Abstract
High temperature proton conductor (HTPC) oxides are attracting extensive attention as electrolyte materials alternative to oxygen-ion conductors for use in solid oxide fuel cells (SOFCs) operating at intermediate temperatures (400–700 °C). The need to lower the operating temperature is dictated by cost reduction for SOFC pervasive use. The major stake for the deployment of this technology is the availability of electrodes able to limit polarization losses at the reduced operation temperature. This review aims to comprehensively describe the state-of-the-art anode and cathode materials that have so far been tested with HTPC oxide electrolytes, offering guidelines and possible strategies to speed up the development of protonic SOFCs.
Introduction
High temperature solid oxide fuel cells
Sustainable energy production, compatible with environment preservation, is one of the major problems that modern societies will face in the near future. The magnitude of the energy challenge is so important that state-of-the-art standard technologies based on fossil fuel combustion will not be sufficient to guarantee future energy requirements. Furthermore, the need to reduce pollutant emissions precludes the extensive use of fossil fuels to generate energy. For these reasons, very pressing is nowadays the demand for new environmental-friendly technologies able to use energy more efficiently, and for renewable sources for energy production and storage at levels far beyond of what is currently possible.
Within this scenario, it is widely recognized that the development and the widespread use of fuel cell (FC) technology might become a keystone in the near future. Fuel cells are electrochemical devices that directly convert chemical energy into electrical energy with extremely high efficiencies owing to the absence of the Carnot limitation of the conventional energy conversion chain [Citation1–3]. Furthermore, compared with internal combustion engines, fuel cells produce small amounts of pollutants and allow large scalability, making the production of energy from mW to hundreds kW possible, while maintaining high efficiency [Citation2]; in other words, fuel cells can be used both for stationary and portable applications.
There are various types of fuel cells, generally classified on the basis of the electrolyte material, such as proton exchange membrane (PEMFC), molten carbonate (MCFC), solid oxide (SOFC) and phosphoric acid (PAFC) [Citation2]. Among them, SOFCs are very promising because they offer flexibility in terms of the type of fuel (hydrogen or hydrocarbons can be used) and do not contain corrosive liquids, as opposed to MCFCs for instance [Citation3].
An SOFC consists of two electrodes in contact with a dense ceramic electrolyte. The electrolyte must allow ions (O2− or H+) to migrate quickly from one electrode to the other, and must have negligible electronic conductivity. Furthermore, the ceramic electrolyte of an SOFC should have a fully dense microstructure to maximize its conductivity and minimize reactant cross-over. Other electrolyte requirements are good thermo-mechanical and chemical compatibility with the contacting materials and the surrounding environment, leading to long-term stability [Citation4, Citation5]. The electrolyte material used for standard SOFC technology is yttria-stabilized zirconia (YSZ), which generally requires high operating temperatures of 700–1000 °C [Citation4].
The electrodes used in SOFCs should possess high electronic conductivity and an open porous microstructure, allowing rapid transport of reactant gases at the triple phase boundary (TPB), which is the region where reactant gases (atmosphere), ions (electrolyte), and electrons (electrode) meet together. Furthermore, the two electrodes should, respectively, exhibit high catalytic activity toward oxidant reduction (positive electrode, cathode) and fuel oxidation (negative electrode, anode) [Citation5].
Nickel is the most frequently used anode material for SOFCs, and generally coupled with an ion-conducting phase to form a composite anode. Nickel provides a conduction path for electrons and shows excellent catalytic activity towards hydrogen oxidation in the temperature range of interest, still offering significant cost saving. The ion-conducting phase is generally used to adjust the thermal expansion coefficient of the anode to that of the ceramic electrolyte, and to extend the TPB to the whole anode volume by creating an ion-conducting pathway. The extension of the TPB means an increase in the number of sites where the anode reactions may occur, thereby resulting in improved electrode performance [Citation5].
The state-of-the-art cathode material for SOFCs based on YSZ is strontium-doped lanthanum manganite (LSM) [Citation4, Citation5]. However, it can only be used above 700 °C because below this temperature, LSM shows large polarization losses due to its limited ionic conductivity [Citation6]. An increase in the TPB length, as in the case of the anode, by forming a composite cathode with an ionic conducting phase, allows improving the cathode performance [Citation7].
Intermediate temperature solid oxide fuel cells
At present, most of the challenges hindering SOFC commercialization arise from their high operating temperature. Therefore, significant research efforts have been recently devoted to decreasing the SOFC operation temperature with the aim of reducing the initial and operating costs [Citation5]. Developing an SOFC able to operate in the so-called intermediate temperature range (IT, 450–650 °C) will result in several advantages: the use of ferrite steel interconnects instead of expensive and brittle ceramic materials, easier and more reliable sealing, a rapid start-up, the reduction of thermal stresses (in particular those caused by thermal expansion mismatches) and a decrease in electrode sintering.
Reducing the SOFC operating temperature leads to two main problems: a decrease in electrolyte conductivity and an increase in electrode polarization losses, because both the ion transport in ceramic electrolytes and the electrochemical reactions at the TPB are thermally activated processes.
The electrolyte resistance can be lowered either by decreasing the electrolyte thickness or using alternative materials with a higher ionic conductivity in the IT range. On the basis of the nature of the charge carriers, two major electrolyte categories can be identified for operation in the IT range: oxygen-ion conductors and high temperature proton conductors (HTPCs). Examples of highly conducting oxygen-ion electrolytes are doped ceria, doped bismuth oxide and doped lanthanum gallate [Citation5, Citation8–11].
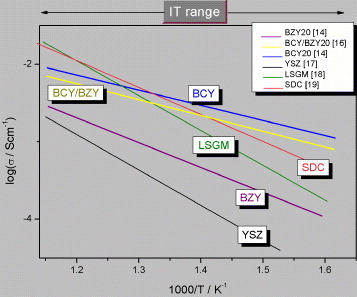
For a couple of decades, HTPCs have been recognized as particularly suitable electrolytes for IT applications, because, compared with oxygen-ion conductors, they show lower activation energy (between 0.4 and 0.6 eV) [Citation12–15] and larger conductivity values in the IT range (figure ) [Citation14, Citation16–19]. Moreover, for HTPC-based FCs, water is produced at the cathode rather than at the anode, avoiding fuel dilution, which reduces the cell efficiency.
