Abstract
We review the solvothermal synthesis, using a mixture of ethylene glycol (EG) and water as the solvent, of zinc oxide (ZnO) particles having spherical and flower-like shapes and hierarchical nanostructures. The preparation conditions of the ZnO particles and the microscopic characterization of the morphology are summarized. We found the following three effects of the ratio of EG to water on the formation of hierarchical structures: (i) EG restricts the growth of ZnO microcrystals, (ii) EG promotes the self-assembly of small crystallites into spheroidal particles and (iii) the high water content of EG results in hollow spheres.
Introduction
Biominerals exhibit high performances because of their hierarchical structures. Shells, which are composites of calcium carbonate plates and organic materials, have excellent fracture resistance compared with the pure calcium carbonate [Citation1]. Nacre has a hierarchical structure consisting of ordered layers of aragonite plates, about 5 μm long and 500 nm thick, within a polymer matrix and the plates are made of nanograins [Citation2]. The exceptional mechanical properties of bones originate from the fiber composite structure and functional adaptation at all levels of the hierarchy [Citation3].
The biomimetic ceramic synthesis inspired by biomineralization is an attractive topic. Complex hierarchical structures can be fabricated by environment-friendly and energy-saving solution processes at low temperatures. Researchers have tried to mimic biological structures and the self-assembly processes. Complex structures found at the surfaces of leaves and butterfly wings are synthesized by chemical routes [Citation4]. Xu et al [Citation5] reviewed the biomimetic growth of inorganic materials and discussed the generation of complex structures with a specific size, shape, orientation, composition and hierarchical organization under ambient conditions in aqueous environments. Koumoto et al [Citation6] summarized the bioinspired patterning of the ceramic thin films by surface modification techniques. The effects of the surface functional groups of the substrates on the deposition of ceramics in aqueous solutions were studied and the site-selective deposition was demonstrated. Two important reports on the biomimetic ceramic synthesis of complex structures have been published recently: one focused on the shape control and organization of minerals and organic–inorganic hybrid materials [Citation7]. Another one featured the solution growth of functional ceramic nanomaterials, particularly the growth mechanisms of electrochemical and chemical bath reactions for various structures [Citation8]. Many inorganic precipitates prepared in solutions have hierarchical structures [Citation5–8].
Zinc oxide (ZnO) is a wide-bandgap semiconductor with a large exciton binding energy; it is widely used for electronic applications [Citation9], such as varistors, surface acoustic wave filters, transparent electrodes and phosphors. Many ZnO fabrication techniques have been developed, such as sputtering [Citation10], chemical vapor deposition [Citation11], pulsed laser deposition [Citation12], spray pyrolysis [Citation13] and electrochemical deposition [Citation14]. ZnO has wurtzite crystal structure and shows spontaneous electrical polarization along its c-axis (figure ). The positive polar face (0001), the c(+)-plane, and the negative polar face (0001−), the c(−)-plane, are rich in zinc and oxygen atoms, respectively. Various properties of ZnO depend on the polarity: surface electronic structure [Citation15], chemical stability [Citation16] and catalytic activity [Citation17, Citation18].
The control of the morphology of ZnO nanostructures is a popular topic in modern materials science [Citation19–24]. A wide variety of ZnO morphologies (e.g. nanowires, rods, plates, stars, flowers, rings and spheres) are observed for precipitates and thin films formed by solution-based processing. Several excellent review papers on the synthesis of ZnO from aqueous solutions have recently been published. Lincot [Citation25] thoroughly reviewed the solution growth of ZnO films and nanostructures. Chemical bath deposition, hydrothermal deposition and electrodeposition of dense and nanoporous structures were studied, and chemical reactions in aqueous solutions were explained using thermodynamic data. Kawano and Imai [Citation26] summarized the various morphologies of ZnO crystals grown from aqueous solutions. They showed that the growth rate, growth sites and growth direction of ZnO crystals can be tuned by varying the degree of supersaturation, the presence of seeds or substrates and the addition of specific organic molecules, respectively. Baruah and Dutta [Citation27] reviewed the hydrothermal growth of ZnO; they presented many examples of nanoparticles, nanowires, nanorods and flower-like and cabbage-like nanostructures and explained the influence of organic additives on ZnO growth. To synthesize hierarchical ZnO structures, which may enhance ZnO functional properties, we can learn from hierarchical structures found in nature. The growth of inorganic materials in solutions has similarities to the formation of biominerals in the morphology and orientation control. Tian et al [Citation19, Citation20] reported such examples where the building blocks of ZnO hierarchical structures grown from simple aqueous solutions resemble the morphology of calcium carbonate units in nacre of red abalone shells as shown in figure , although the former hierarchical structure is monolithic and the latter is a composite of calcium carbonate and organic polymers.
