Abstract
Marine sessile organisms easily adhere to submerged solids such as rocks, metals and plastics, but not to seaweeds and fishes, which are covered with soft and wet ‘hydrogel’. Inspired by this fact, we have studied long-term antifouling properties of hydrogels against marine sessile organisms. Hydrogels, especially those containing hydroxy group and sulfonic group, show excellent antifouling activity against barnacles both in laboratory assays and in the marine environment. The extreme low settlement on hydrogels in vitro and in vivo is mainly caused by antifouling properties against the barnacle cypris.
Introduction
Marine sessile organisms, such as algae, barnacles and sea squirts, can adhere wall-to-wall on submerged surfaces of metals, woods and plastics that has been causing significant economic problems. To prevent the fouling of marine sessile organisms, copper plate was used as antifoulant for ship's bottom early in the Roman times, and until recently, tributyltin (TBT) has been the most popular antifouling paint. However, TBT was banned as an antifouling paint because of its endocrine disruption effect [Citation1]. Therefore, development of pollution-free marine antifouling material is urgently required.
Marine sessile organisms only adhere to hard solid surfaces of rocks, concrete walls, ship's bottom, etc, but not to living organisms such as seaweeds, fishes and jellyfishes. Inspired by this fact, recently, researchers started developing biomimetic antifouling technologies. From the view of chemical composition, several hundred natural antifouling compounds have been found in marine organisms (seaweeds, sea squirts, etc) [Citation2, Citation3]. Researchers prepared an antifouling structure mimicking sharkskin [Citation4, Citation5], and some reports suggested that surface roughness plays important role in biofouling [Citation6, Citation7]. Compared to solid materials, the surface of most marine creatures is rather soft and contains much water. Therefore, soft silicone elastomers [Citation8–11] and hydrogels have been actively investigated and applied as antifouling paint.
In this article, we introduce the antifouling properties against barnacles of hydrogels with different chemical composition and elasticity, tested both in laboratory and marine environment.
Barnacles
Barnacle is one of the most common marine sessile organisms worldwide, and its strong adhesion causes fouling problems. Barnacles spend most of their lives attached to a substrate. Although they look like shells, they are crustaceans and are related to crabs and lobsters (and taste similar).
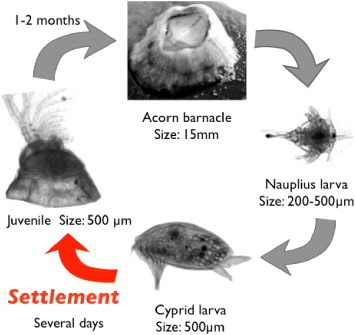
Figure shows the life cycle of barnacles. Barnacles have two larval stages. Nauplius larva is the first-stage larva. It continues growing and molting, and then metamorphoses into cypris larva, which is the second-stage larva. Cypris are non-feeding larvae and live using only nutrient in their body; they were likened to ‘a moving pupa’ by Charles Darwin. Cypris have a pair of antennules, of which the top is sense organ, at anterior inferior part of their body [Citation12], and they walk on the substrate using the antennules before their settlement [Citation13]. This walking is called ‘exploring behavior’, aiming to decide whether the substrate is suitable for settling [Citation14, Citation15]. After the exploring behavior, a cypris attaches firmly and permanently to the substrate by secreting the adhesive proteins [Citation16, Citation17] and then transforms into a juvenile. In the post-settlement stage, barnacles secrete cement proteins to the substrate and broaden their adhesion area [Citation18]. Thus, the development of antifouling technologies is focused on (i) exploring and settlement stages of cypris, and (ii) post-settlement growth.
Hydrogels
Hydrogels mostly consist of water and a polymer network which holds it. They are distinguished from solid materials (metals, plastics, etc) by abundance of water (60–99 wt%) and low elasticity, with the elastic modulus 100–10000 smaller than in common solids. Hydrogels are found, for example, in soft contact lenses and jelly. Human body contains hydrogel, so as marine organisms such as seaweeds, fishes and jellyfishes.
