxidize the bacterial organic matter [Citation26, Citation27]. Alternatively, they can oxidize water to produce hydroxyl OH• radicals which can engage in a series of reactions leading to reactive oxygen species such as superoxides (O2−•), peroxides (O22−) or hydroxides (OH•) radicals as shown by Domènech et al [Citation52]. These oxygen species have potent antibacterial properties [Citation53]. Kormann et al [Citation54] and Doménech and Prieto [Citation55] have shown that a likely product following the initial formation of OH• is hydrogen peroxide H2O2, while Padmavathy and Vijayaraghavan [Citation22] have reported that H2O2, produced via UV photon absorption in ZnO, can penetrate bacteria cell membranes and cause their death. Hydrogen peroxide is also known as an oxidizing agent of zinc oxide and zinc hydroxide [Citation22, Citation52, Citation56]. On the other hand, the ultraviolet illumination of ZnO is also known to lead to the photolysis of the ZnO lattice whereby oxygen gas is released leaving behind very active oxygen vacancy surface defect sites [Citation14, Citation55]. This is akin to a reduction of the surface and compatible with the aforementioned amphoteric character of ZnO. Bikondoa et al have shown unambiguously that such oxygen vacancy sites mediate the photodissociation of water on the surface of rutile TiO2 [Citation10]. Thus, it is reasonable to assume that such a process may also play a role in our experiments since we have shown that a small fraction of the UV light can penetrate the film through to the surface.
The experimental data presented in section 3, contrasting the physical state of reference ZnO films with that of UV treated films actively support the above model. The XPS data clearly showed the evolution near the surface of the wurtzite lattice oxygen into oxygen with lower coordination and an increase in the concentration of oxygen relative to that of zinc. This is compatible with the presence of reactive oxygen species and hydrogen peroxide leading to a further oxidation of the ZnO and an increased concentration of oxygen on the surface and at grain boundary areas. The Raman and the UV-visible transmission data showed the evolution of the ZnO lattice upon UV exposure into a more defective form which also concurs with the above model. Finally, the SEM and AFM images showed the presence of ‘micropitting’ on the surface which is compatible with the presence of active surface sites playing a role in the photocorrosion of the lattice.
Conclusions
We have shown that c-axis oriented ZnO films deposited onto glass substrates at room temperature using pulsed laser deposition have notable bactericidal activity against a biofilm of S. epidermidis when back-illuminated with a UVA light source in an aqueous environment. We have characterized reference and UV exposed ZnO film samples using standard characterization techniques such as XPS, UV-visible absorption, Raman spectroscopy and SEM/AFM microscopies to understand the nature of the mechanisms that are responsible for the bactericidal behavior. A model accounting for all the experimental findings was proposed and according to which the bactericidal action follows from the strongly oxidizing behavior of photogenerated holes on the ZnO surface. In particular, there are good reasons to believe that the photo-holes oxidize water at certain active surface sites leading to the formation of reactive oxygen species with known bactericidal properties. We believe that this study is of particular interest for the acute problem of the disinfection of pathogenic biofilms which form on medical device/implant surfaces. We are currently exploring possible methods based on the present study that could be used for the routine sterilization of such devices.
Acknowledgments
This work was part-supported by Science Foundation Ireland under the Principal Investigator Programme, the National Development Plan (NDP) and the Health Research Board. The authors would like to thank Drs Lisa O’Reilly and Dorotha Wencel for their assistance with some of the experiments.