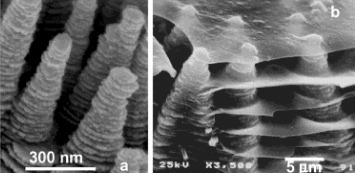
Figure 2 An example of the ZnO hierarchical structure (a) resembling a biomineral (b): oriented ZnO helical columns (a) and bacreous calcium carbonate columns and layers near the growth tips in a young abalone shell (b) [Citation20].
The topic of this review is the solvothermal synthesis of ZnO particles. Here ‘solvothermal’ refers to solution processes which use solvents other than water and include mild heating (to boiling temperature). Many kinds of solvents have been studied for the solvothermal synthesis of ceramic nanoparticles [Citation28]. Because glycols such as ethylene glycol (EG) and diethylene glycol (DEG) have affinity for water, the conditions of the hydrothermal process can be optimized by mixing glycols with water and adjusting the mixing ratio. In particular, we have tuned the solvothermal processes to yield sphere-like ZnO particles such as spheres, doughnuts and flowers using mixtures of water and glycols as solvents. Here we present the produced hierarchical structures of ZnO particles and discuss the effects of the volume ratio of glycol to water on the ZnO morphology.
Solvothermal synthesis of ZnO particles in glycols
Various conditions have been used in the glycol-assisted solvothermal synthesis of ZnO particles with hierarchical structure [Citation27–39], and the concentration of glycol was found to play a key role in the microstructure of ZnO particles. Table lists the preparation conditions of spheroidal ZnO particles using EG and DEG, and the scanning electron microscopy (SEM) images of ZnO particles are shown in figure .
Figure 3 TEM and SEM images of ZnO particles prepared by solvothermal methods using EG or DEG. (a) Spherical particle [Citation30], (b) flower-like particle [Citation31], (c) flower-like hemispheres [Citation35] and (d) spherical Co-doped ZnO particle [Citation36].

Solvothermal synthesis conditions of spheroidal ZnO particles using EG or DEG. Legends: Ref., reference; T, temperature; [Zn], zinc concentration; EG/W, ethylene glycol/water ratio.
In 1995, Jézéquel et al [Citation29] reported the first synthesis of ZnO spherical powders in glycols. They used zinc acetate dihydrate and DEG. When the Zn concentration was less than 0.1 mol l−1 at 180 °C, monodispersed spherical particles 0.2–0.4 μm in diameter were prepared. The particle size depended mainly on the heating rate. The particles were microporous aggregates of crystallites about 10 nm in size.
Tay et al [Citation30] prepared spherical ZnO particles of ∼0.1 μm diameter from a 0.1 mol l−1 solution of zinc acetate dihydrate in DEG at 155–160 °C. The particles consisted of ovoid-like grains 10 nm in size (figure (a)). The authors proposed the assembly mechanism based on the dipole nature of ZnO. Individual nanograins with different polar surfaces are unstable; they form spherical aggregates, thereby minimizing the surface energy.