Figure 1 Comparison between the electrical conductivity values of different HTPC electrolytes, BaZr0.8Y0.2O3-δ (BZY) [Citation14], BaCe0.8Y0.2O3-δ (BCY) [Citation14] and BaCe0.8Y0.2O3-δ/BaZr0.8Y0.2O3-δ (BCY/BZY) bilayer [Citation16], and of the highest performing oxygen-ion conductors, Y-stabilized zirconia (YSZ) [Citation17], La0.8Sr0.2Ga0.8Mg0.2O3 (LSGM) [Citation18] and Ce0.8Sm0.2O1.9-δ (SDC) [Citation19].
The reduction of the SOFC operating temperature affects the electrode performance even more severely than the electrolyte performance, leading to large overpotentials at the electrode/electrolyte interface, both for HTPC and oxygen-ion conducting electrolytes. Indeed, the reduction of cathode overpotentials represents the main obstacle for IT-SOFC development, because the identification of suitable cathode materials with fast charge transfer kinetics at low temperatures is still critical.
The search for cathodes performing with IT-SOFCs based on oxygen-ion conducting electrolytes is extremely active. The IT performance can be enhanced using a composite with an ion-conducting phase, or using mixed ionic/electronic conductors. For example, compounds in the La1−xSrxCo1−yFeyO3−δ (LSCF) series have attracted large interest owing to their good electronic and relatively high ionic conductivity [Citation20, Citation21]. More recently, owing to its high oxygen diffusivity, Ba1−xSrxCo1−yFey O3−δ (BSCF) series has been proposed as a valid cathode material for doped-ceria electrolyte [Citation22]. However, the chemical stability of BSCF in the presence of CO2 and water vapor was questioned [Citation23], indicating limits for practical applications. New materials showing promising performance, such as double perovskite or bismuth ruthenate pyrochlore, have been proposed as alternative cathodes [Citation24, Citation25].
The cathode plays an even more critical role in HTPC electrolytes, because water is produced at the cathode when protons are the charge carriers. However, the research on cathode materials specifically designed for SOFCs based on HTPC electrolytes is still at an early stage. Thus, the most important task for improving the performance of IT-SOFCs based on HTPC electrolytes is the development of purposely tailored cathode materials. It is worth emphasizing that any newly selected electrolyte requires the development of appropriate electrode materials. This concept, apparently trivial, has been somehow neglected for HTPC electrolytes, where cathode materials developed for oxygen-ion conducting electrolytes were utilized with mediocre results.
This article aims to present a comprehensive and critical overview of state-of-the-art electrode materials exploited so far for application in protonic SOFCs. The different strategies followed in the last years to reduce the electrode overpotentials and develop competitive SOFCs based on HTPCs are analyzed and compared, in an attempt of offering useful guidelines to the increasing number of researchers working on protonic SOFCs.
High-temperature proton conductor electrolytes for IT-SOFC application
In the last few years, an ever-growing interest has been directed toward HTPC electrolytes [Citation26]. The discovery of HTPCs dates from the early 1980s, when Iwahara et al reported proton conductivity at elevated temperature (700 °C) in perovskite oxides exposed to hydrogen and/or water vapor containing atmospheres [Citation27]. Still nowadays, the most investigated HTPCs are perovskite-type oxides (ABO3), where alkaline earth elements, such as Ba, Sr and Ca, occupy the A site, and tetravalent elements, usually Ce or Zr, occupy the B site. The creation of oxygen vacancies by doping the B-site with a trivalent element, such as Y, Nd, Sm, Yb, In, Eu, Gd, etc, is crucial for generating the protonic conductivity [Citation19–21]. Mobile protons can be incorporated from molecular hydrogen according to equation (Equation11 ):
1
However, the dissociative adsorption of water (Equation22 ) is considered to be the main reaction leading to the formation of protonic defects [Citation12, Citation28, Citation29]:
2
In this reaction, protons are formed by water dissociation: a hydroxide ion can fill an oxygen vacancy, and a proton can form a covalent bond with lattice oxygen. Because this reaction is exothermic [Citation12, Citation30], proton conduction dominates at low temperatures. At elevated temperatures, where water desorption is favored [Citation31], electronic (p-type) or oxygen-ion conductivity increases [Citation32–37]. The temperature at which dehydration starts depends on the HTPC composition. Generally, highly basic oxides, such as barium cerates, are better at stabilizing protonic defects, and dehydration occurs above 600 °C, whereas less basic oxides, such as barium zirconates, start dehydrating above 400 °C [Citation12]. The most suitable temperature range for proton conduction results as a compromise between sample hydration and proton mobility, and generally peaks around 400–600 °C.
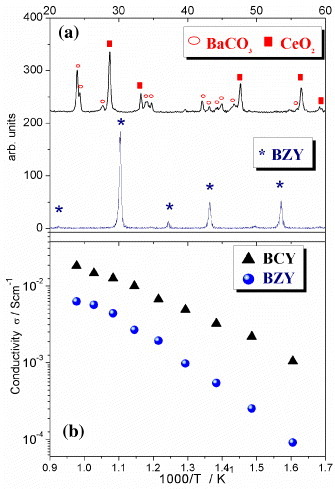
Among the HTPC electrolytes, Y-doped barium cerate (BCY) stands for its high proton conductivity [Citation15, Citation38–40], but suffers from poor chemical stability, reacting with acidic gases (e.g. CO2 and SO2) and steam [Citation41–43]. On the other hand, Y-doped barium zirconate (BZY) shows good chemical stability [Citation12, Citation14, Citation15], but the proton conductivity of the sintered material is insufficient for practical applications owing to the presence of a large volume of poorly conductive grain boundaries, induced by the poor sinterability of BZY. To exemplify this statement, figure (a) shows the x-ray diffraction (XRD) patterns of 20 mol.% Y-doped barium cerate and 20 mol.% Y-doped barium zirconate powders after high-temperature exposure to CO2 atmosphere [Citation14]. While BCY powders completely decomposed into barium carbonate and cerium oxide, BZY samples showed only the reflection lines of the BZY perovskite structure. However, as shown in figure (b), the proton conductivity of a BCY pellet sintered at 1500 °C is almost one order of magnitude larger than that of a BZY pellet sintered at 1600 °C [Citation14].