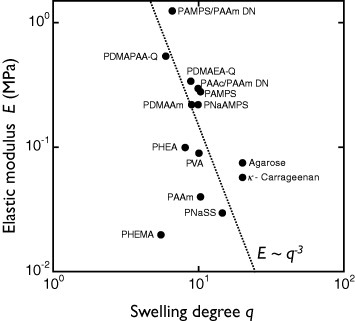
A three-dimensionally crosslinked polymer gel shows entropic elasticity common to rubber. The entropic elasticity is proportional to the volume density of polymer chain segments, so the elasticity of a hydrogel strongly depends on its water content. The water content of hydrogels is expressed by the swelling degree q, which is the volume ratio of the water-swelled and dried states. The elastic modulus E of equilibrated neutral gel in water decreases with q as E∝q−3 [Citation19]. The soft and wet natures of hydrogels are inextricably related, so that it is impossible to change one of those parameters independently.
Most hydrogels are mechanically weak that was limiting their application in various fields. However, hydrogels with mechanical strength and toughness comparable to those of rubbers and living cartilage have been developed recently by several research groups [Citation20–26].
Antifouling properties of hydrogels
Previous investigations addressed the antifouling activity of hydrophilic polymers and hydrogels against some sessile organisms, cells and proteins. Katsuyama, et al [Citation27] described germination inhibition effects of polyelectrolyte hydrogels against zoospores of a seaweed. Rasmussen et al [Citation28] reported that some natural polymer gels and chemical crosslinked polyvinyl alcohol (PVA) gel have antifouling effect against barnacle cypris. Cao et al [Citation29] found that the polysaccharides polymer coatings resist adhesion of proteins, algae and barnacles, and the results of Bowen et al [Citation30] reveal that the antifouling strength depends on the alkyl chain length of polymers.
However, it is still unclear what is the key antifouling factor of hydrogels against cypris larvae and how do cypris larvae detect it. In laboratory assays, hydrogels with deferent elasticity (water content) were prepared from differently charged synthetic and natural polymers, and the cypris settlement on these gels was investigated [Citation31].
Effect of chemical types of hydrogels
A schematic of laboratory settlement test is illustrated in figure , and figure shows the number of barnacles settled on the wall and bottom surfaces, as well as the number of dead barnacles found in the cultivation wells with their bottom surfaces covered by hydrogels. The number of settled barnacles is much lower for hydrogels than solid polystyrene (PS) surface. Furthermore, there is no relationship between the charge of hydrogels and barnacle settlement that indicates cancellation of surface charge on a hydrogel owing to the high ionic strength of seawater. Most cypris larvae which did not settle on hydrogels then attached themselves to PS. On day 1, most of cypris settled on the bottom or walls in bare-PS wells. However, when the well bottom was covered with hydrogels, few settlements were found either on the bottom (gel) or walls (PS) until day 3, that is, cypris selectively settled on PS walls as the result of exploring behavior. Unusual observations were made for some physically crosslinked gels. In the well with κ-carrageenan gel, prompt settlement on the walls was found on day 1, and the total number of settlements (bottom and wall surfaces) was small compared with the well with PVA gel. This behavior might be attributed to the polymer eluted from physical crosslinked gels; however, no effect was observed for agarose, which is also a physically crosslinked gel. The percentage of dead cypris larvae in all wells containing hydrogels (<10%) was almost the same as in bare PS wells, indicating low toxicity of hydrogels to cypris. These findings suggest that the antifouling mechanisms differ for hydrogels and agents that release biocidal chemicals.
Figure 2 Schematic illustrating exploring behavior and settlement of cypris larvae. (Reproduced with permission from [Citation31] ©2009 Taylor and Francis.)
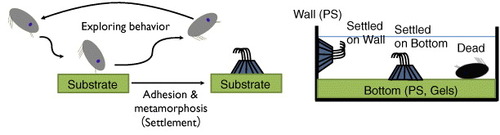
Figure 3 Number of barnacle settlements and dead larvae on several substrates. (Reproduced with permission from [Citation31] ©2009 Taylor and Francis.)
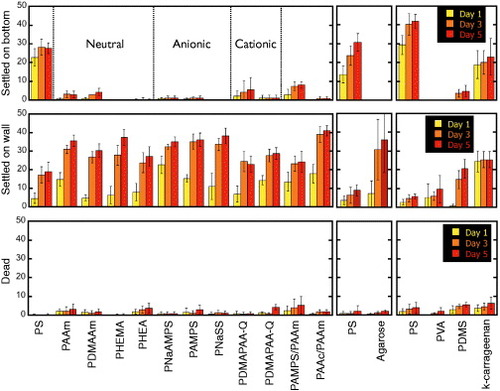
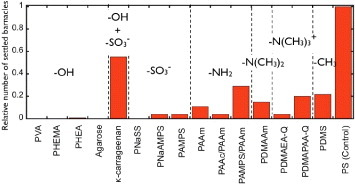
Figure shows the relationship between the functional group of hydrogels and relative number of settlement after 5-day exposure. Excellent antifouling activity against cypris was observed for synthetic polymer gels with hydroxy or sulfonic acid groups, whereas the activity is low for gels with amino groups. In the case of natural polymer gels, there is no settlement on agarose gel with hydroxy groups, whereas the number of settlements is the highest on the κ-carrageenan gel containing both hydroxy and sulfonic acid groups. It is yet unclear why the κ-carrageenan gel behaves differently from other hydrogels that also have hydroxy or sulfonic groups.
