Abstract
Zinc oxide (ZnO) nanorods were grown on a paper support prepared from soft wood pulp. The photocatalytic activity of a sheet of paper with ZnO nanorods embedded in its porous matrix has been studied. ZnO nanorods were firmly attached to cellulose fibers and the photocatalytic paper samples were reused several times with nominal decrease in efficiency. Photodegradation of up to 93% was observed for methylene blue in the presence of paper filled with ZnO nanorods upon irradiation with visible light at 963 Wm–2 for 120 min. Under similar conditions, photodegradation of approximately 35% was observed for methyl orange. Antibacterial tests revealed that the photocatalytic paper inhibits the growth of Escherichia coli under room lighting conditions.
Introduction
Paper is widely used for a variety of applications such as writing, printing, packaging, cleaning, construction and decoration. To further broaden the utility of paper and other nonwoven cellulosic materials, specialty paper was developed targeting specific applications like chromatography, filtration and printing, to name a few. These added functionalities in paper are achieved through different coating technologies. Paper is coated with different compounds to impart qualities like improved surface gloss and smoothness or lower ink absorbency. Photocatalytic paper is a type of specialty paper that was developed to degrade organic pollutants and immobilize microbes [Citation1]. This type of antimicrobial and antifouling paper can have wide applications in health-related activities.
Photocatalysis with different semiconducting metal oxide nanoparticles has been an active area of research following the successful demonstration of the photolysis of water using a titania (TiO2) photoanode [Citation2]. Zinc oxide (ZnO), with its high surface reactivity owing to its large number of active surface defect states, has emerged as a more efficient photocatalyst than TiO2. It has high reaction and mineralization rates [Citation3] because of its more efficient hydroxyl ion generation [Citation4]. ZnO can be structurally modified for visible light absorption paving the way for visible-light photocatalytic activity [Citation5]. Furthermore, Zn2+ ions originating from slow dissolution of ZnO in moist environments can immobilize microbes [Citation6]. As the surface area and surface defects play an important role in the photocatalytic activity of metal oxide nanostructures, one-dimensional nanostructures like nanowires/nanorods are ideal candidates for application to photocatalysis since they offer a larger surface-to-volume ratio than nanoparticulate thin films [Citation5].
Photocatalysis using suspended nanoparticles to remove contaminants in a liquid is an efficient process, but the subsequent removal of the nanoparticles from the liquid can be cumbersome and adds an extra process step. It is therefore important that nanoparticulate photocatalysts should be attached to a support, as freely suspended nanoparticles can enter the human body through skin pores and may lead to health-related problems. Pozzo et al [Citation7] have also discussed the importance of catalyst supports and opined that there is an inverse relationship between the adhesion of a catalyst to a support and its photocatalytic activity. There are only a few reports (including patents) on the use of paper as a catalyst support [Citation1, Citation4, Citation8, Citation9].
Photocatalytic paper was first reported by Matsubara et al [Citation10] in 1995, who presented the catalytic degradation of acetaldehyde in the vapor phase using illumination with a weak fluorescent light. Subsequently, a few publications and patents describing photocatalytic paper have appeared in the literature [Citation11–13]. The majority of available reports on photocatalytic paper mention TiO2 as a photocatalyst, which absorbs light mostly in the ultraviolet region of the electromagnetic spectrum [Citation7]. Ghule et al [Citation14] reported the growth of ZnO nanoparticulate coating on paper support and studied its antibacterial property. ZnO nanorods, which can be strongly attached to any type of substrates through proper surface treatment before seeding [Citation15], are an attractive option for photocatalytic applications. This work is one of the first reports on the in situ growth of ZnO nanorods on a cellulose support with target application as a visible-light photocatalyst.