Ashoka et al prepared flower-like ZnO particles from 0.08 mol l−1 zinc acetate dihydrate in water–EG solvent at 140–160 °C [Citation31]. The flowers had symmetrical shapes with a diameter of about 20 μm. They were self-assembled from sword-like rods about 300–700 nm in diameter (figure (b)). The authors discussed the growth mechanism of flower-like ZnO as follows: the hydroxyl groups of EG adsorb on the positive polar plane of ZnO by the Coulomb interaction. The adsorption slows down the growth along the c-axis, resulting in the formation of polyhedral crystals. Two polyhedral crystals join each other by the action of EG and form twinned crystals with high surface energy along the negative polar plane. The zinc acetate molecules adsorbed on the negative polar plane trigger nucleation and promote the formation of rods around the twinned crystals that result in flower-like ZnO structures.
Wang et al [Citation32] prepared ZnO particles from zinc acetate dihydrate at 170 °C and an EG to water ratio (EG/W) of 4. They studied the effects of Zn concentration on the ZnO morphology. Spherical (∼5 μm diameter) and dumbbell-like particles (∼4 μm length) were obtained at high (0.1–0.15 M) and low (0.025–0.05 M) Zn concentrations, respectively. The microspheres were composed of closely packed ZnO nanorods that self-assembled so as to merge with one end at the particle center.
Doughnut-shaped ZnO particles were obtained at 200 °C at EG/W>1 [Citation33]. The SEM photographs are shown in figures (c)–(h). The particles are 3–15 μm in diameter and consist of clusters of symmetrically arranged small hexagonal nanoplates. Because two planes with high surface energy tend to make an angle with each other to decrease the surface energy, agglomeration of hexagonal plates eventually results in doughnut-shaped particles.
Figure 4 SEM images of the ZnO particles produced at 200 °C at EG/water ratios of (a) 0.2, (b) 0.5, (c–d) 1, (e, f) 2, (g) 3 and (h) 5 [Citation33].

Qi [Citation34] made ZnO flowers at 180 °C from zinc nitrate at EG/W=1. The starting solution was basic and contained Zn(OH)42− ions; the solution pH was controlled by adding NaOH, which differs from others referenced in this review. When the EG content was lowered, the ZnO rods were separated and did not form flower-like shapes.
ZnO hemisphere particles with a hole at their centers were prepared using EG at 200 °C by Zhang et al [Citation35]. The hemispheres had a diameter of about 1 μm and looked like flowers, consisting of numerous nanorods with a diameter of about 50 nm and a length of several hundreds of nanometers. The nanorods were radially aligned (figure (c)). The authors grew similar ZnO hemispherical particles in other glycols such as 1,3-dihydroxypropan instead of EG. They investigated the morphology evolution of ZnO nanostructures with reaction time. At the beginning of the reaction, chain-like precipitates formed and assembled into rounded nanostructures to reduce the surface energy. ZnO nanoparticles in those rounded nanostructures acted as nucleation centers. ZnO hollow hemispheres were formed with preferential growth along the c-axis.
Qiu et al [Citation36] synthesized undoped and Co-doped ZnO spherical particles in EG at 150 °C. The spheres of 1–3 μm diameter were composed of nanorods and nanodiscs for the undoped and Co-doped ZnO, respectively. The nanorods with lengths from 200 to 500 nm were well aligned to the c-axis and grew along the normal to the sphere surface. The Co-doped ZnO nanodiscs had widths ranging from 10 to several tens of nanometers (figure (d)) and their surface was parallel to the c-plane. The authors suggested that the formation of the hollow spheres is assisted by water bubbles. The primary ZnO nuclei are formed from zinc glycolate at a reaction temperature above 100 °C. Water bubbles are generated to provide the assembly centers during the reaction. To minimize the interfacial energy, ZnO nuclei aggregate around the gas−liquid interface, and finally hollow nanorod-based spheres form through an oriented-attachment mechanism. For the growth of the Co-doped ZnO nanodiscs, the authors proposed a self-limiting anisotropic mechanism as follows. Co ions tend to adsorb on the O-terminated surface of ZnO nuclei, thus obstructing the crystal growth along the [001] direction. This leads to the morphological transition to the Co-doped ZnO nanodiscs rather than the formation of undoped ZnO nanorods.
