Abstract
This review on nanoparticles highlights the various biopolymers (proteins and polysaccharides) which have recently revolutionized the world of biocompatible and degradable natural biological materials. The methods of their fabrication, including emulsification, desolvation, coacervation and electrospray drying are described. The characterization of different parameters for a given nanoparticle, such as particle size, surface charge, morphology, stability, structure, cellular uptake, cytotoxicity, drug loading and drug release, is outlined together with the relevant measurement techniques. Applications in the fields of medicine and biotechnology are discussed along with a promising future scope.
Introduction
Nanotechnology refers to the research and technological developments at atomic, molecular, and macromolecular scales, which lead to the controlled manipulation and study of structures and devices with length scales in the range of 1–100 nm [Citation1]. Nanotechnology, the term probably first coined by Taniguchi in Japan [Citation2], is a branch of manufacturing where dimensions on the order of a nanometer are important. Several researchers emphasized the significance of size and revealed the advantages of nanoparticles over microspheres (>1 μm) [Citation3]. Biological nanoparticles are mainly developed for drug delivery systems as an alternative to liposome technology, in order to overcome the problems related to the stability of these vesicles in biological fluids and during storage [Citation4]. The nanoparticle technology used in the recent years has great significance in improving the efficacy of the drugs. The nanoparticles fit into colloidal drug delivery systems, which offer advantages of drug targeting by modified body distribution [Citation5] as well as the enhancement of the cellular uptake [Citation6], which benefits from reduction of undesired toxic side effects of the free drugs [Citation7]. With their easy accessibility in the body, nanoparticles can be transported via the circulation to different body sites [Citation8], thus aiding in systemic treatments. Nanoparticles can be prepared from a variety of materials such as protein, polysaccharides and synthetic polymers. The choice of materials depends on several factors including (i) size and morphology of the nanoparticles; (ii) surface charge and permeability of the nanoparticle; (iii) degree of biodegradability, biocompatibility and cytotoxicity; (iv) drug loading and release profile desired [Citation9, Citation10]. This review details the latest developments in protein and polysaccharide nanoparticles; their different methods of fabrication; their characterization in terms of size, surface charge, morphology, stability, structure, cellular uptake, cytotoxicity, drug loading, drug release; and also utilization of these nanoparticles in medicine and biotechnology.
Biopolymer nanoparticles
Biopolymer nanoparticles were first designed using albumin [Citation11] and non-biodegradable synthetic polymers such as polyacrylamide and poly (methylacrylate) [Citation12, Citation13]. The risks of chronic toxicity due to the intracellular and/or tissue overloading of non-degradable polymers were soon considered as a major limitation for the systemic administration of polyacrylamide and poly (methylacrylate) nanoparticles in humans. As a consequence, the type of nanoparticles that received much attention was designed with synthetic biodegradable polymers including polyalkylcyanoacrylate, poly (lactic-co-glycolic acid) and polyanhydride [Citation14–17].
The therapeutic potential of these biodegradable colloidal systems was investigated for various applications [Citation18–23]. Despite the very interesting results reported in literature, these systems may also be concerned with toxicological problems [Citation24, Citation25]. There is another limitation for the bionanoparticle-based administration of hydrophilic molecules such as peptides, proteins and nucleic acids (oligonucleotide and genes) which are recognized to have great potential in therapeutics. This limitation is mainly because the polymers forming these nanoparticles are mostly hydrophobic, whereas proteins, peptides and nucleic acids are hydrophilic. This leads to difficulties for the drug to efficiently encapsulated and protected against enzymatic degradation [Citation22, Citation26]. Therefore, the preparation of nanoparticles using more hydrophilic and naturally occurring materials has been explored [Citation27–29].
The need for developing biodegradable nanoparticles (liposome, virus-like particle (VLP), protein, etc) as effective drug delivery devices was felt years ago [Citation30]. The reason being in addition to the general advantages of nanoparticles, biopolymer nanoparticles in particular offer several advantages, which include the ease of their preparation from well-understood biodegradable polymers and their high stability in biological fluids and during storage [Citation31]. Nanoparticles made of biodegradable polymers like proteins and polysaccharides can act as efficient drug delivery vehicles for sustained, controlled and targeted release, aiming to improve the therapeutic effects and also to reduce the side effects of the formulated drugs [Citation32].
Protein nanoparticles
The first naturally occurring material used for the preparation of nanoparticles consisted of two proteins, albumin and gelatin [Citation11, Citation16, Citation33, Citation34]. Among the colloidal systems, those based on proteins are very promising because they are biodegradable, less immunogenic [Citation35] and non-toxic; they have greater stability in vivo and during storage [Citation36], are relative easy to prepare and to monitor size distribution [Citation37], and their manufacture can be scaled up [Citation38, Citation39]. In addition, because of the defined primary structure of proteins, the protein-based nanoparticles offer various possibilities for surface modification and covalent drug attachment.
Albumin
Albumin, a protein found in blood plasma, has always been a remarkable molecule owing to its manifold functions and applications. Albumin is a biodegradable, biocompatible and less-immunogenic protein [Citation35, Citation36, Citation40]. The paramount function of albumin is in the circulatory system—to aid in transportation, metabolism, and distribution of exogenous and endogenous ligands [Citation40]. It also has an ability to act as an important extracellular antioxidant [Citation42] and to impart protection from free radicals and other harmful chemical agents [Citation43]. These unique attributes of albumin created a premier place for it in the drug therapy from time immemorial. Literature supports the use of modified serum albumin as a selective agent for tumor detection and/or therapy [Citation44] or as a delivery tool of toxic compounds for elimination of Mycobacterium tuberculosis via receptor-mediated drug delivery [Citation45].
Thus, nanotechnology era also employed the well-established properties of albumin, both human serum albumin (HSA) and bovine serum albumin (BSA), for various purposes as the nanoparticle drug (antibodies, interferon gamma, antiviral compounds) targeting carriers [Citation46–48], therapeutic enhancer of anti-cancer drugs [Citation49], modified vehicles for drug delivery across the brain to the central nervous system and also across blood brain barriers [Citation50, Citation51].
Collagen
Collagen is the structural building material of vertebrates and the most abundant mammalian protein that accounts for 20–30% of total body proteins. Collagen has a unique structure, size and amino acid sequence which results in the formation of triple helix fiber. Collagen is regarded a useful biomaterial because of its excellent biocompatibility, biodegradation and availability [Citation52]. Further, its amenability to modifications paved way for its in numeral applications in nanoparticles fabrication. Those modifications include addition of other proteins, such as elastin, fibronectin and glycosaminoglycans, which results in the improvement of its physicochemical and biological properties [Citation53, Citation54] as well as control of biodegradability and subsequent release of ligand by use of such crosslinking agents as glutaraldehyde, formaldehyde, ultraviolet and gamma radiation [Citation55]. The biodegradable collagen based nanoparticles are thermally stable, readily sterilizable, can be uptaken by the reticuloendothelial system and enable enhanced uptake of drug molecules into the cells [Citation34, Citation56].
Gelatin
Gelatin is a natural water-soluble macromolecule resulting from the heat dissolution and partial hydrolysis of collagen. There are two types of gelatin: type-A gelatin is obtained by acid treatment of collagen with the isoelectric point (pI) between 7.0 and 9.0, whereas Type-B gelatin is produced via alkaline hydrolysis of collagen with the pI between 4.8 and 5.0. Gelatin offers a number of advantages over other synthetic polymers including non-irritability, biocompatibility and biodegradability, which makes it one of the desirable materials as carrier molecule [Citation57, Citation58]. It is a natural macromolecule which is non-toxic and non-carcinogenic, and it shows low immunogenicity and antigenicity [Citation59–62]. Gelatin has large number of functional groups on its surface which aid in chemical crosslinking and derivatization. These advantages led to its application for the synthesis of nanoparticles for drug delivery during the last thirty years [Citation33, Citation34].