Experimental details
Procedure for preparing photocatalytic paper
In a typical process, 30 g of dry bleached kraft soft wood pulp was chopped and dipped in water for 4 h, and the volume of the pulp suspension was increased up to 2000 ml using water (consistency of suspension of 1.5%). The suspension was then disintegrated at a rotation speed of 30 000 rpm using a scan-C 18:65 standard. After disintegration, water was removed from the fiber suspension using a Buchner funnel. For refining, the consistency of the pulp was adjusted at 10%. Pulp was refined using a PFI mill based on scan-C 24:67 standard at 10 000 rpm. To make hand sheets, pulp suspensions were diluted to about 0.2% by adding 15000 ml of water to 30 g of refined pulp. After dewatering using a FORMAX standard sheet mold, a fiber suspension with a consistency of approximately 0.2% was obtained. A circular sheet was made using a scan-C 24:76 standard with a diameter of 15.9 cm and a basis weight of 35g m−2. Photocatalytic paper was prepared through a subsequent post-treatment process, which is normally referred to as `size press treatment’ in papermaking. The thus-prepared sheets were seeded with ZnO nanoparticles through dip coating. They were dipped three times and dried at 90 °C for 15 min after each dipping. The ZnO nanorods were then grown following the procedure detailed in section 2.3.
Synthesis of ZnO seed nanoparticles
ZnO nanoparticles were synthesized in a colloidal solution using ethanol as the solvent following the procedure reported in [Citation16, Citation17]. Briefly, 40 ml of 2 mM zinc acetate dihydrate [(CH3COO)2Zn·2H2O, Merck] solution was heat-treated at 70 °C for 0.5 h; 20 ml of 4 mM sodium hydroxide (NaOH, Merck) solution was then added dropwise, and the admixture was hydrolyzed for 2 h at 60 °C.
Growth of ZnO nanorods
ZnO nanorods were grown using a simple and efficient hydrothermal growth process [Citation18]. Paper sheets were initially seeded by dipping in a colloidal dispersion of ZnO nanoparticles and dried at 90 °C. They were then dipped in an equimolar solution of zinc nitrate hexahydrate [Zn(NO3)2·6H2O, APS Ajax Finechem] and hexamethylenetetramine [(CH2)6N4, Carlo Erba] and maintained at 90 °C for 10 h. Two different concentrations of zinc nitrate and hexamine were used for the nanorod growth: samples 1 (20 mM) and 2 (10 mM). The nanorods grew out of the seeds preferentially along the c-axis of the wurtzite structure [Citation17]. The reaction bath was replenished after 5 h [Citation5]. The sheets were then removed and washed with deionized water several times and dried at 70 °C for 6 h. Nanorod dimensions were quantified using Scion image processing software applied to scanning electron microscopy (SEM) images. The images were obtained with a JEOL JSM-6301F microscope operated at 20 kV. Transmission electron microscopy (TEM) images were taken with a JEOL/JEM 2010 microscope operated at 120 kV.
It is important that a catalyst should remain firmly attached to the support (paper in this case) and that its activity should be repeatable. A simple experiment was carried out to determine whether the ZnO nanorods were firmly attached to the paper. Square sheets (1×1 cm2) of untreated paper and treated paper were air-blown at a pressure of 2 bar. Changes in their weight were monitored to assess the removal of the ZnO nanorods from the paper support.
Photocatalysis tests
Organic dyes
Photocatalysis was studied using two test contaminants, methylene blue (MB, C16H18N3SCl, Carlo Erba) and methyl orange (MO, C14H14N3NaO3S, Carlo Erba). The photodegradation of MB results in the formation of leuco methylene blue (C16H19N3S) [Citation19], while that of MO produces hydrazine (N2H4) and some other reaction intermediates, leading to a change in optical absorption [Citation20]. Solutions of the test contaminants (10 μM) were prepared in deionized water and put in polymethyl methacrylate (PMMA) cuvettes together with a rectangular piece of photocatalytic paper (3×1 cm2). The cuvettes were then placed in front of a 500 W halogen lamp. A glass tank filled with water was installed between the lamp and the cuvettes to absorb UV light and infrared light. The incident light intensity was measured with a Kipp and Zonen pyranometer as 963 Wm−2. Similar cuvettes containing the dyes and untreated paper samples were used as controls. Optical absorption spectra were recorded upon light irradiation at different time intervals using an Ocean Optics spectrophotometer to monitor the rate of decolorization of the test contaminants. The degradation of the dye was estimated in terms of the change in absorption at λmax∼665 nm for MB and at λmax∼460 nm for MO.