Rezapour and Talebian [Citation37] prepared spherical particles at 170 °C from zinc acetate dihydrate (0.025–0.05 M) in EG which contained some HCl. The spheres were 2–10 μm in diameter and were composed of 60 nm crystallites, as deduced by x-ray diffraction (XRD). When 1,4-butanediol was used, nearly hexagonal rod-like ZnO particles were formed.
Recently, our group reported the synthesis of ZnO spherical particles using EG and hexamethylenetetramine (HMT) [Citation38]. HMT is often employed to produce ZnO powders and films in aqueous solutions with a homogeneous precipitation method [Citation21, Citation22]. As the temperature increases, HMT decomposes to formaldehyde and ammonia, which acts as a base and induces ZnO precipitation in aqueous solutions. Zinc acetate anhydride (2.202 g) and HMT (1.682 g) were each dissolved in 20 ml of an EG–water mixture; they were then mixed and heated at 95 °C for 12 h. Spherical ZnO particles were obtained at 95 °C when the solvent contained 87.5 or 95 vol.% EG. These ZnO spheres were obtained at lower temperatures than in previous reports (150−200 °C) [Citation29–37]. This is an advantage of combining the synthesis with the homogeneous precipitation, where hydrolysis is promoted by the decomposition of HMT.
Bitenc and Orel [Citation39] studied the synthesis of ZnO from zinc nitrate at a low temperature of 90 °C, in water–glycol mixtures. Urea was used for increasing the pH of the solvent, which is similar to our usage of HMT [Citation38]. ZnO rods were grown when the solvent contained EG, DEG or tetraethylene glycol. The rods were separated and did not construct round particles. The authors found that nanoparticles precipitated at the early synthesis stage and their aggregates became crystalline rods later when the volume ratio of EG to water was 3:1.
ZnO spherical particles are also obtained from organic solvents other than EG. Shen et al [Citation40] reported the solvothermal synthesis of ZnO microspheres (diameter ∼5 μm) using zinc acetate dihydrate in ethylenediamine in the presence of HAuCl4·4H2O at 160 °C. Zhang et al [Citation41] studied a microwave-assisted solvothermal method in a solvent with a water/methanol ratio of 2 at 160 °C. ZnO spheres with diameters of about 1–2 μm were constructed by self-assembled nanorods.
Effect of water on the nanostructure
Water in glycols plays an important role in the formation of the hierarchical structures, and by controlling the volume ratio of EG to water, one can vary the morphology of the ZnO particles. We find the following three effects of EG in the above-referenced studies (see table ):
EG restricts the growth of ZnO microcrystals, probably via the capping effect.
EG promotes the self-assembly of small crystallites into round particles. At small EG/W ratios the aggregates separate into individual crystallites.
High water content in EG results in hollow spheres, and the cavity size increases with decreasing EG/W ratio.
Figure shows SEM photographs of ZnO particles grown at different EG/W ratios by Ghoshal et al [Citation33]. Microrods with diameters of 4−12 μm and lengths of 10−20 μm were precipitated at EG/W=0.2. With increasing EG content, the growth rate along the c-axis decreased and hexagonal plates were formed due to the capping effect. Hexagonal plates with a diameter of about 5−12 μm and a thickness of about 2−4 μm were obtained at EG/W=0.5, and doughnut-shaped particles were formed at EG/W>1. As the EG/W ratio increased, the sizes of the constituent plates and of the hole in ZnO doughnuts decreased, and at EG/W=5, almost spherical particles with a small hole were obtained.
Table shows the effect of the EG/W ratio on the morphology of ZnO in our experiments [Citation38]. At EG/W<1, the precipitates were hexagonal and contained an unknown phase. Spherical particles precipitated at EG/W=7 or EG/W=19. As the EG/W ratio increased, the lengths of the crystalline domains along both the a- and c-axes decreased, as deduced by XRD, whereas the length ratio for the a- and c-axes did not change much. No precipitation occurred at EG/W=49.
EG/W ratios, phases and shapes, as well as crystallite sizes along the a- and c-directions estimated from the 100 and 002 XRD reflections, respectively [Citation38].