Silk proteins—sericin and fibroin nanoparticles
Silk fibers are primarily made of fibroin and sericin where the structural protein, fibroin is enveloped by the gum-like sticky protein, sericin.
Fibroin
Fibroin—a hydrophobic glycoprotein [Citation63] and one of the ‘core’ proteins—constitutes over 70% of the cocoon. This insoluble protein is almost entirely made of the amino acids glycine, alanine, and serine (-Gly-Ala-Gly-Ala-Gly-Ser-) leading to the formation of antiparallel β-pleated sheet in the fibers [Citation64]. Fibroin is semi-crystalline and consists of two phases: one is the highly crystalline β-pleated sheet phase and the other is non-crystalline phase [Citation65]. Silk fibroin is also histocompatible, less immunogenic and non-toxic [Citation66]. Silk fibroin can be processed into various forms including gels, fibers, membranes, scaffolds, hydrogels and nanoparticles [Citation67–70]. Silk fibroin matrices with high specific surface area, high porosity, good biocompatibility and biodegradability have extensive applications in the field of biomaterials and drug delivery [Citation66, Citation67]. Silk has been used as suture material for many centuries and it has been described as a biopolymer which evokes minimum foreign body response. Moreover silk-based biomaterials are highly biocompatible with various cell types and promote cell growth and proliferation [Citation71–73].
Sericin
Sericins—hydrophilic glycoproteins [Citation74] functioning as a ‘glue’—constitute 20–30% of the cocoon [Citation75]. These hot water soluble proteins comprise different polypeptides ranging in weight from 24 to 400 kDa [Citation76–79] and have unusually high serine content (40%) along with significant amounts of glycine (16%) [Citation76, Citation79]. Sericin is comprised of 35% β-sheet and 63% random coil, and has no α-helical content—hence, its partially unfolded state [Citation80–83]. Sericin nanoparticles, apart from the general advantages of protein nanoparticles, may also offer certain other benefits of the inherent property of sericin. Those include antioxidant [Citation84–87] and antitumor action [Citation88]; enhancement of the bioavailability of such elements as Zn, Mg, Fe, and Ca [Citation89]; as well as suppression of coagulation when sulfated [Citation90]. Sericin is non-toxic to fibroblast cells. Methionine and cysteine content in silk sericin are important factors to promote cell growth and collagen synthesis [Citation91]. Water-soluble silk sericin has no immunogenicity and is also a biocompatible macromolecular protein like silk fibroin [Citation92]. A study of the macrophage response of silk protein concludes that sericin does not usually manifest inflammatory activity when present in soluble form [Citation93]. Recently, Aramwit et al have concluded that sericin promotes wound healing without causing any inflammation [Citation94]. Table shows the milestones in silk protein nanoparticles fabrication and application in chronological order.
Table 1 Milestones of silk protein nanoparticles, preparation and application in chronological order.
Keratin
Keratins are a group of cysteine-rich structural proteins that exhibit a high mechanical strength owing to a large number of disulfide bonds. Keratin has been used very recently as nanosuspension that results in ultrathin, transparent keratin coatings to investigate the in vitro cell proliferation behavior as a potential coating material for standard cultivation [Citation95]. The keratin nanosuspension coatings may provide an inexpensive alternative to materials like collagen or fibronectin. Keratin nanosuspension may also find applications in tissue engineering if it is explored further.
Polysaccharide nanoparticles
Polysaccharide-derived nanoparticles and nanostructured surfaces help to improve biocompatibility of cell toxic material, together with new immobilization approaches, which are currently in development for novel bionano-particle-derived pharmaceutical formulations. Nanoparticles from naturally occurring polysaccharides were designed for the administration of peptides, proteins, and nucleic acids [Citation27, Citation28, Citation96].
Alginate
Alginate is a naturally occurring, water-soluble, linear unbranched polysaccharide extracted from brown seaweed. Alginate is composed of two types of uronic acids, α-L-guluronic acid and β-D-mannuronic acid. The monomeric units are grouped in three ways: blocks of alternating guluronic and mannuronic residues, blocks of guluronic acids and of mannuronic acids [Citation97, Citation98]. Alginate has been reported as mucoadhesive, biocompatible, non-immunogenic substance which undergoes dissolution and biodegradation under normal physiological conditions [Citation99]. The solubility of alginate in water depends on the associated cations. Sodium alginate is soluble in water, whereas calcium induces the formation of a gel [Citation97, Citation100]. Apart from the interaction with calcium, alginate may also form complexes with polycations such as polyenimine (PEI), chitosan, or basic peptides like polylysine and polyarginine [Citation100–102].
Carboxylic groups from the uronic acid confer negative charges to alginate. Chitosan endows nanoparticles with positive surface charge, prolongs the contact time of the active ingredients with the epithelium and enhances absorption via the paracellular transport pathway through the tight junctions [Citation102–104]. Alginate micro and nanoparticles can be easily obtained by inducing gelation with calcium ions [Citation105, Citation106]. This property can be used to produce a pre-gel consisting of very small aggregates of gel particles, followed by the addition of an aqueous polycationic solution to make a polyelectrolyte complex coating [Citation107]. Poly-L-lysine (PLL), a cationic natural polymer, has been used to combine with alginates to prepare nanoparticles. However, PLL is toxic and immunogenic if injected. Recently, chitosan (CS) was selected as an alternative cationic polymer. Table indicates the milestones of alginate and its composite nanoparticles preparation and applications in chronological order.
Table 2 Milestones of alginate and its composite nanoparticles preparation and applications in chronological order.
Chitosan
Chitosan is the second-abundant naturally occurring polysaccharide. Chitosan is made of randomly distributed β-(1-4)-linked D-glucosamine (deacetylated unit) and N-acetyl-D-glucosamine (acetylated unit). It is produced by deacetylation of chitin extracted from shells of crabs, shrimps and krill [Citation108]. Commercially available chitosan is deacetylated between 66 and 95% and has an average molecular weight between 3.8 and 2000 kDa. Chitosan is linear, hydrophilic, positively charged and has mucoadhesive property [Citation108–110]. It is an excellent biopolymer for preparation of microparticles [Citation8] and nanoparticles [Citation111] owing to its excellent biocompatibility and biodegradability [Citation112, Citation113]. In vivo, it is degraded by lysozyme [Citation114]. In addition, the amino groups confer to the molecule a high charge density and are readily available for chemical reactions and salt formation with acids. Chitosan is soluble in various acids, can also interact with polyions to form complexes and gels. These properties are exploited in the fabrication of nanoparticles based either on the spontaneous formation of complexes between chitosan and polyions including DNA [Citation96] or on the gelation of a chitosan solution dispersed in a water-in-oil emulsion. Table details the milestones of chitosan and its composite nanoparticle fabrication and applications in chronological order.
Table 3 Milestones of chitosan and its composite nanoparticle fabrication and applications in chronological order.
Fabrication methodologies
There are three common methods for the preparation of protein and polysaccharide based nanoparticles, namely, emulsification, desolvation and coacervation [Citation115]. Very recently, they were complemented by electrospray drying technique.
Emulsification
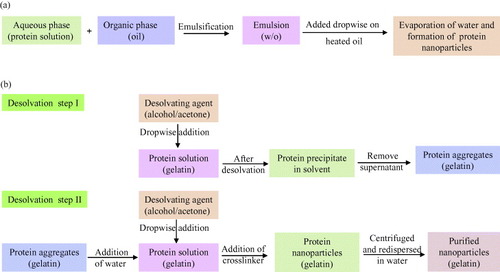
The principle of nano-emulsion formation is based on the spontaneous emulsification that occurs on mixing an organic phase and an aqueous phase (scheme (a)). The organic phase is a homogeneous solution of oil, lipophilic surfactant and water-miscible solvent, whereas the aqueous phase consists of hydrophilic surfactant and water [Citation116]. This method can be described as the dissolution of hydrophobic substances in an organic solvent which is further emulsified with an aqueous solution at very high shear. This results in the formation of very small droplets (50–100 nm). After emulsification, the organic solvent is removed by evaporation, yielding stable dispersions of solid nanoparticles [Citation117–119]. The major disadvantage of this method is the need to add organic solvent and then to remove it. Besides, residues of organic solvent may cause toxic problems.