References
- StobieNDuffyBMcCormackD EColreavyJHidalgoMMcHalePHinderS J 2008 Biomaterials 29 963 http://dx.doi.org/10.1016/j.biomaterials.2007.10.057
- PopatK CEltgrowthMLaTempaT JGrimesC ADesaiT A 2007 Biomaterials 28 4880 http://dx.doi.org/10.1016/j.biomaterials.2007.07.037
- HashimotoKIrieHFujishimaA 2005 Japan. J. Appl. Phys. 44 8269 http://dx.doi.org/10.1143/JJAP.44.8269
- McMurrayT AByrneJ ADunlopP S MMcAdamsE T 2005 J. Appl. Electrochem. 35 723 http://dx.doi.org/10.1007/s10800-005-1397-1
- SunadaKWatanabeTHashimotoK 2003 Environ. Sci. Technol. 37 4785 http://dx.doi.org/10.1021/es034106g
- MachidaMNorimoteKKimuraT 2005 J. Am. Ceram. Soc. 88 95
- KikuchiYSunadaKIyodaTHashimotoKFujishimaA 1997 J. Photochem. Photobiol. A 106 51 http://dx.doi.org/10.1016/S1010-6030(97)00038-5
- DuffyE F et al 2004 Sol. Energy 77 649 http://dx.doi.org/10.1016/j.solener.2004.05.006
- HuangZManessP CBlakeD MWolfrumE JSmolinskiS LJacobyW A 2000 J. Photochem. Photobiol. A 130 163 http://dx.doi.org/10.1016/S1010-6030(99)00205-1
- BikondoaOPangC LIthninRMurynC AOnishiHThorntonG 2006 Nat. Mater. 5 189 http://dx.doi.org/10.1038/nmat1592
- MaedaMWatanabeT 2006 J. Electrochem. Soc. 153 C186 http://dx.doi.org/10.1149/1.2165773
- BaanRStraifKGrosseYSecretanBEl GhissassiFCoglianoV 2006 Lancet Oncol. 27 295 http://dx.doi.org/10.1016/S1470-2045(06)70651-9
- ÖzgürÜAlivov YaLLiuCTkeAReshchikovM ADoğanSAvrutinVChoS JMorkoç H 2005 J. Appl. Phys. 98 041301 http://dx.doi.org/10.1063/1.1992666
- HirschwaldW H 1985 Acc. Chem. Res. 18 228 http://dx.doi.org/10.1021/ar00116a001
- DieboldUVogel KoplitzLDulubO 2004 Appl. Surf. Sci. 237 336
- LimmerS JKulpE ASwitzerJ A 2006 Langmuir 22 10535 http://dx.doi.org/10.1021/la061185b
- XiuF XYangZMandalapuL JLiuJ L 2006 Appl. Phys. Lett. 88 152116 http://dx.doi.org/10.1063/1.2194870
- MoorerW RGenetJ M 1982 Oral Surg. Oral. Med. O 53 508 http://dx.doi.org/10.1016/0030-4220(82)90468-6
- YamamotoOKomatsuMSawaiJNakagawaZ E J 2004 Mater. Sci. Mater. Med. 15 847 http://dx.doi.org/10.1023/B:JMSM.0000036271.35440.36
- YamamotoO 2001 Int. J. Inorg. Mater. 3 643 http://dx.doi.org/10.1016/S1466-6049(01)00197-0
- GhuleKVithal GhuleAChenB JLingY C 2006 Green Chem. 8 1034 http://dx.doi.org/10.1039/b605623g
- PadmavathyNVijayaraghavanR 2008 Sci. Technol. Adv. Mater. 9 035004 http://dx.doi.org/10.1088/1468-6996/9/3/035004
- LiuH LYangT C K 2003 Process. Biochem. 39 475 http://dx.doi.org/10.1016/S0032-9592(03)00084-0
- HariharanC 2006 Appl. Catal. A 304 55 http://dx.doi.org/10.1016/j.apcata.2006.02.020
- YeCBandoYShenGGolbergD J 2006 Phys. Chem. B 110 15146 http://dx.doi.org/10.1021/jp061874w
- RoheBVeemanW STauschM 2006 Nanotechnology 17 277 http://dx.doi.org/10.1088/0957-4484/17/1/047
- AnandanS et al 2006 J. Mol. Catal. A—Chem. 266 149 http://dx.doi.org/10.1016/j.molcata.2006.11.008
- SevenODindarBAydemirSMetinDOzinelM AIcliS 2004 J. Photochem. Photobiol. A 165 103 http://dx.doi.org/10.1016/j.jphotochem.2004.03.005
- SawaiJ 2003 J. Microbiol. Methods 254 177 http://dx.doi.org/10.1016/S0167-7012(03)00037-X