Changes in ZnO morphology with increasing water amount were also reported by Qiu et al [Citation36]. The spherical particles are compact aggregates of nanoparticles in the absence of water and become hollow at EG/W>30. For EG/W ratios between 15 and 7.5, spherical particles were composed of rods and the crystallite size decreases with increasing EG content, which agree with effect (1). At EG/W=60/8, the excess water overly promoted the anisotropic growth, so that the hollow spheres easily fractured forming various hierarchical structures. This observation supports effect (2).
Ashoka et al [Citation31] obtained flower-like ZnO particles at EG/W=2 and randomly distributed hexagonal ZnO rods at EG/W<1. This observation supports effect (2). For EG/W ratios between 0.5 and 2, the size of the constituent ZnO rods did not change with increasing EG/W value. The study by Qi [Citation34] supports effect (1) that the size of ZnO rods decreases with increasing EG content and effect (2) that the aggregates separate into crystallites at small EG/W values. Effect (1) was also reported by Bitenc and Orel [Citation39].
Similar phenomena were observed for other compounds synthesized by the solvothermal process in EG–water mixtures. Cho et al [Citation42] studied the solvothermal synthesis of Fe3O4 particles using EG in the presence of NaOH and dodecylamine and observed a strong effect of the EG/water ratio on the morphology. Hollow Fe3O4 spheres were obtained in the absence of water; they consisted of oriented aggregates of Fe3O4 nanoparticles. The overall shape was polyhedral for EG/W=30 or 15, and it was irregular at EG/W=10.
Characterization of hierarchical structures
The hierarchical structures of the spherical particles were characterized by microscopy. Qiu et al [Citation36] observed the nanorod-based superstructure of ZnO spherical particles. For undoped ZnO, the nanorods were attached at the narrow ends of the nanorod bundles. The nanorods preferentially grew along the c-axis through oriented attachment of nanoparticles. For Co-doped ZnO, the spherical structure was constructed by highly crystalline nanodiscs. The well-defined selected-area electron diffraction (SAED) spots with the [001] zone axis normal to the nanodisc surface indicate the preferential lateral growth to the c-axis.
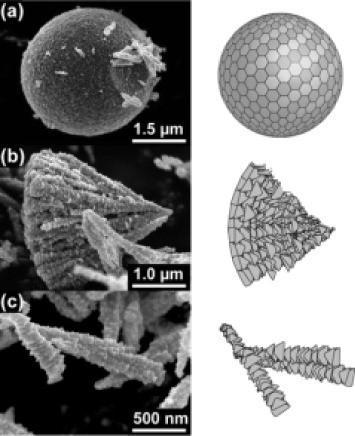
We have clearly observed the hierarchical structure in spherical ZnO particles. Figure shows SEM images of particles prepared in the 87.5 vol.% EG solution and the corresponding illustrations [Citation38]. They are probably fragments of ZnO spheres broken by ultrasonic treatment. The first stage of the hierarchy corresponds to small triangular pyramids 30−100 nm in size that agree with the XRD results. The second stage yields wedge-shaped particles as shown in figure (c), whereas microspheres are produced at the final stage. Cross-sectional transmission electron microscopy observations revealed that the spherical particles were dense and showed radial contrast, implying that they were made of wedge-shaped parts. SAED patterns demonstrated that the crystallites are radially aligned along the c-axis.
Figure 5 SEM images of (a) sphere-, (b) cone- and (c) wedge-shaped parts of the ZnO particles prepared in an 87.5 vol.% EG solution, and the corresponding illustrations [Citation38].
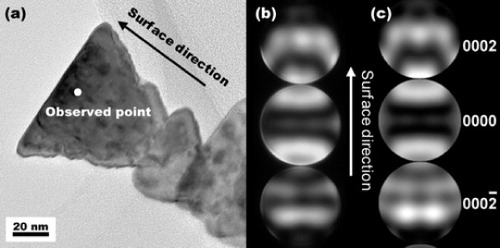
The polarity was determined using convergent-beam electron diffraction (CBED), revealing that the whole surface of the microspheres was a c(+)-plane. The CBED pattern for a wedge-shaped fragment is shown in figure . The incident azimuth was along the m-axis. Simulated CBED patterns were calculated assuming that the base of the wedge is the c(+)-plane. They agreed well with the experimental results, confirming that the surface of the ZnO spherical particles corresponds to the c(+)-plane.