Scheme 1 Schematic representation of nanoparticles preparation by (a) emulsification method [Citation38, Citation119] and (b) two step desolvation method [Citation39, Citation120].
Desolvation
Marty et al [Citation34] used a different method for fabrication of nanoparticles which involved slow addition of a desolvation factor, such as natural salts or alcohol, to the protein solution. The desolvation factor changes the tertiary structure of protein. On reaching the critical level of desolvation, protein clump will be formed which on crosslinking with a chemical substance (e.g. glutaraldehyde) will result in the formation of nanoparticles.
A variation in the desolvation method was developed by Coester et al [Citation39, Citation120] as a two-step desolvation process for the synthesis of gelatin nanoparticles (scheme (b)). In the first desolvation step, the low molecular gelatin fractions present in the supernatant is removed by decanting, and in the second step, high molecular fractions present in the sediment are redissolved and then desolvated again at pH 2.5. The resulting particles can then be easily purified by centrifugation and redispersion.
Coacervation
The coacervation method is similar to desolvation method; it employs mixing of the aqueous protein solution with organic solvent like acetone or ethanol to yield tiny coacervates. These coacervates are limited by the addition of the crosslinking agent, such as glutaraldehyde, etc [Citation121]. The difference of coacervation and desolvation methods is the various parameters which affect the fabrication process to give desired property to the nanoparticles. These parameters include initial protein concentration, temperature, pH, cross linker concentration, agitation speed, molar ratio of protein/organic solvent and organic solvent adding rate [Citation38].
Electrospray drying
The electrospraying method produces relatively monodisperse and biologically active protein particles. This method involves preparation of protein solution by dissolving the dry powder in an electrosprayable solution. Dispersion of the solution followed by solvent evaporation leaves dry residues collected on suitable deposition substrates. Insulin nanoparticles sized between 88 and 110 nm were prepared by this method [Citation122]. Higher production rate of the nanoparticles also increases their size. The biological activity of the electrosprayed protein-based nanoparticles is not affected by the process conditions [Citation122].
Characterization of nanoparticles
Nanoparticles are generally characterized by their size, morphology and surface charge, using such advanced microscopic techniques as scanning electron microscopy (SEM), transmission electron microscopy (TEM) and atomic force microscopy (AFM). The average particle diameter, their size distribution and charge affect the physical stability and the in vivo distribution of the nanoparticles. Electron microscopy techniques are very useful in ascertaining the overall shape of polymeric nanoparticles, which may determine their toxicity. The surface charge of the nanoparticles affects the physical stability and redispersibility of the polymer dispersion as well as their in vivo performance [Citation31].
Particle size
The major application of nanoparticles is in drug release and drug targeting. It has been found that particle size affects the drug release. Smaller particles offer larger surface area. As a result, most of the drug loaded onto them will be exposed to the particle surface leading to fast drug release. On the contrary, drugs slowly diffuse inside larger particles [Citation123]. As a drawback, smaller particles tend to aggregate during storage and transportation of nanoparticle dispersion. Hence, there is a compromise between a small size and maximum stability of nanoparticles [Citation124].
Another major application of nanoparticles is in the field of biosensors by immobilizing enzymes. Reducing the size of carrier materials generally improves the efficiency of immobilized enzymes, as the smaller particles provide large surface area for surface attachment of enzymes and limit the resistance to the diffusion of substrates. In addition, the physical characteristics of nanoparticles, such as enhanced diffusion and particle motility, can impact the inherent catalytic activity of attached enzymes [Citation125]. Polymer degradation can also be affected by the particle size. For instance, the degradation rate of poly(lactic-co-glycolic acid) was found to increase with increasing particle size in vitro [Citation126].
There are a several tools for determining nanoparticle size as discussed below.
Dynamic light scattering
Currently, the fastest and most popular method of determining particle size is photon-correlation spectroscopy (PCS) or dynamic light scattering (DLS). DLS is widely used to determine the size of Brownian nanoparticles in colloidal suspensions in the nano and submicron ranges [Citation127]. Shining monochromatic light (laser) onto a solution of spherical particles in Brownian motion causes a Doppler shift when the light hits the moving particle, changing the wavelength of the incoming light. This change is related to the size of the particle. It is possible to extract the size distribution and give a description of the particle's motion in the medium, measuring the diffusion coefficient of the particle and using the autocorrelation function. This method has several advantages: the experiment is quick, is almost automatic and does not require extensive experience. Moreover, this method has modest development costs. The advantage of using dynamic scattering is the possibility to analyze samples containing broad distributions of species of widely differing molecular masses (e.g. a native protein and various sizes of aggregates), and to detect very small amounts of the higher mass species (<0.01% in many cases). PCS determines the average particle size and polydispersity index (PI) which is a range of measurement of the particle sizes within measured samples [Citation128]. Observation of larger particles compared to smaller particles reveals that for a given temperature, the larger particles move more slowly than the smaller ones.
Nanoparticle tracking analysis
A slight modification to photon-correlation spectroscopy, nanoparticle tracking analysis (NTA) is a technique developed by NanoSight Ltd to determine the size distribution profile of small particles in a liquid suspension. The technique is used in conjunction with an ultramicroscope which allows small particles in liquid suspension to be visualized moving under Brownian motion. Computer software is then used to track particles’ movements and subsequently estimate their hydrodynamic radius, using the Stokes-Einstein equation. Also, as the samples require minimal preparation, the time required to process one sample is much reduced.
Particle morphology
The size and morphology of nanoparticles exert a profound influence on the physical and chemical properties that determine their interaction with the environment and biological systems. There are certain techniques to analyze the morphology of nanoparticles. Microscopic techniques like SEM, TEM and AFM, along with particle size and distribution analysis, also determine other parameters like morphology or surface roughness of the nanoparticles.
Scanning electron microscope
For SEM characterization, nanoparticles solution should be first converted into a dry powder, which is then mounted on a sample holder followed by coating with a conductive metal, such as gold, using a sputter coater. The sample is then scanned with a focused fine beam of electrons. The surface characteristics of the sample are obtained from the secondary electrons emitted from the sample surface. The nanoparticles must be able to withstand vacuum, and the electron beam can damage the polymer. The mean size obtained by SEM is comparable with results obtained by dynamic light scattering.
Transmission electron microscope
TEM operates on different principle than SEM, yet it often brings same type of data. The sample preparation for TEM is complex and time consuming because of its requirement to be ultra thin for the electron transmittance. The nanoparticles dispersion is deposited onto support grids or films. To make nanoparticles withstand the instrument vacuum and facilitate handling, they are fixed using either a negative staining material, such as phosphotungstic acid or derivatives, uranyl acetate, etc, or by plastic embedding. Alternate method is to expose the sample to liquid nitrogen temperatures after embedding in vitreous ice [Citation129]. The surface characteristics of the sample are obtained when a beam of electrons is transmitted through an ultra thin sample, interacting with the sample as it passes through.
Atomic force microscopy
AFM is yet another tool used to characterize variety of surfaces, including nanoparticles, at the atomic level and it is one of the primary forms of scanning probe microscopes [Citation130]. The prime advantage of AFM is its ability to image non-conducting samples without any specific treatment, thus allowing imaging of delicate biological and polymeric nano and microstructures. AFM requires minimal sample preparation and can be performed in ambient conditions [Citation131]. Scanning with a sharp probe across its surface and then monitoring and compiling the tip-sample interactions provide the images of the sample surface.