- ChakrabartiSDoggettBO’HaireRMcGlynnEHenryM OMeaneyAMosnierJ P 2006 Electron. Lett. 42 1181 http://dx.doi.org/10.1049/el:20062161
- DuclèreJ R et al 2005 J. Mater. Sci. Mater. El. 16 421 http://dx.doi.org/10.1007/s10854-005-2308-2
- EasonR 2007 Pulsed Laser Deposition of Thin Films: Applications-Led Growth of Functional Materials Hoboken, NJ Wiley
- AltmanHSteinbergDPoratYMorAFriedmanDFriedmanMBachrachG 2006 J. Antimicrob. Chemother. 58 198 http://dx.doi.org/10.1093/jac/dkl181
- AutyM A EGardinerG EMcBreartyS JO’SullivanE OMulvihillD MCollinsJ KFitzgeraldG FStantonCRossR P 2001 Appl. Environ. Microbiol. 67 420 http://dx.doi.org/10.1128/AEM.67.1.420-425.2001
- GouveaC A KWypychFMoraesS GDuranNNagataNPeralta-ZamoraP 2000 Chemosphere 240 433 http://dx.doi.org/10.1016/S0045-6535(99)00313-6
- HopeC KClementsDWilsonM 2002 J. Appl. Microbiol. 93 448 http://dx.doi.org/10.1046/j.1365-2672.2002.01703.x
- HopeC KWilsonM 2003 J. Microbiol. Methods 54 403 http://dx.doi.org/10.1016/S0167-7012(03)00085-X
- OosterhofJ J HBuijssenK J D ABusscherH Jvan der LaanB F A Mvan der MeiH C 2006 Appl. Environ. Microbiol. 72 3673 http://dx.doi.org/10.1128/AEM.72.5.3673-3677.2006
- TakenakaSIwakuMHoshinoE 2001 J. Infect. Chemother. 7 87 http://dx.doi.org/10.1007/s101560100014
- WebbJ SThompsonL SJamesSCharltonTTolker-NielsenTKochBGivskovB MKjellebergS 2003 J. Bacteriol. 185 4585 http://dx.doi.org/10.1128/JB.185.15.4585-4592.2003
- NakataYOkadaTMaedaM 2002 Appl. Surf. Sci. 197–198 368 http://dx.doi.org/10.1016/S0169-4332(02)00426-9
- NatsumeYSakataH 2002 Mater. Chem. Phys. 78 170 http://dx.doi.org/10.1016/S0254-0584(02)00314-0
- LopatiukOChernyakLOsinskyAXieJ QChowP P 2005 Appl. Phys. Lett. 87 162103 http://dx.doi.org/10.1063/1.2106001
- ManjonF JMariBSerranoJRomeroA H 2005 J. Appl. Phys. 97 053516 http://dx.doi.org/10.1063/1.1856222
- EmelieP YPhillipsJ DBullerBVenkateswaranU D 2006 J. Electron. Mater. 35 525 http://dx.doi.org/10.1007/s11664-006-0094-0
- Ashkenov et al 2003 J. Appl. Phys. 93 126 http://dx.doi.org/10.1063/1.1526935
- KunatMGirol StGBeckerTBurghausUWollC 2002 Phys. Rev. B 66 081402 http://dx.doi.org/10.1103/PhysRevB.66.081402
- GrunzeMHirschwaldWThullE 1976 Thin Solid Films 37 351 http://dx.doi.org/10.1016/0040-6090(76)90605-2
- MosbackerH LStrzhemechnyY MWhiteB DSmithP ELookD CReynoldsD CLittonC WBrillsonL J 2005 Appl. Phys. Lett. 87 012102 http://dx.doi.org/10.1063/1.1984089
- DupinJ CGonbeauDVinatierPLevasseurA 2000 Phys. Chem. Chem. Phys. 2 1319 http://dx.doi.org/10.1039/a908800h
- DakeL SBaerD RZacharaJ M 1989 Surf. Interface Anal. 14 71 http://dx.doi.org/10.1002/sia.740140115
- DomènechXAyllónJ APeralJ 2001 Environ. Sci. Pollut. Res. 8 1 http://dx.doi.org/10.1007/BF02987409
- GauntFBeggsC BGeorghiuG E 2006 IEEE Trans. Plasma Sci. 34 1257 http://dx.doi.org/10.1109/TPS.2006.878381
- KormannCBahnemanD WHoffmannM R 1988 Environ. Sci. Technol. 22 798 http://dx.doi.org/10.1021/es00172a009
- DoménechJPrietoA 1986 J. Phys. Chem. 90 1123 http://dx.doi.org/10.1021/j100278a031
- UekawaNMochizukiNKajiwaraJMoriFJunWuYKakegawaK 2003 Phys. Chem. Chem. Phys. 5 929 http://dx.doi.org/10.1039/b210990e