The degradation efficiency was calculated using
1 where I0 is the initial absorption intensity of MB at λmax=665 nm or of MO at λmax=460 nm, I is the intensity at λmax after illumination at time t, C0 is the initial concentration of the dyes and is C the concentration after illumination at time t. The photocatalytic tests with MB and MO dyes were repeated after rinsing the used photocatalytic paper with deionized water and drying at it 70 °C.
Antibacterial activity
The microbicidal property of the photocatalytic paper was monitored using commonly available strains of the bacterium Escherichia Coli (E. coli), some of which can cause severe food poisoning in humans [Citation21]. Wild-type E. coli (TISTR 073) cells were cultivated on nutrient agar (source) and incubated at 37 °C for 18 h using the streak plate method [Citation22]. After harvesting the cells incubated for 18 h by scraping from the nutrient agar, they were mixed in phosphate buffered saline by a vortex mixer until they were uniformly dispersed. Then the suspension was centrifuged at 4000 rpm for 10 min and the supernatant was discarded. This process was repeated twice and the cell pellet was mixed with 1 ml of deionized water for the experiments. The E. coli cells thus prepared were redispersed in 10 ml of Milli-Q water, and the optical density of the suspension was kept at 0.6 (600 nm wavelength) for all the tests.
To observe antibacterial activity, three sheets (1.5×1.5cm2) of both the untreated paper and ZnO-treated paper were taken and dried for 10 min in a laminar airflow. Antibacterial activity was assessed via the zone of inhibition, i.e. the absence of viable E. coli cells, around the paper samples. For this purpose, 100 μ of E. coli cell suspension was spread on nutrient agar, and the square paper samples were placed on it in a triangular formation. The temperature inside the incubation box was maintained at 37 °C and incubation was continued for 72 h. The area of inhibition surrounding the square paper samples of 2.25 cm2 area was then measured. E. coli cells were stained with safranin dye before imaging. Photocatalysis tests were carried out under illumination using a tungsten halogen lamp (light intensity ∼11 W m–2 on the sample) and also in the dark as a control.
Results and discussion
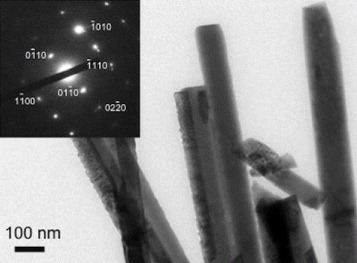
The porous (void) structure of paper prepared from cellulose pulp is attractive as a catalyst support as it allows higher loading of photocatalysts. The enhanced decomposition of volatile organic compounds as a result of the increased porosity of paper has been reported in the literature [Citation23] An SEM image of as-prepared paper is shown in figure (a) where a porous structure can be observed. The process of affixing presynthesized ZnO nanoparticles in colloidal form allows nanoparticles to percolate through gaps of porous cellulose. As a result, ZnO nanorods grow even deep inside the pores, thereby increasing loading in a sample. ZnO nanorods grown inside a porous structure of paper can be seen in figure (b). Figure (c) shows ZnO nanorods on the top surface of the paper, and figure (d) shows a close-up view of the ZnO nanorods. Measurements carried out on twenty samples revealed the width and length of the nanorods for sample 1 as 260 and 2000 nm, and the corresponding values for sample 2 as 80 and 600 nm, respectively. A TEM image of the ZnO nanorods is shown in figure . The nanorods are highly crystalline, having the wurtzite structure, as is evident from the electron diffraction pattern shown in the inset of figure . The diffraction pattern could be indexed to different planes of the wurtzite structure. The formation of wurtzite under hydrothermal growth conditions and the presence of inherent structural defects were also observed by Morin et al [Citation24] using TEM and electron diffraction analysis. The wurtzite structure is ideal for hydrothermal anisotropic growth owing to the presence of polar and non-polar surfaces, with a natural tendency to minimize the polar surface in order to reduce surface energy [Citation18].