Particle stability
The colloidal stability is analyzed through zeta potential of nanoparticles. This potential is an indirect measure of the surface charge. It corresponds to potential difference between the outer Helmholtz plane and the surface of shear. Laser Doppler anemometry is the technique used to measure the zeta potential. It is based on the evaluation of the velocity of particles by the shift caused in the interference fringe, which is produced by the intersection of two laser beams. The electrophoresis mobility is then transformed into zeta potential. Most colloidal particles have negative zeta potential values ranging from about −100 to −5 mV. Surface charges prevent the agglomeration of nanoparticles polymer dispersions because of strong electrostatic repulsion, thereby enhancing the stability of the nanoparticles. The zeta potential can also provide information regarding the nature of material encapsulated within the nanocapsule or coated onto the surface [Citation132].
Particle structure
Analysis of structure changes of the free protein sample and protein nanoparticles is imperative to understand the nature of modifications taking place in the protein in terms of confirmation, folding, chemical bonding, etc, during the synthesis of nanoparticles.
X-ray diffraction
One of the techniques for this purpose is x-ray diffraction (XRD) which is the primary tool for investigating the structure of crystalline materials, from atomic arrangement to crystallite size and imperfections. XRD also analyzes the phase composition, crystallite size and shape, lattice distortions and faulting, composition variations, orientation and in situ structure development of the nanoparticles. Usually, the XRD pattern is obtained by illuminating the sample with an x-rays source (Copper Kα line) with wavelength of 1.54 Å and scanning the diffraction within a certain range of the angle 2θ.
Fourier transform infrared spectroscopy
Another technique to supplement XRD is Fourier transform infrared spectroscopy (FTIR). The advantage of FTIR over crystallographic techniques is its capability to provide information about the structural details of proteins in solution with greater spatial and temporal resolution [Citation133]. Sample used for characterization is usually lyophilized nanoparticles in minute quantities. The basic principle that governs is that the bonds and groups of bonds vibrate at characteristic frequencies. A molecule that is exposed to infrared rays absorbs infrared energy at frequencies which are characteristic to that molecule. FTIR analysis is carried out by illuminating the sample with a modulated IR beam. The sample transmittance and reflectance of the infrared rays at different frequencies is translated into an IR absorption plot, which is then analyzed and matched with known signatures of identified materials in the FTIR library.
Cellular uptake and cytotoxicity
Apart from these characterizations of nanoparticles, it is important to assess their cellular uptake and cytotoxicity, both in vitro and in vivo. Cellular uptake of nanoparticles is determined by tagging the nanoparticles with fluorescent tags like fluorescence isothiocyanate (FITC) followed by incubating these fluorescence-tagged nanoparticles with cells and their visualization under confocal laser scanning microscope [Citation67]. Cytotoxicity analysis is usually performed by incubating nanoparticles with cells and carrying out 3-(4,5-dimethylthiazol-2-yl)-2, 5-diphenyl tetrazolium bromide (MTT) assay [Citation134], a common method to evaluate toxicity of the biomaterials based on the mitochondrial activity. The underlying principle is the reduction of yellow to purple formazan in living cells [Citation135, Citation136]. Followed by the addition of a solubilization solution (usually either dimethyl sulfoxide, an acidified ethanol solution, or a solution of the detergent sodium dodecyl sulfate in diluted hydrochloric acid) to dissolve the insoluble purple formazan product into a colored solution. Then, the absorbance of this colored solution is quantified at a certain wavelength (depending on the employed solvent) using a spectrophotometer. These reductions take place only when reductase enzymes are active, and therefore, conversion is often used as a measure of viable cells.
Drug loading and drug release
In addition to the above characterization, bionanoparticles may be evaluated for their property of drug loading and drug release. The drug loading of the nanoparticles is generally defined as the amount of drug bound per mass of polymer (usually moles of drug per mg polymer or mg drug per mg polymer); it could also be given as percentage relative to the polymer. The technique used for this analysis is classical analytical methods like UV spectroscopy or high performance liquid chromatography (HPLC) after ultracentrifugation, ultra filtration, gel filtration, or centrifugal ultrafiltration. The encapsulation efficiency refers to the ratio of the amount of encapsulated/absorbed drug to the total (theoretical) amount of drug used, with regard to the final drug delivery system of the dispersion of nanoparticles. Quantification is performed with the UV spectroscopy or HPLC. Drug release assays are also similar to drug loading assay which is assessed for a period of time to analyze the mechanism of drug release.
Application of nanoparticles in drug delivery
Nanoparticle-based delivery systems have the potential power to improve drug stability, increase the duration of the therapeutic effect and permit enteral or parenteral administration, which may prevent or minimize the drug degradation and metabolism as well as cellular efflux [Citation104, Citation105, Citation107, Citation137]. Protein nanoparticles (figure ) can transport a number of drugs across the blood brain barrier that normally cannot cross this barrier after intravenous injection. A number of authors have demonstrated a considerable tendency for an accumulation of protein nanoparticles in certain tumors. The binding of a variety of cytotoxic drugs, 5-fluorouracil, paclitaxel and doxorubicin to albumin or gelatin nanoparticles significantly enhanced the efficacy against experimental tumors or human tumors transplanted to nude mice in comparison to free drug.
Figure 1 Schematic outline of the preparation of protein nanoparticles and its biomedical applications. In this layout, silk is shown as a representative protein. Silks are mainly obtained from the lepidopteron insects of the family Bombycidae (e.g. Bombyx mori) and Saturniidae (e.g. Antheraea mylitta). Silk proteins can be isolated from the cocoons and mature 5th instar larvae. Silk proteins are of two types: the fibrous protein fibroin and the glue protein sericin. The isolated silk proteins are further processed and engineered to obtain the regenerated silk protein solution. The regenerated silk solutions are then further subjected to processes like desolvation, coacervation, emulsification and electrospraying to obtain the silk-based nanoparticles. These silk-based nanoparticles are further utilized in such biomedical applications as drug delivery, enzyme immobilization, growth factors delivery, in vivo bioimaging, gene delivery, etc [Citation67].
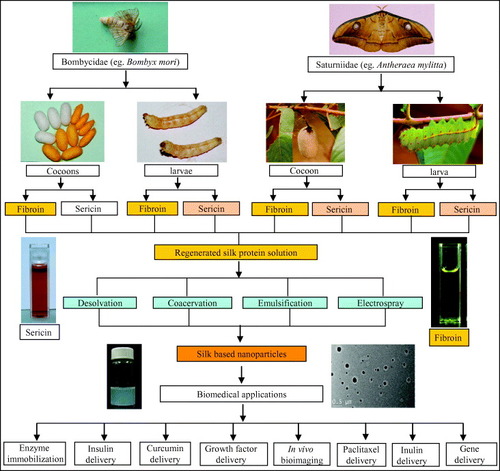
Gelatin nanoparticles loaded with a hydrophilic (pilocarpine HCl) and a hydrophobic (hydrocortisone) drug presented increased bioavailability of topical ophthalmic drug [Citation138] and also for other drugs such as doxorubicin [Citation139]. Later, gelatin nanoparticles were employed for peptide drug delivery system [Citation140]. Nanoparticles provide tremendous potential as gene delivery vehicles. They can easily direct and control gene expression kinetics by altering various processing parameters used to make the nanoparticles, as showed by the works where gelatin was used as efficient carrier system for plasmid vector DNA and proteins as model drugs [Citation141].