Figure 2 XRD plots of BCY (top) and BZY (bottom) powders after exposure to CO2 atmosphere at 900 °C for 3 h (a); proton conductivity of a BCY and BZY pellets after sintering at 1500 and 1600 °C, respectively (b) [Citation14].
Therefore, the main challenge related to HTPC electrolyte development is to achieve high proton conductivity while preserving chemical stability. Promising results have recently been reported [Citation14, Citation44–47], but these efforts will have little benefit without the ad-hoc development of anode and cathode materials for proton conducting oxides.
Anode materials for HTPC electrolytes
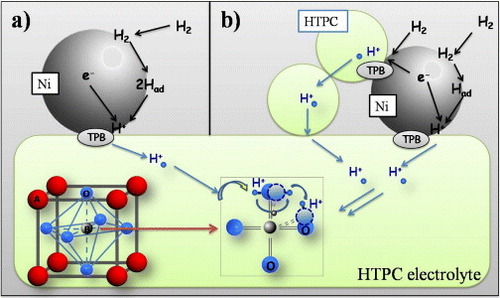
Most of the protonic SOFCs utilize composite anodes fabricated by mixing Ni with the HTPC material used as the electrolyte. Figure shows an illustration of the anode reactions when Ni or a composite Ni-HTPC is used as anode materials with a proton-conducting electrolyte. The figure clearly illustrates the increase in the number of electrochemically active sites when a composite anode is used. The anode specific surface area plays an important role in determining its electrochemical performance; the larger the surface area, the larger the TPB length and the faster the reaction rate. Large surface areas can be achieved by producing composite anodes using powders with very small average grain size.
Figure 3 Illustration of the possible anode reactions for an SOFC, based on a proton conducting electrolyte, in the case of Ni (a) or a composite Ni-protonic conductor anode (b).
It is worth emphasizing that the electrochemical and morphological characterization of the Ni-HTPC composite anodes has received little attention so far. The most significant results in this field are reviewed and compared here.
Essoumhi et al [Citation48] investigated the porosity and the electrical conductivity of two different anode cermets made of Ni-BCY, containing 35 and 45 vol.% of Ni (corresponding to 50 and 60 wt% of NiO). The anodes were prepared by mixing commercial NiO powders with BCY powders produced by flash combustion method. Significant increases in the anode porosity (from 22 to 48%) and electrical conductivity (from 70 to 500 S cm−1 at 25 °C) with increasing Ni content were reported. Furthermore, in a symmetrical cell configuration, the area specific resistance (ASR) decreased from 0.32 to 0.06 Ωcm2 when the Ni content of the anode was increased from 35 to 45 vol.%, at 600 °C in wet 10% H2 in N2 atmosphere. These results highlight that the presence of a percolation pathway through the Ni particles is critical to ensure suitable fuel cell performance. Interestingly, the conductivity of the BCY electrolyte was smaller for the anode containing 35 vol.% of Ni [Citation48]. As suggested by the authors, this could be due to the low anode porosity, which led to reduced gas diffusion, and, in turn, to insufficient electrolyte hydration. Similar effect of the anode materials on the protonic electrolyte conductivity has been reported elsewhere [Citation49].
Chevallier et al [Citation49] produced the NiO-BCY anode by dispersing a BCY nanocrystalline powder in a Ni nitrate solution and then firing at 1000 °C. The ASR of the anode cermet, measured in a symmetrical cell configuration with BCY as the electrolyte, was quite large, reaching 0.6 Ωcm2 at 700 °C in wet Ar containing 5% of H2. Furthermore, the authors reported significant anode degradation after exposure to CO2 at 700 °C. This implies that BCY-based anodes are not suitable for application in hydrocarbon fuelled protonic SOFCs.
Mather et al [Citation50] reported the ASR of a different Ni-HTPC composite anode, made of Ni and SrCe0.9Yb0.1O3-δ (SCYb) containing 33 wt% of Ni. They produced the anode powders by combustion method starting from a mixture of molten nitrates and urea. The anode powders were co-pressed on both sides of a green SCYb pellet in symmetrical cell configuration and co-fired at 1250 °C. The reported ASR values in wet H2 atmosphere were extremely large (5 Ωcm2 at 700 °C) owing to the formation of insulating phases at the electrode/electrolyte interface. In particular, the authors observed the presence of Sr and Yb-rich crystals in the electrolyte and at the interfacial layers. Furthermore, accumulation of Co, used as sintering additive for the electrolyte, was observed at the electrode/electrolyte interface.
Chemical reactions leading to the formation of secondary phases at the electrode/electrolyte interfaces can limit the ionic migration, thereby increasing the electrode polarization [Citation51]. Agarwal and Liu [Citation52] observed very large bulk and interfacial resistances for an anode-supported fuel cell. The cell was made of a thin BaCe0.8Gd0.2O3-δ (BCG) electrolyte, which was deposited on a NiO-BCG anode co-fired at 1200 °C. Chemical reactions at the anode/electrolyte interface reduced the conductivity and the ionic transport number of the BCG electrolyte, resulting in low power density output and open circuit voltage. Differently, the formation of secondary phases has not been reported in studies of composite anodes made of Ni/doped barium cerate [Citation49, Citation53, Citation54], Ni/BaCe0.7Zr0.1Y0.2O3-δ (BCZY) [Citation55–58], and Ni/BZY [Citation59], even after thermal treatments at temperatures as high as 1400 °C.
Despite Ni-based anodes are the most commonly used for SOFC applications, several drawbacks in the Ni use are reported at the current SOFC operating temperatures of 500–800 °C [Citation1]. Ni tends to agglomerate, resulting in the deterioration of anode performance with time. A second problem is the coke formation when hydrocarbons are used as fuel that hinders the anode performance. However, only few alternative anode materials have been proposed for HTPC-based SOFCs. Few papers report the use of hydrogen-permeable metal membranes [Citation60, Citation61] as anodic supporting structure for protonic SOFCs. In particular, the use of a supporting Pd membrane resulted in a power density of 1.4 W cm-2 at 600 °C [Citation61], the largest power density output reported so far for a protonic SOFC.