Figure 1 Scanning electron micrographs showing (a) porous structure of the paper, (b) ZnO nanorods growing in the paper pores, (c) ZnO nanorods on the top surface of the paper and (d) close up view of the ZnO nanorods.
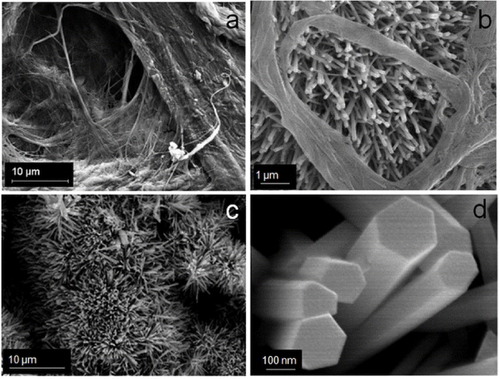
Figure 2 Transmission electron micrograph showing a section of a ZnO nanorod cluster. Inset: electron diffraction pattern from one ZnO nanorod.
The attachment of the ZnO nanorods to the cellulose support is important for preventing the removal of nanostructures from the test solution. The air blowing experiment detailed in section 2.3 revealed that after an initial weight loss, the sample weight stabilized (figure ). The initial weight loss can be attributed to the removal of loosely bound ZnO agglomerates and cigarlike structures resulting from secondary growth [Citation18], which were not removed by washing. The slight decrease in the weight of the bare substrate is due to the removal of broken bits of fibers resulting from the cutting process. All the photocatalysis experiments reported below were carried out after blowing air onto the samples at a pressure of 2 bar for 10 min.
Figure 3 Weight loss of 1×1cm2 paper samples with ZnO nanorods (samples 1 and 2) and without ZnO (untreated) after blowing air at a pressure of 2 bar. The weight loss is plotted as a function of time of air blowing. The weight of the samples became almost constant after 2 min of blowing indicating the firm attachment of the ZnO nanorods.
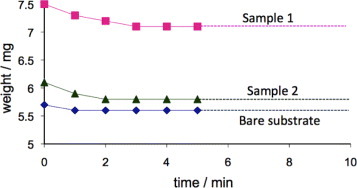
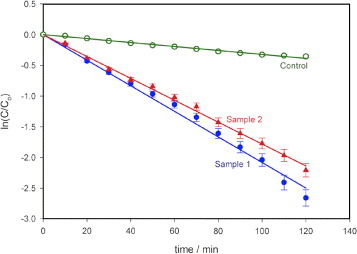
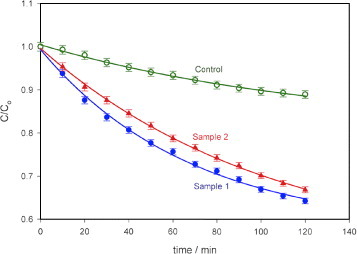
Figure shows the results of the photodegradation of MB using the photocatalytic paper with ZnO nanorods (samples 1 and 2). As reported in [Citation25], the degradation of MB is exponential with time. The degradation rate constant k for MB was obtained from the semi-logarithmic plot in figure using
2 The degradation rate constants are comparable for both the samples with decomposed MB fractions of 93% (k∼0.0208 min−1, sample 1) and 89% (k∼0.0178 min−1, Sample 2) upon illumination for 120 min. During this period, the control (10 μM MB solution only) degraded by 30% (k∼0.0032 min−1).
Figure 4 Photodegradation of MB using photocatalytic paper with ZnO nanorods of different dimensions (samples 1 and 2). Sample 1 with thicker and longer nanorods showed higher photoreactivity (93% degradation after 120 min) than sample 2 (89% degradation after 120 min). The control sample of paper without ZnO nanorods showed 30% degradation in 120 min. Light intensity at the sample: 963 Wm–2, error bar: 5%.