Figure 4 Relative number of settlements on day 5 for hydrogels with different functional groups. (Reproduced with permission from [Citation31] ©2009 Taylor and Francis.)
Effect of physical properties of hydrogels
Figure shows the relationship between the relative number of settlements and elasticity of hydrogels. Gels with hydroxy or sulfonic groups show very high antifouling activities, regardless of elasticity, whereas the number of settlements increases with elasticity for gels with amino groups; these differences are not understood yet. Dahlström et al [Citation32] reported lower number of settlements on hydrophilic PS or grass than on hydrophobic PS or glass surfaces, indicating that different surface wettability for various functional groups may affect barnacle settlement. As described above, there is a negative correlation between elasticity and water content of hydrogels, and these two parameters cannot be changed independently. Thus, a decrease in water content may promote settlement via increase of elasticity. A scaling law between elasticity E and swelling degree q is observed experimentally for hydrogels in seawater, regardless of their chemical structure and charge (figure ). This fact indicates that charged gels behave like neutral gels because of the charge screening in seawater. Eshet et al [Citation33] reported that the deposition rate of bacteria depends on the swelling degree that might have the same explanation as the above results.
Figure 5 Relative number of settlements on the bottom surface after 5 days of exposure versus elastic modulus for several substrates. (Reproduced with permission from [Citation31] ©2009 Taylor and Francis.)
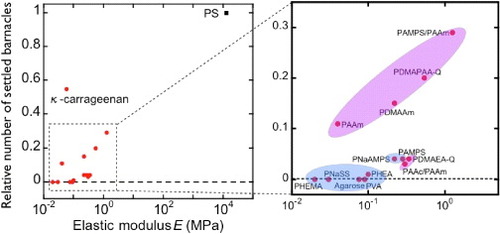
Figure 6 Elastic modulus (E) versus swelling degree (q) for several hydrogels. The dotted line shows the scaling relationship E∼q−3. (Reproduced with permission from [Citation31] ©2009 Taylor and Francis.)
The findings from laboratory assays suggest that cypris larvae sense the chemical structure and elasticity (water content) of hydrogels via exploring behavior, and that the key antifouling factor of hydrogels is low elasticity (high water content). These results should help us create new antifouling materials.
Long-term fouling test in marine environment
Several factors are considered when choosing a material for long-term fouling test in marine environment: the material must be environmentally benign, relatively soft and durable. Hydrogels are soft and environmentally benign, and showed high antifouling activity against barnacle in laboratory assays. However, it was unclear whether they can tolerate the strong water currents and temperature changes during long-term in vivo experiments. For example, while the PHEMA hydrogel was found efficient against diatom and algae in laboratory and marine environments over 1 month period, it is unclear whether it would withstand oceanic conditions [Citation34].
The two main challenges of long-term experiments in marine environment are the durability of a hydrogel and the technique of attaching it in the experimental setup. The recently developed PAMPS/PAAm double-network (DN) gel [Citation22] and physically crosslinked PVA gels are considered sufficiently robust for such experiments [Citation35]. Besides investigating the antifouling properties of these hydrogels, the base plate morphology of barnacle was also studied.
Antifouling properties of hydrogels against marine sessile organisms
PVA gel (10×10 cm2 area), PAMPS/PAAm DN gel (12×12 cm2 area) and polyethylene (PE, control sample) plates were fixed to a stainless frame and immersed to a depth of 5–8 m below the sea surface with nylon ropes from a raft. The thickness of the gels was 1 cm. Data were collected from two specimens of each gel sample up to 330 days.