Yamaguchi et al compared the voltage losses of BCY-based SOFCs using Pd, Pd-Ag, and porous Ni as anodes [Citation60]. The use of Pd-Ag membrane resulted in the largest voltage losses, probably because of the low permeation flux of protons through the anode. Pd membrane based cells showed a better performance with respect to porous Ni-based cells above 600 °C. For a Ni-based protonic SOFC, the anode reaction is given by (3), while in a hydrogen-permeable membrane-based protonic SOFC, because hydrogen exists in the membrane in the atomic form, the expected reaction is given by (4) [Citation60]:
3
4 This could justify why the anode reaction for the hydrogen-permeable membrane becomes faster at higher temperatures [Citation60].
Considering the use of alternative anodes, Hibino et al [Citation62] compared the measured overpotentials of different metallic anodes, namely Fe, Pd, Ni, Cu, Ru and Pt, using BCY as electrolyte. The smallest overpotential was observed for the Fe anode, whose performance was further improved by impregnating 3 wt% Pd on the FeO surface. This could represent a valid alternative to Ni-based anodes, with the further advantage of reducing coke formation in the presence of hydrocarbon fuels.
A few papers reported that adding an HTPC to oxygen-ion conductor-based anode cermets improved the chemical stability, especially in the presence of hydrocarbons. As an example, Jin et al [Citation63] reported that Ni-YSZ anodes infiltrated with SrZr0.95Y0.05O3-δ, a proton conducting compound, showed lower overpotential and improved operational stability in dry methane when compared to standard Ni-YSZ anodes.
The direct hydrocarbon feeding of SOFCs might cause anode poisoning by sulfur, a major impurity in fuels. An investigation on sulfur tolerance of the Ni-BCZY anodes has been reported by Fang et al [Citation64]. It was observed that the hydrogen permeation flux through Ni-BCZY dense membranes significantly decreased with increasing the H2S concentration in the gas environment owing to the formation of BaS, CeO2, Ni3S2 and Ce2O2S compounds, as revealed by XRD analysis.
In contrast, a recent work claimed that Ni-BaCe0.7Zr0.1Y0.1Yb0.1O3-δ (BCZYYb) anodes show excellent tolerance towards sulfur and even CO2 [Citation65]. The only difference between these anodes and those investigated by Fang et al [Citation64] was the doping content: 10 mol% Y and 10 mol% Yb in [Citation65] and 20 mol% Y in [Citation64]. This implies that the chemical stability derives from the presence of 10 mol% Yb. To explain their results, Yang et al [Citation65] stated that ‘the two dopants on the B-site function in a cooperative fashion to improve the ionic conductivity and the catalytic activity for reforming or oxidation of hydrocarbons as well as conversion of H2S to SO2’. Ni-BCZYYb anodes also showed a surprising chemical stability towards CO2: recent results revealed that BCZY strongly decomposed when exposed to 3% CO2 at 600 °C for 3 h [Citation66], while adding Yb was claimed to ensure the stability of BCZYYb for 300 h at 750 °C in H2 atmosphere containing 50 vol% CO2 [Citation65].
Cathode materials for HTPC electrolytes
If the requirements for a cathode working with an oxygen-ion conducting electrolyte are severe and complex, for a cathode working in a protonic SOFC it is also necessary to take into account the reaction leading to water generation (Equation55 ):
5
Moreover, for proton conducting electrolytes the requirement of a porous cathode microstructure is stringent to allow water evaporation, unless the cathode material also exhibits proton conductivity.
Theoretically, the most unfavorable cathode materials are metals or mixed electronic/oxygen-ion conductors, because in these cases the cathode reaction can only take place at the cathode/electrolyte interface (figure (a)). Table summarizes the elementary steps involved in the cathode reaction occurring at the interface with an HTPC electrolyte.
Figure 4 Possible reactions at the cathode using a metal or an O2-/e- mixed conductor (a), an H+/e- mixed conductor (b) and a composite cathode made of a proton conductor phase and an O2-/e- mixed conductor phase (c).
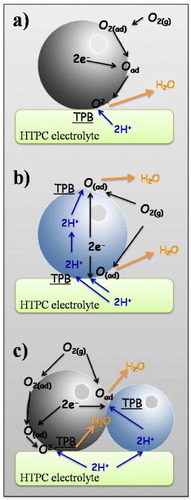
Table 1 Elementary cathode reaction steps in a protonic SOFC using an electronic (a) or a mixed oxygen-ion/electronic (b) conductor as the cathode material.
Noble metals such as Pt exhibited large overpotential with HTPCs, despite their good catalytic activity; in addition, they are too expensive for large-scale practical application [Citation14, Citation67]. Concerning electronic or mixed oxygen-ion/electronic conducting oxides, Iwahara et al [Citation68] reported that among Ca0.85Ce0.15MnO3−δ, La0.6Ba0.4MnO3−δ, and La0.6Ba0.6CoO3-δ, the latter showed the best performance in a protonic SOFC, but without any significant improvement compared with Pt. Hibino et al [Citation62] systematically investigated the cathodic overpotential of several perovskite materials applied on a BCY electrolyte, reporting better performance for Ba05.Pr0.5CoO3-δ than for Ba0.5La0.5CoO3-δ, La0.5Sr0.5CoO3-δ, Sm0.5Sr0.5CoO3-δ and Pt. Yamaura et al [Citation69] studied the overpotential of strontium-doped lanthanum ferrite, La0.7Sr0.3MO3-δ, where M=Fe, Mn and Co, reporting the smallest overpotential for La0.7Sr0.3FeO3-δ. However, because the cathode microstructure strongly affects the cathode performance by determining the extension of the reaction zone, it is not possible to compare different cathode materials unless their microstructure is accurately described, which was lacking in the above cited papers. Furthermore, as discussed for the anode, chemical reactions with the electrolyte can strongly reduce the electrode performance, blocking the ionic transport at the cathode/electrolyte interface. For example, Yamaura et al [Citation69] reported chemical reaction between SrCe0.95Yb0.05O3-δ (SCYb) electrolyte and La0.7Sr0.3FeO3-δ cathode upon heating at 1000 °C. Decreasing the co-firing temperature can avoid the formation of secondary phases and reduce cathode coarsening, but it usually leads to poor adhesion between the cathode and the electrolyte. Formation of secondary phases between SCYb and another lanthanum ferrite-based cathode (La0.6Sr0.4Fe0.8Co0.2O3-δ, LSCF) after co-firing at 1100 °C was also reported by Fabbri et al [Citation70]. Differently, no chemical reactions were observed between BaCe0.9Yb0.1O3-δ and LSCF after the same co-firing treatment. Therefore, Ba-based HTPCs seem to have better chemical compatibility with lanthanum-based cathodes than Sr-based HTPCs. For instance, BZY also hardly reacted with LaCoO3, and only limited reactivity was observed with LaFeO3 and La2NiO4, resulting in the formation of small amounts of a La2Zr2O7 phase [Citation71]. The formation of La2Zr2O7 was also observed between YSZ and lanthanum-based cathodes, resulting in cell performance degradation due to the low oxygen-ion conductivity of this compound [Citation72]. Because La2Zr2O7 also has poor proton conductivity (about two orders of magnitude lower than that of BaCeO3 at 400 °C [Citation12]), its formation at the electrode/electrolyte interface is also expected to reduce the performance of protonic SOFCs.