The photocatalytic degradation of MO is also exponential, as reported in [Citation26, Citation27]. Our observations of the degradation of MO using the photocatalytic paper could be fitted with equation (Equation33 ), and the degradation rate constants were calculated using equation (Equation4
4 ).
3
4 The degradation of MO is shown in figure as a plot of C/C0 versus time of illumination. The decomposed fraction of MO was much lower (between 30 and 35% in 120 min) than that of MB. The rate constants (k) for MO are calculated as 0.0067min−1 for sample 1, 0.0046 min−1 for sample 2 and 0.0015 min–1 for the control.
Figure 5 Photodegradation of MO using photocatalytic paper with ZnO nanorods of different dimensions (samples 1 and 2). Sample 1 with thicker and longer nanorods showed higher photoreactivity (35% after 120 min) than sample 2 (30% in 120 min). The control paper sample without ZnO nanorods showed 11% degradation in 120 min. Light intensity at the sample: 963 Wm–2, error bar: 1%.
A repeatability test was carried out three times using sample 1 for both MB and MO and the results are shown in figure . The reductions in MB degradation efficiency after 120 min of exposure to visible light were ∼3.2% for the second trial and ∼5.4% for the third trial, and the rate constants were 0.0185 and 0.0169 min−1, respectively. The corresponding values for MO were 6.4%, 13%, 0.0064 and 0.0055min–1. The decrease in efficiency is probably due to the photodegradation product molecules that did not desorb during the washing process thereby decreasing the number of active sites on the surface. The desorption of the molecules on the ZnO nanorod surfaces by changing the pH of the solution, for example, should lead to a better repeatability.
Figure 6 Repeatability test on the photocatalytic paper (sample 1) on (a) MB and (b) MO. Degradation efficiency of MB decreased by approximately 3.2% in the second trial and 5.4% in the third trial (error bar: 5%). The corresponding values for MO were 6.4% and 13%. Light intensity at the sample: 963 Wm–2, error bar: 2%.
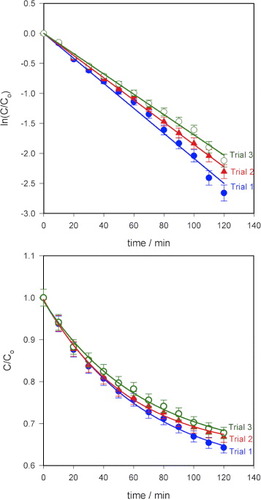
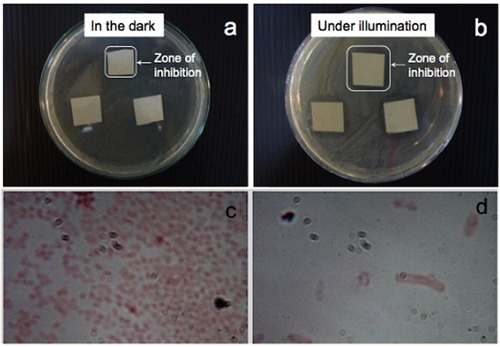
Figure shows the results of the antimicrobial tests with E. coli as a graph showing a comparison of the inhibition zone surrounding the paper sample for different conditions. Experiments were carried out in the dark as a control since ZnO itself is a bactericidal material. In moist environments, ZnO undergoes a slow dissolution and releases Zn2+ ions [Citation28]. Zn2+ ions can rupture the bacterial cell wall [Citation29] and can move inside bacterial cells, thereby inhibiting their growth [Citation6]. Cho et al [Citation30] reported the inhibition of E. coli growth in the presence of Zn2+ ions, and the use of ZnO nanoparticles to suppress the growth of Streptococcus mutans, a grampositive bacterium, in the dark was documented in [Citation31]. The increase in the inhibition zone upon illumination with visible light is indicative of photocatalytic immobilization. Free electrons from electron–hole pairs generated by the illumination of ZnO can be injected into a bacterium and rupture its cell wall [Citation32]. Not only do bacteria become immobilized on the surface of photocatalytic paper but they also cannot survive in the vicinity of ZnO-treated paper. No inhibition was observed in either the dark or illuminated condition for the control sample (paper with no ZnO nanorods) indicating the lack of microbicidal property of the paper. Figure indicates that an inhibition zone could be observed for both samples 1 (3.2 cm2) and 2 (2.8 cm2) after incubation for 72 h in the dark. This zone increased upon illumination to 4.9 cm2 (sample 1) and 4.2 cm2 (sample 2) as a result of the photocatalytic activity of the ZnO nanorods. After a 24 h incubation under illumination, the inhibition zones were 4.4 cm2 for sample 1 and 3.8cm2 for sample 2. The inhibition zones can be clearly observed in the digital photographs in figure showing sample 2 after incubation for 48 h in the dark and under illumination from a tungsten halogen lamp. Images of the bacterial cells viewed under an optical microscope at a magnification of 1000× inside and outside the zone of inhibition are shown in figures (c) and (d), respectively.