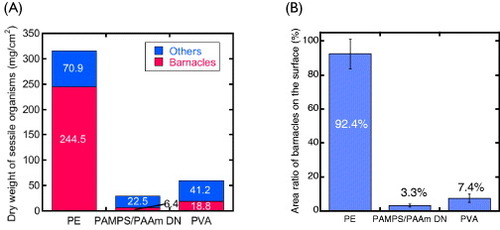
Figure (A) shows the dry weight per unit area of the barnacles and other marine sessile organisms (sea squirt, sponge, seaweed, etc) adhered to the substrates. It reveals low adhesion to the gel of not only barnacle but also other micro or macro organisms (sea squirt, sponge and seaweed, etc). The area ratio of barnacles to the surface they were attached to is shown in figure (B). Whereas the PE surface was almost completely covered with barnacles (92.4% area ratio), the ratio was much smaller for gel-covered plates: 7.4% for PVA and 3.3% for PAMPS/PAAm DN. The larger value for PE is caused both by the higher density of barnacle settlements and the larger size of the individual barnacles on PE. The density of barnacles was 0.98, 0.17 and 0.11 cm−2, for PE, PVA gel and PAMPS/PAAm DN gel.
Figure 7 (A) Dry weight of sessile organisms and (B) area ratio of barnacles settled on various substrates after 330-day exposure. (Reproduced with permission from [Citation35] ©2009 Taylor and Francis.)
Morphology of settled barnacles on each substrate
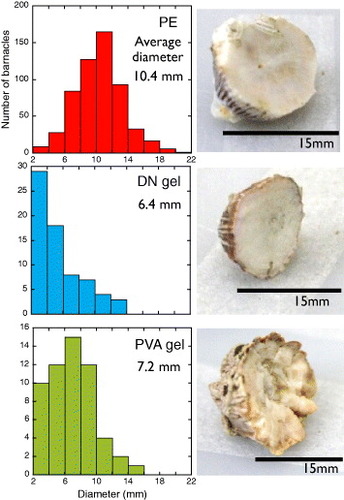
As shown in figure , barnacles had a flat basal surface when attached to hard (E=151 MPa) PE and a very rough and concave basal surface on the soft (E=0.09 MPa) PVA gel. A slightly concave surface was observed on the PAMPS/PAAm DN gel, which is relatively rigid (E=1.25 MPa) compared with the PVA gel. These results are in agreement with the literature reports that barnacle bases were flat on rigid PMMA but showed a concave shape on relatively soft polydimethylsiloxane (PDMS) [Citation36, Citation37]. It has been reported that the amount of adhesive substance was almost same for PDMS and PMMA. In the case of hydrogels, the amount of adhesive substance could not be related to the hydrogel type.
Figure 8 Basal diameter distribution of barnacles settled on various surfaces, and photographs of the basal surfaces after exposure for 330 days. (Reproduced with permission from [Citation35] ©2009 Taylor and Francis.)
Barnacle growth model on hydrogels in marine environment
Figure shows the histogram of the basal diameter of barnacles on different substrates. Normal (Gaussian) distribution is observed on PE because of the strong adhesion. The smaller size and lower number of large barnacles on the DN gel can be related either to their easy detachment during the growth, due to the weak adhesion to the DN gels, or to the growth inhibition on the DN gels. A similar tendency is observed for the PVA gel and can be attributed to the wettability and softness of hydrogels.
Effect of wettability of hydrogels
Figure shows the components of adult barnacle from a lateral view. The growth of an adult barnacle on a solid surface (such as PE) is illustrated in figure (A). When an adult barnacle secretes new cement onto the surface, its muscles contract so that the base is subjected to a pull-up force and the shell wall presses the substrate. If the adhesive strength between the base and substrate is stronger than the pull-up force, a new cement layer is produced without detachment (figure (B)). This scenario is observed on PE. As a result, barnacles can easily grow on PE surfaces. On the other hand, because of the high water content (about 85–90 wt%) of the gel, the secreted cementing proteins (molecular weight 14–165 kDa [Citation38]) might not form a layer on the gel surface, and instead diffuse into the gel network (pore size >10 nm). Therefore, the adhesion strength on gels might be lower than the barnacle's contractile force, resulting in easy detachment of the barnacle.
Figure 9 Illustrations of the growth of (A) an adult barnacle on a solid surface (adapted from [Citation39]) and (B) barnacles on substrates with different elasticity. (Reproduced with permission from [Citation35] ©2009 Taylor and Francis.)