Figure 6 (a) TEM image of, and (b) experimental and (c) simulated CBED patterns of, the wedge-shape fragment of a ZnO particle prepared in an 87.5 vol.% EG solution [Citation38].
Summary
Biominerals are produced via environment-friendly, energy-saving solution processes at low temperatures. Their hierarchical structures result in remarkable mechanical and optical properties. Complex hierarchical structures inspired by nature can also be prepared in the laboratory. We have reviewed solvothermal synthesis, which uses glycols and water as the solvent, of spherical and flower-like ZnO particles with hierarchical nanostructures. The preparation conditions and morphological details of the ZnO particles were summarized. Water in glycols plays an important role in the formation of the hierarchical structures. We summarize the three effects of EG as follows: (i) EG restricts the growth of ZnO microcrystals, probably via the capping effect; (ii) EG promotes self-assembly of small crystallites into round particles; at small EG concentrations the aggregates disperse into crystallites; and (iii) high water content in EG results in hollow spheres, and the cavity size increases with decreasing EG concentration.
References
- KakisawaHSumitomoTInoueRKagawaY 2010 Comput. Sci. Technol. 70 161 http://dx.doi.org/10.1016/j.compscitech.2009.10.003
- SumitomoTKakisawaHOwakiYKagawaY 2008 J. Mater. Res. 23 3213 http://dx.doi.org/10.1557/JMR.2008.0389
- WeinkamerRFratzlP 2011 Mater. Sci. Eng. C 31 1164 http://dx.doi.org/10.1016/j.msec.2010.12.002
- LiuaKJiangK 2011 Nano Today 6 155 http://dx.doi.org/10.1016/j.nantod.2011.02.002
- XuAWMaYCölfenH 2007 J. Mater. Chem. 17 415 http://dx.doi.org/10.1039/b611918m
- KoumotoKSaitoNGaoYFMasudaYZhuP X 2008 Bull. Chem. Soc. Japan 81 1337 http://dx.doi.org/10.1246/bcsj.81.1337
- Special issue 2010 MRS Bull. 35 116–149 http://dx.doi.org/10.1557/mrs2010.630
- Special issue 2010 MRS Bull. 35 743–796
- MoulsonA JHerbertJ M 1990 Electroceramics London Chapman and Hall
- MinamiTNantoHTanakaS 1982 Appl. Phys. Lett. 41 958 http://dx.doi.org/10.1063/1.93355
- ShiosakiTYamamotoTYagiMKawabataA 1981 Appl. Phys. Lett. 39 399 http://dx.doi.org/10.1063/1.92751
- AdachiYOhashiNOhnishiTOhgakiTSakaguchiIHanedaHLippmaaM 2008 J. Mater. Res. 23 3269 http://dx.doi.org/10.1557/JMR.2008.0404
- LiDHanedaHHishitaSOhashiN 2005 Chem. Mater. 17 2588 http://dx.doi.org/10.1021/cm049100k
- IzakiMOmiT 1996 Appl. Phys. Lett. 68 2439 http://dx.doi.org/10.1063/1.116160
- WanderASchedinFSteadmanPNorrisAMcGrathRTurnerT SThorntonGHarrisonN M 2001 Phys. Rev. Lett. 86 3811 http://dx.doi.org/10.1103/PhysRevLett.86.3811
- OhashiNTakahashiKHishitaSSakaguchiIFunakuboHHanedaH 2007 J. Electrochem. Soc. 154 D82 http://dx.doi.org/10.1149/1.2402991