Figure 7 Increase in zone of inhibition for E.coli upon incubation in the dark and under illumination in the presence of photocatalytic paper. No inhibition zone was observed for the control sample without ZnO nanorods. The antibacterial activity increased by almost threefold upon illumination with a tungsten halogen lamp at an intensity of only 11 Wm–2 at the sample position. The activity in the dark is due to the bactericidal properties of zinc oxide.
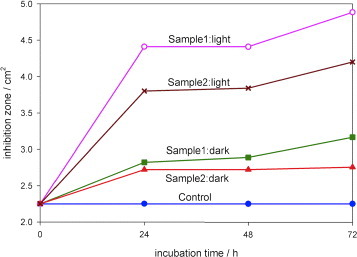
Figure 8 Results of antibacterial experiments carried out using photocatalytic paper (Sample 1) after incubation for 48 h (a) in the dark and (b) under illumination with a tungsten halogen lamp. The inhibition zone increased from 1.7×1.7 cm2 in the dark to 2.1×2.1 cm2 under visible-light illumination. Optical images taken at a magnification of 1000× (c) outside the inhibition zone and (d) inside the inhibition zone.
Conclusions
ZnO nanorods have been successfully grown in paper supports and their photocatalytic activity was determined. The efficient photodegradation of organic dyes (methylene blue and methyl orange) and the photocatalytic immobilization of common bacterium, E. coli, were studied under visible-light irradiation. A 93% photodegradation was observed for methylene blue and a 35% photodegradation was observed for methyl orange in the presence of the photocatalyst paper upon white-light irradiation at 963 Wm−2. Repeatability tests showed that the photocatalytic paper could be reused several times with a nominal decrease in efficiency. Antibacterial tests revealed that the photocatalytic paper is capable of completely eliminating E. coli in its vicinity even under room lighting condition.
Acknowledgments
The authors would like to acknowledge partial financial support from the National Nanotechnology Center, of the National Science and Technology Development Agency (NSTDA), Ministry of Science and Technology (MOST), Thailand and the Centre of Excellence in Nanotechnology at the Asian Institute of Technology, Thailand.