Surface modification of gelatin nanoparticles by covalent attachment of biotin-binding proteins, enabling the binding of biotinylated drug targeting ligands by avidin-biotin-complex formation, was also one of the strategies employed [Citation142–144]. Thiolated gelatin nanoparticles were produced to release the incorporated nucleic acid molecule in a highly reducing environment [Citation145]. These nanoparticles can meet specific controlled release needs due to their versatility in chemical modification and crosslinking. This was further demonstrated by Kushibiki and Tabata in 2005 [Citation146] using DNA-containing poly(ethylene glycol)-modified (PEGylated) gelatin nanoparticles. PEGylation of gelatin also proved beneficial to long-circulating delivery system in vivo and also for targeting tumor cells, such as BT/20 human breast cancer cells [Citation147].
Chitosan has been used in the pharmaceutical field in various forms, such as films, beads, intragastric floating tablets, microspheres, and nanoparticles [Citation8, Citation96, Citation148–150]. It was employed as a vehicle for drug delivery for sustained release systems [Citation152–155] and mucoadhesive formulations [Citation112, Citation113, Citation156]. Chitosan was also used for the improvement of the dissolution rate of poorly soluble drugs [Citation157], drug targeting [Citation158] and enhancement of peptide drug absorption [Citation111, Citation156].
The advantage of chitosan nanoparticles is its hydrophilic nature which results in its longer circulation in blood as observed by Allemann et al [Citation17] for the hydrophilic nanoparticles. Therefore, hydrophilic systems not only control the rate of drug administration that prolongs the duration of the therapeutic effect, but also deliver the drug to specific sites [Citation160]. Surface-modified chitosan nanoparticles are also suitable for the entrapment and controlled release of proteins and vaccines, such as ethylene oxide propylene oxide block copolymer [Citation96].
Conclusions
Nanoparticles can be developed from a variety of materials such as synthetic polymers, proteins and polysaccharides. Biopolymeric nanomaterials are advantageous compared to the synthetic polymers, as they are biodegradable, biocompatible and non-toxic in nature.
Biopolymeric nanoparticles (proteins and polysaccharides) are fabricated by four different types of methods, namely emulsification, desolvation, coacervation and electrospray drying.
Biopolymeric nanoparticles are generally characterized in terms of size and morphology (by DLS, SEM, TEM and AFM techniques); particle stability (DLS and Zeta analyzer); particle structure (XRD and FTIR), cytotoxicity and biocompatibility.
Biopolymeric nanoparticles hold enormous applications as vehicle for administration of drugs and vaccines. Bioactive molecules within the nanoparticles can target specific sites (tumors) and deliver at those desired locations.
Future scope
Several challenges remain for further development of biopolymer-based nanoparticles:
The preparations strategies of protein and polysaccharide based nanostructures need to be enhanced for obtaining evenly dispersed and stable nanoparticles.
The properties and applications of protein as well as polysaccharide based nanoparticles are still in the developing stage for drug delivery and therapeutic purposes.
The cytotoxicity, biodegradability, biocompatibility and immune response of these biopolymeric nanoparticles need to be evaluated for their potential applications in clinical drug delivery.
The development and the production of the biopolymeric nanoparticles are still at the laboratory scale, biologists and chemical engineers need to collaborate for the large-scale production of these nanoparticles.
The combination of bioengineering, chemical modification and nanomaterials sciences in designing nano-particulate structures will improve the pharmacokinetics and biodistribution of the drug delivery system.
Acknowledgements
Our laboratory is financially supported by Department of Biotechnology (Indo-Australian Biotechnology Fund), Department of Science and Technology (Indo-Russian Joint programme), Government of India and Indo-US Science and Technology Forum, New Delhi.
References
- GaoJ HXuB 2009 Nano Today 4 37 http://dx.doi.org/10.1016/j.nantod.2008.10.009
- TaniguchiN 1983 Ann. CIRF 2 573 http://dx.doi.org/10.1016/S0007-8506(07)60185-1
- MecleanSProcesserEO'MalleyDClarkNRamtoolaZBraydenD 1998 Eur. J. Pharm. Sci. 6 153 http://dx.doi.org/10.1016/S0928-0987(97)10007-0
- OppenheimR C 1981 Int. J. Pharm. 8 217 http://dx.doi.org/10.1016/0378-5173(81)90100-9
- KreuterJ 1983 Pharm. Acta Helv. 58 217
- SchaferVvon BriesenHAndreesenRSteffanA MRoyerCTrosterSKreuterJRubsamen-WaigmannH 1992 Pharm. Res. 9 541 http://dx.doi.org/10.1023/A:1015852732512
- NarayaniRRaoK P 1993 Int. J. Pharm. 95 85 http://dx.doi.org/10.1016/0378-5173(93)90393-T
- BertholdACremerKKreuterJ 1996 J. Control. Release 39 17 http://dx.doi.org/10.1016/0168-3659(95)00129-8
- KreuterJ 1994 Nanoparticles in Colloidal Drug Delivery Systems New York Marcel Dekker p 219
- KreuterJ 1995 J. Drug Target 3 171 http://dx.doi.org/10.3109/10611869509015940
- ScheffelURhodesB ANatarajanT KWagnerH N 1972 J. Nucl. Med. 13 498
- BirrenbachGSpeiserP 1976 J. Pharm. Sci. 65 1763 http://dx.doi.org/10.1002/jps.2600651217
- KreuterJSpeiserP P 1976 Infect. Immun. 13 204
- CouvreurPKanteBRolandMGuiotPBauduinPSpeiserP 1979 J. Pharm. Pharmacol. 31 311
- GurnyR 1981 Drug Dev. Ind. Pharm. 7 1 http://dx.doi.org/10.3109/03639048109055684
- Vauthier-HoltzschererCBenabbouSSpenlehauerGVeillardMCouvreurP 1991 STP Pharm. Sci. 1 109