- KawanoKKomatsuMYajimaYHanedaHMakiHYamamotoT 2002 Appl. Surf. Sci. 189 265 http://dx.doi.org/10.1016/S0169-4332(01)01022-4
- MclarenAValdes-SolisTLiGTsangS C 2009 J. Am. Chem. Soc. 131 12540 http://dx.doi.org/10.1021/ja9052703
- TianZ RVoigtJ ALiuJMckenzieBMcdermottM JRodriguezM AKonishiHXuH 2003 Nat. Mater. 2 821 http://dx.doi.org/10.1038/nmat1014
- TianZ RVoigtJ ALiuJMcKenzieMMcDermottM J 2002 J. Amer. Chem. Soc. 124 12954 http://dx.doi.org/10.1021/ja0279545
- VayssieresL 2003 Adv. Mater. 15 464 http://dx.doi.org/10.1002/adma.200390108
- MasudaYKatoK 2008 Cryst. Growth Des. 8 2633 http://dx.doi.org/10.1021/cg060607c
- SaitoNHanedaHSekiguchiTOhashiNSakaguchiIKoumotoK 2002 Adv. Mater. 14 418 http://dx.doi.org/10.1002/(ISSN)1521-4095
- AubertTGrassetFPotelMNazabalVCardinalTPechevSSaitoNOhashiNHanedaH 2010 Sci. Technol. Adv. Mater. 11 044401 http://dx.doi.org/10.1088/1468-6996/11/4/044401
- LincotD 2010 MRS Bull. 35 778 http://dx.doi.org/10.1557/mrs2010.507
- KawanoTImaiH 2010 J. Ceram. Soc. Japan 118 969 http://dx.doi.org/10.2109/jcersj2.118.969
- BaruahSDuttaJ 2009 Sci. Technol. Adv. Mater. 10 013001 http://dx.doi.org/10.1088/1468-6996/10/1/013001
- InoueMOtsuHKominamiHInuiT 1995 J. Alloys Compd. 226 146 http://dx.doi.org/10.1016/0925-8388(95)01632-5
- JézéquelDGuenotaJJouiniaNFiévetF 1995 J. Mater. Res. 10 77 http://dx.doi.org/10.1557/JMR.1995.0077
- TayY YLiSBoeyFChengY HLiangM H 2007 Physica B 394 372 http://dx.doi.org/10.1016/j.physb.2006.12.062
- AshokaSNagarajuGTharamaniC NChandrappG T 2009 Mater. Lett. 63 873 http://dx.doi.org/10.1016/j.matlet.2009.01.054
- WangL BFanY PBalaHSunG 2011 Micro Nano Lett. 6 741 http://dx.doi.org/10.1049/mnl.2011.0149
- GhoshalTKarSChaudhuriS 2007 Cryst. Growth Des. 7 136 http://dx.doi.org/10.1021/cg060289h
- QiX 2009 Powder Technol. 189 103 http://dx.doi.org/10.1016/j.powtec.2008.06.008
- ZhangHWuJZhaiCDuNMaXYangD 2007 Nanotechnology 18 455604 http://dx.doi.org/10.1088/0957-4484/18/45/455604
- QiuYChenWYangSZhangBZhangX XZhongY CWongK S 2010 Cryst. Growth Des. 10 177 http://dx.doi.org/10.1021/cg900832m
- RezapourMTalebianN 2011 Mater. Chem. Phys. 129 249 http://dx.doi.org/10.1016/j.matchemphys.2011.04.012
- MatsumotoKSaitoNMitateTHojoJInadaMHanedaH 2009 Cryst. Growth Des. 9 5014 http://dx.doi.org/10.1021/cg901216g
- BitencMOrelZ C 2009 Mater. Res. Bull. 44 381 http://dx.doi.org/10.1016/j.materresbull.2008.05.005
- ShenLBaoNYanagisawaKGuptaADomenKGrimesC A 2007 Cryst. Growth Des. 7 2742 http://dx.doi.org/10.1021/cg0705409
- ZhangLZhuY JCaoS W 2008 Chem. Lett. 37 1002 http://dx.doi.org/10.1246/cl.2008.1002
- ChoSNohJParkSLimD YChoiS H 2007 J. Mater. Sci. 42 4877 http://dx.doi.org/10.1007/s10853-006-0685-4