Cation diffusion from the cathode to the electrolyte was observed by Lin et al [Citation73] between Ba0.5Sr0.5Co0.8Fe0.2O3-δ, a highly performing cathode for oxygen-ion based cell and BCY electrolyte. In particular, they reported barium diffusion from BCY to BSCF after co-firing at 900 °C. Interestingly, they found that the change in Ba content did not significantly affect the electrode ASR, but the Ba deficiency of the electrolyte caused an increase in the BCY ohmic resistance and led to a blocking effect for proton transfer at the electrode/electrolyte interface. Similar cation diffusion was reported in a recent paper of Li et al [Citation74] between BCZY and PrBaCo2O5+δ (PBC), a double-layered perovskite cathode with high oxygen diffusion and surface exchange coefficients [Citation75]. After co-firing BCZY and PBC up to 1000 °C, no other phases appeared in the XRD patterns, but shifts in the peak positions indicated a change in composition. The authors explained this finding by the diffusion of cobalt from PBC to BCZY accompanied by the incorporation of Y3+ into the Pr site of PBC. The resulting Co-doped BCZY electrolyte showed smaller conductivity, while the incorporation of Y into the PBC structure lowered the ASR of the cathode owing to a faster charge transfer process [Citation74].
Other double-layered perovskite cathodes have recently been used for protonic SOFC applications. Unfortunately, most of the associated papers do not report the chemical compatibility and ASR measurements. They merely allow a comparison of the power density output of protonic SOFCs based on BCZY electrolyte using different double-perovskite cathodes; the best performance was obtained with SmBa0.5Sr0.5Co2O5+δ (245 mW cm-2 at 600 °C) [Citation56].
Cobalt-free cathode materials (BaCe0.5Fe0.5O3-δ and BaCe0.5Bi0.5O3-δ) have been developed for protonic SOFCs, but with rather low power density output (192 and 125 mW cm-2 at 600 °C, respectively) [Citation75, Citation76].
In theory, the most desirable cathode material for use in a protonic SOFC should present mixed electronic and protonic conductivity to extend the TPB from the electrode/electrolyte interface to the whole cathode bulk. Overall, there are two ways to simultaneously achieve high electronic and protonic conductivity; one is to modify a proton conductor by introducing a multivalent element into its structure, and the other is to produce a composite electrode made of a proton-conducting phase with electronic or mixed electronic/oxygen-ion conduction. For mixed protonic/electronic conductors, the H+ ions should be transferred from the electrolyte to the electrode and be able to rapidly migrate through the bulk electrode reacting with the O2 in the surrounding atmosphere (figure (b)). For composite cathodes, the reaction steps are the same as reported in table , with the difference that the TPB is extended to the whole cathode (figure (c)).
Fabbri et al [Citation37] have attempted to increase the p-type conductivity of barium cerate proton conductor by using multivalent dopants, such as Sm, Eu and Yb. BaCe0.9Yb0.1O3–δ (BCYb) was identified as a promising mixed proton/electron–hole conductor under dry, high po2 atmosphere. However, the electron–hole conductivity was reduced by the introduction of water vapor in the atmosphere that corresponds to the cathode operating conditions in a protonic SOFC. As a result of the insufficient electronic conductivity under wet conditions, the ASR of this cathode material was extremely large. Mukundan et al [Citation77] also tried to develop a mixed proton/electron-hole conductor with the BaCe0.8-yPryGd0.2O3-δ composition; the lowest overpotential was measured for BaPr0.8Gd0.2O3–δ compound, which was later studied in detail by Magraso et al [Citation78]. This compound showed mainly p-type conduction, with a minor protonic contribution even in wet atmosphere. Therefore, up to now, high protonic conductivity in perovskite-type materials seems to prevent a concurrent high electronic conductivity. To overcome this problem, composite cathodes have been developed in the last few years.
Composite cathodes made of Sm0.5Sr0.5Co0.2O3-δ (SSC) and BaCe0.8Sm0.2O3-δ (BCS) have recently been investigated [Citation79]. Optimization of the cathode composition (60 wt% of SSC) and of the fabrication process (co-firing at 1050 °C) resulted in an ASR of 0.21 Ω cm2 at 700 °C. A slightly lower ASR value (0.14 Ω·cm2 at 700 °C) was observed for the composite cathode made of LSCF and BCYb, mixed in a 1:1 weight ratio [Citation70]. Both SSC-BCS [Citation79] and LSCF-BCYb [Citation70] composite cathodes have been characterized by electrochemical impedance spectroscopy (EIS). In particular, ASR measurements performed at different partial pressures of oxygen allowed correlating each semicircle in the complex impedance plot to a specific electrode process [Citation80]. While for the SSC-BCS only one semicircle could be observed in the low-frequency region, two semicircles appeared for LSCF-BCYb under similar experimental conditions (intermediate temperature, wet air atmosphere). In both cases, the rate-limiting step was associated with the dissociative adsorption of oxygen.