- AllemannELerouxJ CGurnyRDoelkerE 1993 Pharm. Res. 10 1732 http://dx.doi.org/10.1023/A:1018970030327
- MathiowitzE et al 1997 Nature 386 410 http://dx.doi.org/10.1038/386410a0
- CouvreurPVauthierC 1991 J. Control. Release 17 187 http://dx.doi.org/10.1016/0168-3659(91)90058-L
- CouvreurPVauthierC 1994 Drug Absorption Enhancement Concepts, Limitations and Trends A Gde Boer Leiden Amsterdam Harwood Academic p 457
- CouvreurPDubernetCPuisieuxF 1995 Eur. J. Pharm. Biopharm. 41 2
- FattalEVauthierCAynieINakadaYLambertGMalvyCCouvreurP 1998 J. Control. Release 53 137 http://dx.doi.org/10.1016/S0168-3659(97)00246-0
- LabhasetwarVSongCLevyR J 1997 Adv. Drug Deliv. Rev. 24 63 http://dx.doi.org/10.1016/S0169-409X(96)00483-8
- MaassenSFattalEMüllerR HCouvreurP 1993 STP Pharma. 3 11
- Fernandez-UrrusunoRFattalEPorquetDFegerJCouvreurP 1995 Toxicol. Appl. Pharmacol. 130 272 http://dx.doi.org/10.1006/taap.1995.1032
- EmileCBazileDHermanFHeleneCVeillardM 1996 Drug Deliv. 3 187 http://dx.doi.org/10.3109/10717549609029449
- RajaonarivonyMVauthierCCouarrazeGPuisieuxFCouvreurP 1993 J. Pharm. Sci. 82 912 http://dx.doi.org/10.1002/jps.2600820909
- WangNWuX S 1997 Pharm. Dev. Technol. 2 135 http://dx.doi.org/10.3109/10837459709022618
- CalvoPRemunan-LopezCVila-JatoJ LAlonsoM J 1997 Pharm. Res. 14 1431 http://dx.doi.org/10.1023/A:1012128907225
- KumareshS STejrajM AAnandraoR KWalterE R 2001 J. Control. Release 70 1 http://dx.doi.org/10.1016/S0168-3659(00)00339-4
- MuaLSeowcP H 2006 Colloids Surf. B 47 90 http://dx.doi.org/10.1016/j.colsurfb.2005.08.016
- CouvreurPGrefRAndrieuxKMalvyC 2006 Prog. Solid State Chem. 34 231 http://dx.doi.org/10.1016/j.progsolidstchem.2005.11.009
- KreuterJ 1978 Pharm. Acta Helv. 53 33
- MartyJ JOppenheimerR CSpeiserP 1978 Pharm. Acta Helv. 53 17
- RubinoO PKowalskyRSwarbrickJ 1993 Pharm. Res. 10 1059 http://dx.doi.org/10.1023/A:1018979126326
- LinWCoombesG AGarnettCDaviesCSchachtEDavisSIllunL 1994 Pharm. Res. 11 1588 http://dx.doi.org/10.1023/A:1018957704209
- MacAdamA BShafiZ BJamesS LMarriottCMartinG P 1997 Int. J. Pharm. 151 47 http://dx.doi.org/10.1016/S0378-5173(97)04886-2
- JahanshahiMNajafpourGRahimnejadM 2008 Afr. J. Biotechnol. 7 362
- CoesterCNayyarPSamuelJ 2006 Eur. J. Pharm. Biopharm. 62 306 http://dx.doi.org/10.1016/j.ejpb.2005.09.009
- LinWGarnettM CDaviesM CBignottiFFerrutiPDavisS SIllumL 1997 Biomaterials 18 559 http://dx.doi.org/10.1016/S0142-9612(96)00176-7
- CarterD CHoJ X 1994 Adv. Protein Chem. 45 153 http://dx.doi.org/10.1016/S0065-3233(08)60640-3
- HalliwellB 1988 Biochem. Pharmacol. 37 569 http://dx.doi.org/10.1016/0006-2952(88)90126-8
- EmersonT E 1989 Crit. Care Med. 17 690 http://dx.doi.org/10.1097/00003246-198907000-00020
- SinnHSchrenkH HFriedrichE ASchillingUMaier-BorstW 1990 Nucl. Med. Biol. 17 819
- MajumdarSBasuS K 1991 Antimicrob. Agents Chemother. 35 135
- NakagawaYTakayamaKUedaHMachidaYNagaiT 1987 Drug Des. Deliv. 2 99
- SeguraSEspuelasSRenedoM JIracheJ M 2005 Drug Dev. Ind. Pharm. 31 271
- IracheJ MMerodioMArnedoACamapaneroM AMirshahiMEspuelasS 2005 Mini-Rev. Med. Chem. 5 293
- SanthiKDhanarajS AJosephVPonnusankarSSureshB 2002 Drug Dev. Ind. Pharm. 28 1171 http://dx.doi.org/10.1081/DDC-120014584
- KreuterJHekmataraTDreisSVogelTGelperinaSLangerK 2007 J. Control. Release 118 54 http://dx.doi.org/10.1016/j.jconrel.2006.12.012
- MerodioMIracheJ MEclancherFMirshahiMVillarroyaH 2000 J. Drug Target. 8 289 http://dx.doi.org/10.3109/10611860008997907
- LynnA KYannasI VBonfieldW 2004 J. Biomed. Mater. Res. B: Appl. Biomater. B 71 343
- BarbaniNGiustiPLazzeriLPolaccoGPizziraniG 1995 J. Biomater. Sci. Polym. Ed. 7 461 http://dx.doi.org/10.1163/156856295X00535
- LefebvreFGoreckiSBareilleRAmedeeJBordenaveLRabaudM 1992 Biomaterials 13 28 http://dx.doi.org/10.1016/0142-9612(92)90091-2
- RudermanR JWadeC W RShepardW DLeonardF 1973 J. Biomed. Mater. Res. 7 263 http://dx.doi.org/10.1002/jbm.820070213
- BenderAvon BriesenHKreuterJDuncanI BRubsamen-WaigmannH 1996 Antimicrob. Agents Chemother. 40 1467
- SchwickH GHeideK 1969 Bibl. Haematol. 3 111
- WardA GCourtsA 1977 The Science and Technology of Gelatin New York Academic
- BajpaiA KChoubeyJ 2006 J. Mater. Sci: Mater. Med. 17 345 http://dx.doi.org/10.1007/s10856-006-8235-9
- StevensK REinersonN JBurmaniaJ AKaoW J 2002 J. Biomater. Sci. Polym. Ed. 13 1353 http://dx.doi.org/10.1163/15685620260449741
- MariosYChakfeNDengXMariosMHowTKingWGuidoinR 1995 Biomaterials 16 1131 http://dx.doi.org/10.1016/0142-9612(95)93576-Y
- DiSilvioLCourtney-HarrisR GDownesS 1994 J. Mater. Sci., Mater. Med. 5 819 http://dx.doi.org/10.1007/BF00213141
- SinoharaHAsanoYFukuiA 1971 Biochem. Biophys. Acta 237 273
- AsakuraTKaplanD L 1994 Encyclopedia of Agricultural Science vol 4, C JArntzen E MRitter New York Academic p 1
- LotzBColonna-CesariF 1979 Biochemie 61 205 http://dx.doi.org/10.1016/S0300-9084(79)80067-X
- AltmanG HDiazFJakubaCCalabroTHoranR LChenJLuHRichmondJKaplanD L 2003 Biomaterials 24 401 http://dx.doi.org/10.1016/S0142-9612(02)00353-8
- KunduJChungY IKimY HTaeGKunduS C 2010 Int. J. Pharm. doi: 10.1016/j.ijpharm.2009.12.052
- VepariCKaplanD L 2007 Prog. Polym. Sci. 32 991 http://dx.doi.org/10.1016/j.progpolymsci.2007.05.013
- KunduJPatraCKunduS C 2008 Mater. Sci. Eng. C 28 1376 http://dx.doi.org/10.1016/j.msec.2008.03.004
- KunduJDewanMGhoshalSKunduS C 2008 J. Mater. Sci., Mater. Med. 19 2679 http://dx.doi.org/10.1007/s10856-008-3398-1
- KaplanD LMelloC MArcidiaconoSFosseySSenecalKMullerW 1997 Protein-Based Materials KMcGrath D LKaplan Boston Birkhauser p 103