References
- PeltonRGengXBrookM 2006 Adv. Colloid Interface Sci. 127 43 http://dx.doi.org/10.1016/j.cis.2006.08.002
- FujishimaAHondaK 1972 Nature 238 37 http://dx.doi.org/10.1038/238037a0
- PouliosIMakriDProhaskaX 1999 Global Nest: The Int. J. 1 55
- CarrawayE RHoffmanA JHoffmannM R 1994 Environ. Sci. Technol. 28 786 http://dx.doi.org/10.1021/es00054a007
- BaruahSRafiqueR FDuttaJ 2008 Nano 3 8 http://dx.doi.org/10.1142/S179329200800126X
- ChoY HLeeS JLeeJ YKimS WLeeC BLeeW YYoonM S 2002 Int. J. Antimicrob. Agents 19 576 http://dx.doi.org/10.1016/S0924-8579(02)00115-2
- PozzoR LBaltanasM ACassanoA E 1997 Catal. Today 39 219 http://dx.doi.org/10.1016/S0920-5861(97)00103-X
- OkazakiM 2000 Kinoshi Kenkyu Kaishi 38 93
- AguedachABrosillonSMorvanJLhadiE K 2005 Appl. Catal. B 57 55 http://dx.doi.org/10.1016/j.apcatb.2004.10.009
- MatsubaraHTakadaMKoyamaSHashimotoKFujishimaA 1995 Chem. Lett. 9 767 http://dx.doi.org/10.1246/cl.1995.767
- IguchiYIchiuraHKitaokaTTanakaH 2003 Chemosphere 53 1193 http://dx.doi.org/10.1016/S0045-6535(03)00582-4
- KogaKKawakatsuHSakamotoT 1999 Kenkyu Hokoku-Fukuoka-ken Kogyo Gijutsu Senta 9 63
- RaillardCHaquetVLeCloirecLegrandJ 2004 J. Photochem. Photobiol. A 163 425 http://dx.doi.org/10.1016/j.jphotochem.2004.01.014
- GhuleKGhuleA VChenB JLingY C 2006 Green Chem. 8 1034 http://dx.doi.org/10.1039/b605623g
- BaruahSThanachayanontCDuttaJ 2008 Sci. Technol. Adv. Mater. 9 025009 http://dx.doi.org/10.1088/1468-6996/9/2/025009
- UllahRDuttaJ 2008 J. Hazard. Mater. 156 194 http://dx.doi.org/10.1016/j.jhazmat.2007.12.033
- SugunanAWaradH CBomanMDuttaJ 2006 J. Sol–Gel Sci. Technol. 39 49 http://dx.doi.org/10.1007/s10971-006-6969-y
- BaruahSDuttaJ 2009 Sci. Technol. Adv. Mater. 10 013001 http://dx.doi.org/10.1088/1468-6996/10/1/013001
- www.udel.edu/pchem/C446/Experiments/exp7.pdf
- QuintanaMRicraERodriguezJEstradaW 2002 Catal. Today 76 141 http://dx.doi.org/10.1016/S0920-5861(02)00214-6
- VogtR LDippoldL 2005 Public Health Reports 120 174
- SoperR 2005 Biological Science Cambridge Cambridge University Press
- FukahoriSIguchiYIchiuraHKitaokaTTanakaHWariishiH 2007 Chemosphere 66 2136 http://dx.doi.org/10.1016/j.chemosphere.2006.09.022
- MorinS ABiermanM JTongJJinS 2010 Science 328 476 http://dx.doi.org/10.1126/science.1182977
- XuNShiZFanYDongJShiJHuM Z C 1999 Ind. Eng. Chem. Res. 38 373 http://dx.doi.org/10.1021/ie980378u
- ChenJ QWangDZhuM XGaoC J 2006 J. Hazard. Mater. 138 182 http://dx.doi.org/10.1016/j.jhazmat.2006.05.049
- XuJ CLuMGuoX YLiH L 2005 J. Mol. Catal. A: Chem. 226 123 http://dx.doi.org/10.1016/j.molcata.2004.09.051
- HanJQiuWGaoW 2010 J. Hazard. Mater. 178 115 http://dx.doi.org/10.1016/j.jhazmat.2010.01.050
- AtmacaSGulKClcekR 1998 Turk. J. Med. Sci. 28 595
- ChoY HLeeS JLeeJ YKimS WLeeC BLeeW YYoonM S 2002 Int. J. Antimicrob. Agents 19 576 http://dx.doi.org/10.1016/S0924-8579(02)00115-2
- Hernandez-SierraJ FRuizFCruzPenaMartinez-GutierrezFMartinezA EdeJesusPozosGuillenTapia-PerezHMartinez CastanonG 2008 Nanomed. Nanotechnol. Biol. Med. 4 237 http://dx.doi.org/10.1016/j.nano.2008.04.005
- HuangZManessP CBlakeD MWolfrumE JSmolinskiS LJacobyW A 2000 J. Photochem. Photobiol. A 130 163 http://dx.doi.org/10.1016/S1010-6030(99)00205-1