Other composite cathode materials have been simply tested in protonic SOFCs; Ba0.5Sr0.5Co0.8Fe0.2O3-δ mixed with BCZY in a 3:2 weight ratio [Citation81], Ba0.5Sr0.5Zn0.2Fe0.8O3-δ mixed with Ba0.5Ce0.5Zr0.3Y0.16Zn0.04O3-δ [Citation82] and SSC mixed with BZCY in a 3 : 2 weight ratio [Citation83]. The cell using the last cathode showed the largest power density among anode-supported protonic SOFCs. The authors suggested that the formation of secondary phases between SSC and BCZY, namely BaCoO3 and Sm2Zr2O7, is beneficial for the oxygen reduction because both phases are mixed electronic/ionic conductors. However, Ishihara et al [Citation84] reported that BaCoO3 only exhibits a low cathodic overpotential when Ba is heavily substituted with La (between 30 and 50 mol%). Furthermore, even though Sm2Zr2O7-based compounds are proton conductors, their conductivity is about two orders of magnitude smaller than that of BZCY [Citation85, Citation86]; thus, it is expected that Sm2Zr2O7 formation would reduce the cell performance.
All these findings indicate that chemical reactions, microstructure characteristics and EIS analysis in different atmospheres are essential for optimizing the performance of cathode materials used in protonic SOFCs, and thus, a thorough characterization in this direction is crucial for rationally improving fuel cell performance.
Present performance of proton conducting SOFCs and future guidelines
The best performance of a fuel cell based on an HTPC electrolyte has probably been reported by Ito et al [Citation61]. Power density outputs of 1.4 and 0.9 W cm-2 were achieved at 600 and 400 °C, respectively, depositing by pulsed laser deposition (PLD) a 0.7 μm thick BCY film on a 40 μm thick anodic Pd membrane. These large power densities are likely to result from the use of a Pd membrane through which hydrogen can diffuse in the atomic form, even though the thermo-mechanical stability of such metallic-ceramic system might be questionable for practical use. Using PLD, BCY thin films were grown on dense NiO-BCY anodes and subsequently reduced to obtain a porous substrate [Citation87]. However, in contrast to the previous case, poor power output values were obtained owing to large ohmic losses. Still, using BCY as a thin electrolyte membrane, large power density output was reported by Balachandran et al [Citation88], reaching about 1.5 W cm-2 at 800 °C.
Up to now, the most popular electrolyte for protonic SOFCs is definitively BCZY [Citation57, Citation59, Citation76, Citation80, Citation83], which has been claimed to exhibit high conductivity and good chemical stability for fuel cell application [Citation85], even though later works demonstrated that this compound is unstable in the presence of CO2 [Citation14, Citation66]. In these works, BCZY layers, with a thickness ranging between 10 and 65 μm, were deposited onto a NiO-BCZY supporting anode by co-pressing, drop-coating, screen printing, or spray-coating, in some cases adding a pore former to the anode powder to achieve a greater anode porosity [Citation57, Citation76, Citation80]. Interestingly, the largest power density has been reported for a cell made with the thickest BCZY membrane. In particular, the best performance was obtained using a 65 μm thick BCZY membrane fabricated by co-pressing method, SSC-BCZY composite cathode, and a NiO-BZCY anode with the two phases in a 1:1.92 weight ratio and rice starch as the pore former, to the best of our knowledge [Citation83]. The worst performance reported for a BCZY-based SOFC was obtained, to the best of our knowledge, using a 25 μm thick BCZY membrane fabricated by co-pressing, a BaCe0.5Bi0.5O3-δ cathode, and a NiO-BCZY anode with the two phases in a 3:2 weight ratio and the addition of 20 wt% of corn starch [Citation76]. The comparison of these two results shows how the cathode plays a critical role in improving the performance of SOFCs. Therefore, besides developing more protonic SOFCs, deeper investigations on cathode materials might help boosting the performance of protonic SOFCs, which is still quite poor compared with that of SOFCs based on oxygen-ion conducting electrolytes [Citation89].
Conclusions
High temperature protonic conductors show tremendous promise for application in IT-SOFCs. However, their practical use is yet to be realized, owing to several drawbacks, including the very large electrode overpotentials usually observed at low temperatures, especially at the cathode site. Because HTPCs are extremely promising for operation below 600 °C, finding suitable electrode materials becomes crucial for enabling the widespread use of portable SOFCs. However, a systematic approach clarifying the effects of the chemical composition and microstructure on the electrochemical properties of electrodes for HTPC electrolytes has been often neglected up to now, favoring a more empirical approach of trial and errors during fuel cell tests, as revealed by the literature survey of the recent years. A more fundamental approach will be needed to successfully provide novel electrode materials for protonic SOFCs.