- SofiaSMcCarthyM BGronowiczGKaplanD L 2001 J. Biomed. Mater. Res. 54 139 http://dx.doi.org/10.1002/1097-4636(200101)54:1<139::AID-JBM17>3.0.CO;2-7
- ShaoZVollrathF 2002 Nature 418 741 http://dx.doi.org/10.1038/418741a
- SinoharaH 1979 Comput. Biochem. Physiol. 6311 87 http://dx.doi.org/10.1016/0305-0491(79)90239-6
- KunduS CDashB CDashRKaplanD L 2008 Prog. Polym. Sci. 33 998 http://dx.doi.org/10.1016/j.progpolymsci.2008.08.002
- GamoTInokuchiTLauferH 1977 Insect Biochem. Mol. Biol. 7 285
- TokutakeS 1980 Biochem. J. 187 413
- TakasuYYamadaHTsubouchiK 2002 Biosci. Biotechnol. Biochem. 66 2715 http://dx.doi.org/10.1271/bbb.66.2715
- MichailleJ JCoublePPrudhommeJ CGarelA 1986 Biochemie 68 1165 http://dx.doi.org/10.1016/S0300-9084(86)80060-8
- TsukadaMBertholonG 1981 Bull. Sci. Inst. Text. Fr. 10 141
- MandalB BPriyaA SKunduS C 2009 Acta Biomater. 5 3007 http://dx.doi.org/10.1016/j.actbio.2009.03.026
- DashB CMandalB BKunduS C 2009 J. Biotechnol. 144 321 http://dx.doi.org/10.1016/j.jbiotec.2009.09.019
- BunningT JJiangHAdamsW WCraneR LFarmerBKaplanD L 1993 Silk Polymers-Material Science and Biotechnology: ACS Symposium Series 544 D LKaplan W WAdams BFarmer CViney Washington, DC American Chemical Society p 353
- MandalB BKunduS C 2009 Nanotechnology 20 355101 http://dx.doi.org/10.1088/0957-4484/20/35/355101
- SuzukiN et al 2004 Biofactors 21 329 http://dx.doi.org/10.1002/biof.552210164
- DashRAcharyaCBinduP CKunduS C 2008 Biochem. Mol. Biol. Rep. 41 236
- DashRMandalMGhoshS KKunduS C 2008 Mol. Cell. Biochem. 311 111 http://dx.doi.org/10.1007/s11010-008-9702-z
- ZhaorigetuSMasahiroSWatanabeHKatoN 2001 Biosci. Biotechnol. Biochem. 65 2181 http://dx.doi.org/10.1271/bbb.65.2181
- SasakiMYamadaHKatoN 2000 Nutr. Res. 20 1505 http://dx.doi.org/10.1016/S0271-5317(00)80031-7
- TamadaYSanoMNiwaKImaiTYoshinoG 2004 J. Biomater. Sci. Polym. Ed. 15 971 http://dx.doi.org/10.1163/1568562041526469
- AramwitPKanokpanontSDe-EknamkulWKameiKSrichanaT 2009 J. Biomater. Sci. Polym. Ed. 20 1295 http://dx.doi.org/10.1163/156856209X453006
- ZhangY Q 2002 Biotechnol. Adv. 20 91 http://dx.doi.org/10.1016/S0734-9750(02)00003-4
- ZhangY QMaYXiaY YShenW DMaoJ PXueR Y 2006 J. Control. Release 115 307 http://dx.doi.org/10.1016/j.jconrel.2006.08.019
- AramwitPKanokpanontSDe-EknamkulWSrichanaT 2009 J. Biosci. Bioengg. 107 556 http://dx.doi.org/10.1016/j.jbiosc.2008.12.012
- ReichlS 2009 Biomaterials 30 6854 http://dx.doi.org/10.1016/j.biomaterials.2009.08.051
- CalvoPRemunan-LopezCVila-JatoJ LAlosoM J 1997 J. Appl. Polym. Sci. 63 125 http://dx.doi.org/10.1002/(SICI)1097-4628(19970103)63:1<125::AID-APP13>3.0.CO;2-4
- GacesaP 1988 Carbohydr. Polym. 8 161 http://dx.doi.org/10.1016/0144-8617(88)90001-X
- GuiselyK B 1989 Enzyme Microb. Technol. 11 706 http://dx.doi.org/10.1016/0141-0229(89)90119-1
- GombotzW RWeeS F 1998 Adv. Drug Deliv. Rev. 31 267 http://dx.doi.org/10.1016/S0169-409X(97)00124-5
- ReesD AWelshE J 1977 Angew. Chem. Int. Ed. Engl. 16 214 http://dx.doi.org/10.1002/anie.197702141
- TakahashiTTakayamaKMachidaYNagaiT 1990 Int. J. Pharm. 61 35 http://dx.doi.org/10.1016/0378-5173(90)90041-2
- BystrickySMalovikovaASticzayT 1991 Carbohydr. Polym. 15 299 http://dx.doi.org/10.1016/0144-8617(91)90044-D
- KotzeA FThanouM MLuebetaenH Lde BoerA GVerhoefJ CJungingerH E 1999 J. Pharm. Sci. 88 253 http://dx.doi.org/10.1021/js980233c
- SarmentoBRibeiroAVeigaFSampaioPNeufeldRFerreiraD 2007 Pharm. Res. 24 2198 http://dx.doi.org/10.1007/s11095-007-9367-4
- PanYLiY JZhaoH YZhengJ MXuHWeiGHaoJ SCuiF D 2002 Int. J. Pharm. 249 139 http://dx.doi.org/10.1016/S0378-5173(02)00486-6
- MladenovskaKCruaudORichommePBelamieERaickiR SVenier-JulienneM CPopovskiEBenoitJ PGoracinovaK 2007 Int. J. Pharm. 345 59 http://dx.doi.org/10.1016/j.ijpharm.2007.05.059
- DeSRobinsonD 2003 J. Control. Release 89 101 http://dx.doi.org/10.1016/S0168-3659(03)00098-1
- KasH S 1997 J. Microencapsul. 14 689 http://dx.doi.org/10.3109/02652049709006820
- BerschtP CNiesBLiebendorferAKreuterJ 1994 Biomaterials 15 593 http://dx.doi.org/10.1016/0142-9612(94)90209-7
- HiranoSSeinoHAkiyamaYNonakaI 1988 Polym. Eng. Sci. 59 897
- JinC 1996 Chin. J. Biomed. Engg. 15 102
- LehrC MBouwstraJ ASchachtE HJungingerH E 1992 Int. J. Pharm. 78 43 http://dx.doi.org/10.1016/0378-5173(92)90353-4
- LuelenH LLehrC MRentelC ONoachA B JBoerA GVerhoefJ CJungingerH E 1994 J. Control. Release 29 329 http://dx.doi.org/10.1016/0168-3659(94)90078-7
- NordtveitR JVårumK MSmidsr⊘dO 1996 Carbohydr. Polym. 29 163 http://dx.doi.org/10.1016/0144-8617(96)00003-3
- LangerKBalthasarSVogelVDinauerNVon BriesenHSchubertD 2003 Int. J. Pharm. 257 169 http://dx.doi.org/10.1016/S0378-5173(03)00134-0
- BouchemalKBriançonSPerrierEFessiH 2004 Int. J. Pharm. 280 241 http://dx.doi.org/10.1016/j.ijpharm.2004.05.016
- SjöströmBKaplunATalmonYCabaneB 1995 Pharm. Res. 12 39 http://dx.doi.org/10.1023/A:1016278302046
- GaoZShuklaA JJohnsonJ RCrowleyW R 1995 Pharm. Res. 12 857 http://dx.doi.org/10.1023/A:1016209020160
- JahanshahiMBabaeiZ 2008 Afr. J. Biotechnol. 7 4926
- CoesterC JLangerKVon BriesenHKreuterJ 2000 J. Microencapsul. 17 187 http://dx.doi.org/10.1080/026520400288427
- LinWCoombesADaviesMDavisSIllumL 1993 J. Drug Target. 1 237 http://dx.doi.org/10.3109/10611869308996081
- GomezABinghamDDe JuanLTangK 1999 Mater. Res. Soc. Symp. Proc. 550 101
- RedheadH MDavisS SIllumL 2001 J. Control. Release 70 353 http://dx.doi.org/10.1016/S0168-3659(00)00367-9
- JahanshahiMSanatiM HMinuchehrZHajizadehSBabaeiZ 2007 Am. Inst. Phys. 228 929
- BetancorLLuckariftH R 2008 Trends Biotechnol. 26 566 http://dx.doi.org/10.1016/j.tibtech.2008.06.009
- DunneMCorriganO IRamtoolaZ 2000 Biomaterials 21 1659 http://dx.doi.org/10.1016/S0142-9612(00)00040-5