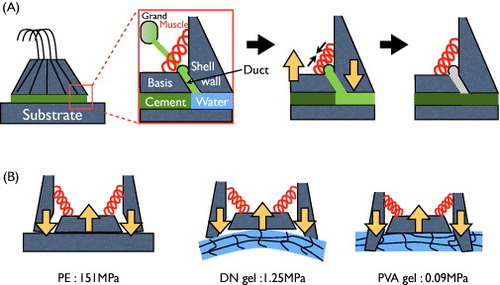
Effect of softness of hydrogels
In the growing process, the detachment of barnacle from the surface not only depends on the adhesion strength between the base of the barnacle and the substrate, but also on the elastic modulus of the substrate. The elastic modulus of PE is 151 MPa. The adhesion strength was sufficient for barnacles not to detach from the substrate during the muscle contractions that persist in the adult form. Furthermore, the substrate was not deformed by the contractile force because of the high elastic modulus of PE, as revealed by the flat morphology of basal surfaces of barnacles on PE in figure . On the other hand, PAMPS/PAAm DN gel (E=1.25 MPa), which is about 100 times softer than PE, was deformed by pressure developed by the shell wall during the barnacle growth. As the adhesion strength was weak because of the high water content in the gel, the barnacle was easily detached upon bending from the DN gel. This model is justified by the concave shape of the basal surface of the barnacles (figure ). In comparison with DN gel, PVA gel is much softer (E=0.09 MPa). Therefore, the shell wall could cut through the PVA gel and embed into it during muscle contraction. The adhesion strength on PVA gel might be as low as on the PAMPS/PAAm DN gel; however, this embedding of the shell wall might mechanically lock the shell wall to the PVA gel, thereby hindering the detachment. This model explains the higher number of barnacles settled on PVA gel than on PAMPS/PAAm DN gel in the long-term test.
Conclusions
Hydrogels show excellent antifouling activity against barnacle cypris, which results in low density of settlement on hydrogels in vitro and in vivo. This activity depends on the functional groups of the gel polymer and is higher for hydroxy and sulfonic groups. The mechanism of this action is not well understood, and some hints may be found by investigating the surfaces of marine organisms such as algae and sea squirts.
The hydration of polymer chain may interrupt the adhesion of cementing protein, thereby affecting the cypris settlement behavior and the barnacle growth. We are studying various substrates, including hydrogels, to elucidate the effect of substrate properties such as hydrophobicity/hydrophilicity and elasticity on the barnacle growth. These in vitro results may help understanding the barnacle growth process and adhesion to hydrogels.
Owing to the high water content and lack of toxicity hydrogels are environmentally benign antifouling materials. We believe that hydrogels, having a soft and wet structure similar to that of marine organisms, will help reduce economic loss in marine fields and preserve marine environment.
Acknowledgments
This research was financially supported by a grant-in-aid for a seeds innovation project from Japan Science and Technology Agency, and Specially Promoted Research (no. 18002002) from the Ministry of Education, Science, Sports and Culture of Japan. The authors thank A Kakugo, T Kurokawa and the graduate students in LSW for their contributions, as well as H Furukawa, Y Osada, Y Nogata, K Matsumura, N Fusetani and K Hashimoto for useful discussions and contributions to this work.
References
- International Marine Organization 1999 Resolutions Summaries A 895 (21)
- FusetaniN 2004 Nat. Prod. Rep. 21 94 http://dx.doi.org/10.1039/b302231p
- FusetaniN 2011 Nat. Prod. Rep. 28 400 http://dx.doi.org/10.1039/c0np00034e
- ChungK KSchumacherJ FSampsonE MBurneR AAntonelliP JBrennanA B 2007 Biointerphases 2 89 http://dx.doi.org/10.1116/1.2751405