References
- AtkinsonABarnettSGorteR JIrvineJ T SMcEvoyA JMogensenMSinghalS CVohsJ 2004 Nat. Mater. 3 17 http://dx.doi.org/10.1038/nmat1040
- Boudghene StambouliATraversaE 2002 Renew. Sustain. Energy Rev. 6 295 http://dx.doi.org/10.1016/S1364-0321(01)00015-6
- Boudghene StambouliATraversaE 2002 Renew. Sustain. Energy Rev. 6 433 http://dx.doi.org/10.1016/S1364-0321(02)00014-X
- BrettD J LAtkinsonABrandonN PSkinnerS J 2008 Chem. Soc. Rev. 37 1568 http://dx.doi.org/10.1039/b612060c
- SinghalS CKendallK 2003 High-Temperature Solid Oxide Fuel Cells: Fundamentals, Design and Applications Oxford Elsevier
- WangW GMogensenM 2005 Solid State Ion. 176 457 http://dx.doi.org/10.1016/j.ssi.2004.09.007
- HoritaKYamajiNSakaiYXiongTKatoHYokokawaTKawadaT 2002 J. Power Sources 106 224 http://dx.doi.org/10.1016/S0378-7753(01)01017-5
- SteeleB C HHeinzelA 2001 Nature 414 345 http://dx.doi.org/10.1038/35104620
- YamamotoO 2000 Electrochim. Acta 45 2423 http://dx.doi.org/10.1016/S0013-4686(00)00330-3
- HaileS M 2003 Acta Mater. 51 5981 http://dx.doi.org/10.1016/j.actamat.2003.08.004
- TarancónA 2009 Energies 2 1130 http://dx.doi.org/10.3390/en20401130
- KreuerK D 2003 Annu. Rev. Mater. Res. 33 333 http://dx.doi.org/10.1146/annurev.matsci.33.022802.091825
- BohnH GSchoberT 2000 J. Am. Ceram. Soc. 83 768
- FabbriED'EpifanioADi BartolomeoELicocciaSTraversaE 2008 Solid State Ion. 179 558 http://dx.doi.org/10.1016/j.ssi.2008.04.002
- KatahiraKKohchiYShimuraTIwaharaH 2000 Solid State Ion. 138 91 http://dx.doi.org/10.1016/S0167-2738(00)00777-3
- FabbriEPergolesiDD'EpifanioADi BartolomeoEBalestrinoGLicocciaSTraversaE 2008 Energy Environ. Sci. 1 355 http://dx.doi.org/10.1039/b806655h
- KwonO HChoiG M 2006 Solid State Ion. 177 3057 http://dx.doi.org/10.1016/j.ssi.2006.07.039
- IshiharaTShibayamaTHondaMNishiguchiHTakitaY 2000 J. Electrochem. Soc. 147 1332 http://dx.doi.org/10.1149/1.1393358
- EspositoVTraversaE 2008 J. Am. Ceram. Soc. 91 1037 http://dx.doi.org/10.1111/j.1551-2916.2008.02347.x
- Perry MurrayESeverM JBarnettS A 2002 Solid State Ion. 148 27 http://dx.doi.org/10.1016/S0167-2738(02)00102-9
- EsquirolAKilnerJBrandonN 2004 Solid State Ion. 175 63 http://dx.doi.org/10.1016/j.ssi.2004.09.013
- ShaoZHaileS M 2004 Nature 431 170 http://dx.doi.org/10.1038/nature02863
- BucherEEggerACaramanG BSitteW 2008 J. Electrochem. Soc. 155 B1218 http://dx.doi.org/10.1149/1.2981024
- KimGWangSJacobsonA JReimusLBrodersenPMimsC A 2007 Mater. Chem. 17 2500 http://dx.doi.org/10.1039/b618345j
- EspositoVLuongB HDi BartolomeoEWachsmanE DTraversaE 2006 J. Electrochem. Soc. 153 A2232 http://dx.doi.org/10.1149/1.2358088
- FabbriEPergolesiDTraversaE 2010 Chem. Soc. Rev. submitted
- IwaharaHEsakaTUchidaHMaedaN 1981 Solid State Ion. 3/4 359 http://dx.doi.org/10.1016/0167-2738(81)90113-2
- HaileS MStaneffGRyuK H 2001 J. Mater. Sci. 36 1149 http://dx.doi.org/10.1023/A:1004877708871
- BonanosN 2001 Solid State Ion. 145 265 http://dx.doi.org/10.1016/S0167-2738(01)00951-1
- ScherbanTVilleneuveRAlbelloLLucazeauG 1993 Solid State Ion. 61 93 http://dx.doi.org/10.1016/0167-2738(93)90339-5
- StevensonD AJiangNBuchananR MHennF E G 1993 Solid State Ion. 62 279 http://dx.doi.org/10.1016/0167-2738(93)90383-E
- SongS JWachsmanE DDorrisS EBalachandranU 2003 J. Electrochem. Soc. 150 A790 http://dx.doi.org/10.1149/1.1574031
- BonanosN 1992 Solid State Ion. 53–56 967 http://dx.doi.org/10.1016/0167-2738(92)90278-W
- KhartonV VMarozauI PMatherG CNaumovichE NFradeJ R 2006 Electrochim. Acta 51 6389 http://dx.doi.org/10.1016/j.electacta.2006.04.023
- PasierbPWierzbickaMKomornickiSRekasM J 2007 J. Power Sources 173 681 http://dx.doi.org/10.1016/j.jpowsour.2007.05.057
- SchoberTSchillingWWenzlH 1996 Solid State Ion. 86–88 653 http://dx.doi.org/10.1016/0167-2738(96)00230-5
- FabbriEOhTLicocciaSTraversaEWachsmanE D 2009 J. Electrochem. Soc. 156 B38 http://dx.doi.org/10.1149/1.3005781
- TaniguchiNHatohKNiikuraJGamoT 1992 Solid State Ion. 53–56 998 http://dx.doi.org/10.1016/0167-2738(92)90283-U
- MaGShimuraTIwaharaH 1998 Solid State Ion. 110 103 http://dx.doi.org/10.1016/S0167-2738(98)00130-1
- ShimaDHaileS M 1997 Solid State Ion. 97 443 http://dx.doi.org/10.1016/S0167-2738(97)00029-5
- ZakowskyNWilliamsonSIrvineJ T S 2005 Solid State Ion. 176 3019 http://dx.doi.org/10.1016/j.ssi.2005.09.040
- BhideS VVirkarA V 1999 J. Electrochem. Soc. 146 2038 http://dx.doi.org/10.1149/1.1391888
- ChenFS⊘rensenO TMengGPengD J 1997 Mater. Chem. 7 481 http://dx.doi.org/10.1039/a605377g
- TaniguchiNNishimuraCKatoJ 2001 Solid State Ion. 145 349 http://dx.doi.org/10.1016/S0167-2738(01)00930-4
- AzadA KIrvineJ T S 2007 Solid State Ion. 178 635 http://dx.doi.org/10.1016/j.ssi.2007.02.004