- BerneB JPecoraR 1975 Dynamic Light Scattering New York Wiley
- TakahashiKKatoHSaitoTMatsuyamaSKinugasaS 2008 Part. Part. Syst. Charact. 25 31 http://dx.doi.org/10.1002/ppsc.200700015
- AmzallagAVaillantCJacobMUnserMBednarJKahnJDubochetJStasiakAMaddocksJ H 2006 Nucleic Acids Res. 34 125 http://dx.doi.org/10.1093/nar/gkl675
- BlanchardC R 1996 Chem. Educ. 5 1 http://dx.doi.org/10.1007/s00897960059a
- MagonovS N 1993 Appl. Spectrosc. Rev. 28 1 http://dx.doi.org/10.1080/05704929308021499
- MohanrajV JChenY 2006 Trop. J. Pharm. Res. 5 561
- BerthomieuCHienerwadelR 2009 Photosynth. Res. 101 157 http://dx.doi.org/10.1007/s11120-009-9439-x
- WilsonA P 2000 Cytotoxicity and Viability Assays in Animal Cell Culture: A Practical Approach vol 1, 3rd edn J R WMasters London Oxford University Press
- DobruckiB T 2002 J. Cytometry 47 236 http://dx.doi.org/10.1002/cyto.10080
- MosmannT 1983 J. Immunol. Methods 65 55 http://dx.doi.org/10.1016/0022-1759(83)90303-4
- FlorenceA 1997 Pharm. Res. 14 259 http://dx.doi.org/10.1023/A:1012029517394
- VandervoortVLudwigA 2004 Eur. J. Pharm. Biopharm 57 251 http://dx.doi.org/10.1016/S0939-6411(03)00187-5
- LeoEArlettiRForniFCameroniR 1997 Farmaco 52 385
- LiJ KWangNWuX S 1998 J. Microencapsul. 15 163 http://dx.doi.org/10.3109/02652049809006846
- MladenovskaKKumbaradziE FDodovG MMakraduliLGoracinovaK 2002 Int. J. Pharm. 242 247 http://dx.doi.org/10.1016/S0378-5173(02)00167-9
- WartlickHMichaelisKBalthasarSStrebhardtKKreuterJLangerK 2004 J. Drug Target. 12 461 http://dx.doi.org/10.1080/10611860400010697
- LangerKCoesterCWeberCvon BriesenHKreuterJ 2000 Eur. J. Pharm. Biopharm. 49 303 http://dx.doi.org/10.1016/S0939-6411(00)00068-0
- CoesterCKreuterJvon BriesenHLangerK 2000 Int. J. Pharm. 196 147 http://dx.doi.org/10.1016/S0378-5173(99)00409-3
- KommareddySAmijiM 2005 Bioconjug. Chem. 16 1423 http://dx.doi.org/10.1021/bc050146t
- KushibikiTTabataY 2005 J. Biomater. Sci. Polym. 16 1447 http://dx.doi.org/10.1163/156856205774472326
- KaulGAmijiM 2002 Pharm. Res. 197 1061 http://dx.doi.org/10.1023/A:1016486910719
- FeltOBuriPGurnyR 1998 Drug Dev. Ind. Pharm. 24 979 http://dx.doi.org/10.3109/03639049809089942
- GiunchediPGentaIContiBMuzzarelliR A AConteU 1998 Biomaterials 19 157 http://dx.doi.org/10.1016/S0142-9612(97)00181-6
- IllumL 1998 Pharm. Res. 15 1326 http://dx.doi.org/10.1023/A:1011929016601
- WuYWuQWangY NMaJ B 2003 Acta Chem. Sin. 61 614
- HouW MMiyazakiSTakadaMKomaiT 1985 Chem. Pharm. Bull. 33 3986
- KawashimaYHandaTKasaiAKasaiATakenakaHLinS YAndoY 1985 J. Pharm. Sci. 74 264 http://dx.doi.org/10.1002/jps.2600740308
- MiyazakiSYamaguchiHYokouchiCTakadaMHouW M 1988 Chem. Pharm. Bull. 36 4033
- ShiraishiSImaiTOtagiriM 1993 J. Control. Release 25 217 http://dx.doi.org/10.1016/0168-3659(93)90080-O
- IllumLFarrajN FDavisS S 1994 Pharm. Res. 11 1186 http://dx.doi.org/10.1023/A:1018901302450
- MiyazakiSIshiKNadaiT 1981 Chem. Pharm. Bull. 29 3067
- GalloJ MHassanE E 1988 Pharm. Res. 5 300 http://dx.doi.org/10.1023/A:1015978704810
- HassanE EParishR CGalloJ M 1992 Pharm. Res. 9 390 http://dx.doi.org/10.1023/A:1015803321609
- WuYYangWWangCHuJFuS 2005 Int. J. Pharm. 295 235 http://dx.doi.org/10.1016/j.ijpharm.2005.01.042
- ZhangY QShenW DXiangR LZhugeL JGaoW JWangW B 2007 J. Nanopart. Res. 9 885 http://dx.doi.org/10.1007/s11051-006-9162-x
- ZhangY QXiangR LYanH BChenX X 2008 Chem. J. Chin. Univ. 29 628
- MyungS JKimH SKimYChenPJinH J 2008 Macromol. Res. 16 604
- YanH BZhangY QMaY LZhouL X 2009 J. Nanopart. Res. 11 1937 http://dx.doi.org/10.1007/s11051-008-9549-y
- GuptaVAsehARíosC NAggarwalB BMathurA B 2009 Int. J. Nanomedicine 4 115
- NomuraMYamadaH 1992 Sen-i Gakkaishi 48 305
- ChoK Y et al 2003 Int. J. Biol. Macromol. 32 36 http://dx.doi.org/10.1016/S0141-8130(03)00023-0
- SongYJinYSunJWeiD 2006 Polym. Int. 55 1350 http://dx.doi.org/10.1002/pi.2093
- AynieIVauthierCChacunHFattalECouvreurP 1999 Antisense Nucleic Acid Drug Dev. 9 301
- YiY MYangT YPanW M 1999 World J. Gastroenterol. 5 57
- YouJ OPengC A 2004 Macromol. Symp. 219 147 http://dx.doi.org/10.1002/masy.200550113
- SarmentoBFerreiraDVeigaFRibeiroA 2006 Carbohydr. Polym. 66 1 http://dx.doi.org/10.1016/j.carbpol.2006.02.008
- SarmentoBRibeiroA JVeigaFFerreiraD CNeufeldR J 2007 J. Nanosci. Nanotechnol. 7 2833 http://dx.doi.org/10.1166/jnn.2007.609
- SonavaneG SDevarajanP V 2007 J. Biomed. Nanotech. 3 160 http://dx.doi.org/10.1166/jbn.2007.005
- MotwaniS KChopraSTalegaonkarSKohliKAhmadF JKharR K 2008 Eur. J. Pharm. Biopharm. 68 513
- MaoH Q et al 2001 J. Control. Release 70 399 http://dx.doi.org/10.1016/S0168-3659(00)00361-8
- JanesK AFresneauM PMarazuelaAFabraAAlonsoM J 2001 J. Control. Release 73 255 http://dx.doi.org/10.1016/S0168-3659(01)00294-2
- SoppimathK SAminabhaviT MKulkarniA RRudzinskiW E 2001 J. Control. Release 70 1 http://dx.doi.org/10.1016/S0168-3659(00)00339-4
- BanerjeeTMitraSKumar SinghAKumar SharmaRMaitraA 2002 Int. J. Pharm. 243 93 http://dx.doi.org/10.1016/S0378-5173(02)00267-3
- VilaASanchezAJanesK ABehrensIKisselTVila-JatoJ L 2004 Eur. J. Pharm. Biopharm. 57 123 http://dx.doi.org/10.1016/j.ejpb.2003.09.006
- MansouriSLavignePCorsiKBenderdourMBeaumontEFernandesJ C 2004 Eur. J. Pharm. Biopharm. 57 1 http://dx.doi.org/10.1016/S0939-6411(03)00155-3
- MaZLimT MLimL Y 2005 Int. J. Pharm. 293 271 http://dx.doi.org/10.1016/j.ijpharm.2004.12.025
- YouJ OLiuY CPengC A 2006 Int. J. Nanomedicine 1 173 http://dx.doi.org/10.2147/nano.2006.1.2.173
- SharmaB VAliMBabootaSAliJ 2007 Indian J. Pharm. Sci. 69 712
- RekhaM RSharmaC P 2009 J. Control. Release 135 144 http://dx.doi.org/10.1016/j.jconrel.2009.01.011