- MaginC MFinlayJ AClayGCallowM ECallowJ ABrennanA B 2011 Biomacromolecules 12 915
- PetronisSBerntssonK MGoldJGatenholmP 2000 J. Biomater. Sci. Polym. Edn. 11 1051 http://dx.doi.org/10.1163/156856200743571
- BerntssonK MJonssonP RLejhallMGatenholmP 2000 J. Exp. Mar. Biol. Ecol. 251 59 http://dx.doi.org/10.1016/S0022-0981(00)00210-0
- BradyR FGriffithJ RLoveK SFieldD E 1987 J. Coat. Technol. 59 113
- BradyR FJr 2001 Prog. Org. Coat. 43 188 http://dx.doi.org/10.1016/S0300-9440(01)00180-1
- KavanaghC J 2005 J. Adhes. 81 843 http://dx.doi.org/10.1080/00218460500189331
- AndersonC et al 2004 J. Mar. Des. Oper. B4 11
- LagerssonN C et al 2003 J. Mar. Biol. Ass. UK 83 361 http://dx.doi.org/10.1017/S0025315403007203h
- LagerssonN CH⊘egJ T 2002 Mar. Biol. 141 513 http://dx.doi.org/10.1007/s00227-002-0854-1
- PrendergastaG SZurnC MBersVHeadR MHanssonL JThomasonJ C 2008 Biofouling 24 449 http://dx.doi.org/10.1080/08927010802340135
- CrispD J 1960 J. Exp. Biol. 38 429
- PhangI YAldredNLingX YHuskinsJClareA SVancsoG J 2010 J. R. Soc. Interface 7 285 http://dx.doi.org/10.1098/rsif.2009.0127
- ÖdlingKAlbertssonCRussellJ TandMårtensson 2006 J. Exp. Biol. 209 956 http://dx.doi.org/10.1242/jeb.02031
- AldredNClareA S 2008 Biofouling 24 351 http://dx.doi.org/10.1080/08927010802256117
- de GennesP G 1979 Scaling Concepts in Polymer Physics Ithaca Cornell University Press p 128
- OkumuraYItoK 2001 Adv. Mater. 13 485 http://dx.doi.org/10.1002/(ISSN)1521-4095
- HaraguchiKTakehisaT 2002 Adv. Mater. 14 1120 http://dx.doi.org/10.1002/1521-4095(20020816)14:16<1120::AID-ADMA1120>3.0.CO;2-9
- GongJ PKatsuyamaYKurokawaTOsadaY 2003 Adv. Mater. 15 1155 http://dx.doi.org/10.1002/adma.200304907
- SakaiTMatsunagaTYamamotoYItoCYoshidaRSuzukiSSasakiNShibayamaMChungU 2008 Macromolecules 41 5379 http://dx.doi.org/10.1021/ma800476x
- YasudaK et al 2009 Macromol. Biosci. 9 307 http://dx.doi.org/10.1002/mabi.v9:4
- HagiwaraYPutraAKakugoAFurukawaHGongJ P 2010 Cellulose 17 93 http://dx.doi.org/10.1007/s10570-009-9357-2
- WangQMynarJ LYoshidaMLeeELeeMOkuroKKinbaraKAidaT 2010 Nature 463 339 http://dx.doi.org/10.1038/nature08693
- KatsuyamaYKurokawaTKanekoTGongJ POsadaYYotsukuraNMotomuraT 2002 Macromol. Biosci. 2 163 http://dx.doi.org/10.1002/1616-5195(20020501)2:4<163::AID-MABI163>3.0.CO;2-2
- RasmussenKWillemsenP RØstgaardK 2002 Biofouling 18 177 http://dx.doi.org/10.1080/08927010220152038
- CaoX et al 2009 Biomacromolecules 10 907 http://dx.doi.org/10.1021/bm8014208
- BowenJPettittM EKendallKLeggettG JPreeceJ ACallowM ECallowJ A 2007 J. R. Soc. Interface 4 473 http://dx.doi.org/10.1098/rsif.2006.0191
- MurosakiT et al 2009 Biofouling 25 313 http://dx.doi.org/10.1080/08927010902730516
- DahlströmMJonssonHJonssonP RElwingH 2004 J. Exp. Mar. Biol. Ecol. 305 223 http://dx.doi.org/10.1016/j.jembe.2003.12.013
- EshetIFregerVKasherRHerzbergMLeiJUlbrichtM 2011 Biomacromolecules 12 2681 http://dx.doi.org/10.1021/bm200476g
- CowlingM JHodgkiessTParrA C SSmithM JMarrsS J 2000 Sci. Total Environ. 258 129 http://dx.doi.org/10.1016/S0048-9697(00)00513-1
- MurosakiTNoguchiTHashimotoKKakugoAKurokawaTSaitoJChenY MFurukawaHGongJ P 2009 Biofouling 25 657 http://dx.doi.org/10.1080/08927010903082628
- BerglinMGatenholmP 2003 Colloids Surf. B 28 107 http://dx.doi.org/10.1016/S0927-7765(02)00149-2
- WendtD EKowalkeG LKimJSingerI L 2006 Biofouling 22 1 http://dx.doi.org/10.1080/08927010500499563
- KaminoK 2008 Mar. Biotechnol. 10 111 http://dx.doi.org/10.1007/s10126-007-9076-3
- SaroyanJ RLindnerEDooleyC A 1970 Biol. Bull. 139 333 http://dx.doi.org/10.2307/1540088