- BabiloPHaileS M 2005 J. Am. Ceram. Soc. 88 2362 http://dx.doi.org/10.1111/j.1551-2916.2005.00449.x
- XieKYanRXuXLiuXMengG 2009 J. Power Sources 187 403 http://dx.doi.org/10.1016/j.jpowsour.2008.11.007
- EssoumhiATailladesGTaillades-JacquinMJonesD JRozièreJ 2008 Solid State Ion. 179 2155 http://dx.doi.org/10.1016/j.ssi.2008.07.025
- ChevallierLZunicMEspositoVDi BartolomeoETraversaE 2009 Solid State Ion. 180 715 http://dx.doi.org/10.1016/j.ssi.2009.03.005
- MatherG CFigueiredoF MFaggD PNorbyTJuradoJ RFradeJ R 2003 Solid State Ion. 158 333 http://dx.doi.org/10.1016/S0167-2738(02)00904-9
- EspositoVTraversaEWachsmanE D 2005 J. Electrochem. Soc. 152 A2300 http://dx.doi.org/10.1149/1.2097036
- AgarwalVLiuM 1997 J. Electrochem. Soc. 144 1035 http://dx.doi.org/10.1149/1.1837526
- RanranPYanWLizhaiYZongqiangM 2006 Solid State Ion. 177 389 http://dx.doi.org/10.1016/j.ssi.2005.11.020
- ZunicMChevallierLDeganelloFD'EpifanioALicocciaSDi BartolomeoEandT 2009 J. Power Sources 190 417 http://dx.doi.org/10.1016/j.jpowsour.2009.01.046
- SunWYanLLinBZhangSLiuW 2010 J. Power Sources 195 3155 http://dx.doi.org/10.1016/j.jpowsour.2009.11.100
- DingHXueXLiuXMengG 2010 J. Power Sources 195 775 http://dx.doi.org/10.1016/j.jpowsour.2009.08.022
- ZhaoLHeBNianQXunZPengRMengGLiuX 2009 J. Power Sources 194 291 http://dx.doi.org/10.1016/j.jpowsour.2009.05.001
- LinBDongYYanRZhangSHuMZhouYMengG 2009 J. Power Sources 186 446 http://dx.doi.org/10.1016/j.jpowsour.2008.09.120
- FabbriESannaSD'EpifanioADi BartolomeoELicocciaSBalestrinoGTraversaE 2010 Energy Environ. Sci. 3 618 http://dx.doi.org/10.1039/c001316a
- YamaguchiSShishidoTYugamiHYumamotoSHaraS 2003 Solid State Ion. 162–163 291 http://dx.doi.org/10.1016/S0167-2738(03)00221-2
- ItoNIijimaMKimuraKIguchiS 2005 J. Power Sources 152 200 http://dx.doi.org/10.1016/j.jpowsour.2005.01.009
- HibinoTHashimotoASuzukiMSanoM 2002 J. Electrochem. Soc. 149 A1503 http://dx.doi.org/10.1149/1.1513983
- JinYSaitoHYamaharaKIharaM 2009 Electrochem. Solid-State Lett. 12 B8 http://dx.doi.org/10.1149/1.3028636
- FangSBiLWuXGaoHChenCLiuW 2008 J. Power Sources 183 126 http://dx.doi.org/10.1016/j.jpowsour.2008.05.015
- YangLWangSBlinnKLiuZChengZLiuM 2009 Science 326 126 http://dx.doi.org/10.1126/science.1174811
- BiLTaoZLiuCSunWWangHLiuW 2009 J. Membrane Sci. 336 1 http://dx.doi.org/10.1016/j.memsci.2009.03.042
- UchidaHTanakaSIwaharaH 1985 J. Appl. Electrochem. 15 93 http://dx.doi.org/10.1007/BF00617745
- IwaharaHYajimaTHibinoTUshidaH 1993 J. Electrochem. Soc. 140 1687 http://dx.doi.org/10.1149/1.2221624
- YamauraHIkutaTYahiroHOkadaG 2005 Solid State Ion. 176 269 http://dx.doi.org/10.1016/j.ssi.2004.08.008
- FabbriELicocciaSTraversaEWachsmanE D 2009 Fuel Cells 9 128 http://dx.doi.org/10.1002/fuce.200800126
- TolchardJ RGrandeT 2007 Solid State Ion. 178 593 http://dx.doi.org/10.1016/j.ssi.2007.01.018
- JiYKilnerJ ACarolanM F 2005 Solid State Ion. 176 937 http://dx.doi.org/10.1016/j.ssi.2004.11.019
- LinYRanRZhengYShaoZJinWXuNAhnJ 2008 J. Power Sources 180 15 http://dx.doi.org/10.1016/j.jpowsour.2008.02.044
- LinYRanRZhangCCaiRShaoZ 2010 J. Phys. Chem. A 114 3764 http://dx.doi.org/10.1021/jp9042599
- TaoZBiLZhuZLiuW 2009 J. Power Sources 194 801 http://dx.doi.org/10.1016/j.jpowsour.2009.06.071
- TaoZBiLYanLSunWZhuZPengRLiuW 2009 Electrochem. Commun. 11 688 http://dx.doi.org/10.1016/j.elecom.2009.01.012
- MukundanRDaviesP KWorrelW L 2001 J. Electrochem. Soc. 148 A82 http://dx.doi.org/10.1149/1.1344520
- MagrasoAHaugsrudRSegarraMNorbyT 2009 J. Electroceram. 23 80 http://dx.doi.org/10.1007/s10832-008-9541-z
- WuTPengRXiaC 2008 Solid State Ion. 179 1505 http://dx.doi.org/10.1016/j.ssi.2007.12.005
- BrandonN PKilnerJ AMogensenM J 2004 J. Electrochem. Soc. 151 A1847 http://dx.doi.org/10.1149/1.1799391
- LinBDingHDongYWangSZhangXFangDMengG 2009 J. Power Sources 186 58 http://dx.doi.org/10.1016/j.jpowsour.2008.09.041
- LuXDingYChenY 2009 J. Alloys Compd. 484 856 http://dx.doi.org/10.1016/j.jallcom.2009.05.065
- YangLZuoCWangSChengZLiuM 2008 Adv. Mater. 20 3280 http://dx.doi.org/10.1002/adma.200702762
- IshiharaTFukuiSNishiguchiHTakitaY 2002 J. Electrochem. Soc. 149 A823 http://dx.doi.org/10.1149/1.1480015
- ZuoCZhaSLiuMHatanoMUchiyamaM 2006 Adv. Mater. 18 3318 http://dx.doi.org/10.1002/adma.200601366
- ShimuraTKomoriMIwaharaH 1996 Solid State Ion. 86–88 685 http://dx.doi.org/10.1016/0167-2738(96)00148-8
- MatsumotoHNomuraIOkadaSIshiharaT 2008 Solid State Ion. 179 1486 http://dx.doi.org/10.1016/j.ssi.2008.02.048
- BalachandranULeeT HDorrisS E 2007 ECS Trans. 7 987 http://dx.doi.org/10.1149/1.2729194
- AhnJ SPergolesiDCamarattaM AYoonHLeeB WLeeK TJungD WTraversaEWachsmanE D 2009 Electrochem. Commun. 11 1504 http://dx.doi.org/10.1016/j.elecom.2009.05.041