Abstract
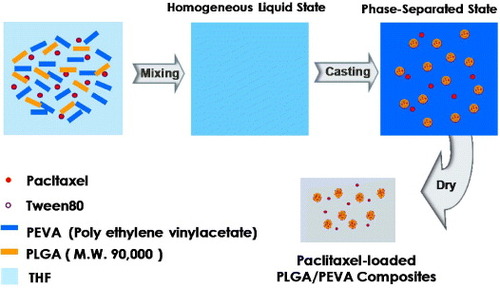
The mixture of poly(lactide-co-glycolide) (PLGA) and poly(ethylene vinyl acetate) (PEVA) forms a homogeneous liquid in an organic solvent such as tetrahydrofuran, and a phase-separated PLGA/PEVA composite can be prepared from it by evaporating the organic solvent. Exploiting this phenomenon, we designed a novel method of preparing a drug-loaded PLGA/PEVA composite and used it for coating drug-eluting stents (DESs). Paclitaxel (PTX), an anticancer drug, was chosen as a model drug. PLGA acts as a microdepot for PTX, and PEVA provides mechanical strength to the coating material. The presence of PLGA in the PLGA/PEVA composite suppressed PTX crystallization in the coating material, and PTX showed a sustained release rate over more than 30 days. The mechanical strength of the PLGA/PEVA composite was better than that of PEVA used as a control. After coating the stent with a PLGA/PEVA composite using ultrasonic atomizing spray, the morphology of the coated material was observed by scanning electron microscopy, and the release pattern of PTX was measured by high-performance liquid chromatography.
Introduction
Metallic stents have been successfully used to treat obstructive symptoms caused by the structure of nonvascular organs, such as the urethra, esophagus and trachea [Citation1–3]. However, tissue hyperplasia after implantation of bare metal stents, leading to in-stent restenosis, has emerged as a new obstacle [Citation4].
To overcome this problem, drug-eluting stents (DESs) with polymer-coated metallic scaffolds have been designed and characterized. DESs are usually made of metallic scaffolds, which are coated to increase their biocompatibility and provide local therapeutic delivery of antirestenotic agents [Citation5]. A number of studies have been performed on polymer-based, DES coating technologies, which enable the preparation of a variety of drug/carrier (polymer) coatings [Citation6]. Carriers can be durable or biodegradable, and are coated with either a single or multiple layers [Citation7].
In this study, a new type of coating material for DES based on a PLGA/PEVA composite has been designed and characterized. Poly(lactide-co-glycolide) (PLGA), which has good biocompatibility and biodegradability, was previously examined for this purpose [Citation8, Citation9], and numerous studies were reported on DES coated with poly(ethylene vinyl acetate) (PEVA) [Citation10–12]. The combination of PEVA and PLGA, based on the phase separation phenomenon, will provide a new form of coating materials for DES. The mixture of PLGA and PEVA forms a homogeneous liquid in an organic solvent such as tetrahydrofuran (THF), and a phase-separated PLGA/PEVA composite can be prepared from it by evaporating the organic solvent. On the basis on this phenomenon, we designed a novel method of preparing a drug-loaded PLGA/PEVA composite and used it for coating DESs. Paclitaxel (PTX), an anticancer drug, was chosen as a model drug. It was reported that the parenteral drug Taxol is unstable, as evidenced by turbidity or haziness, and, upon storage of aqueous solutions, by the formation of insoluble precipitates due to the low solubility of PTX in aqueous media [Citation13]. When PTX was loaded in a polymer matrix, it crystallized. This hindered the release of PTX from the matrix and decreased the mechanical strength of the matrix. To overcome these difficulties, a PLGA/PEVA composite was designed and characterized as a coating material for DES. The mechanical strength of the composite was measured as a function of the PLGA/PEVA ratio using sheet samples. After coating the stent with the composite using ultrasonic atomizing spray, the morphology of the coated material was observed by scanning electron microscopy (SEM), and the release pattern of PTX was measured by high-performance liquid chromatography (HPLC).
Materials and methods
Materials
An 18-mm-long stent was made of annealed L-605 cobalt-chromium alloy (surgical grade) using laser cutting methods. Anhydrous PTX was purchased from Samyang Genex Co. (Daejeon, Korea). PLGA (75 mol% lactide, molecular weight 90 000) was purchased from Boelinger Ingelheim (Germany). All the other reagents used in this study were of analytical grade.
Preparation of spraying solutions
The spraying solutions were prepared individually by placing 90 mg of polymers with various PEVA/PLGA ratios, 5 mg of PTX, and 5 mg of Tween 80 into 20 ml vials, and then immediately pouring the mixture into 10 ml of THF.
To observe the formation of the PTX-loaded PLGA/PEVA composite by SEM (JEOL JSM-6700F, Japan), the solution mixture was cast onto a glass plate to evaporate the THF. The thus prepared sheet samples were used for further optimization of the composite materials.
Measurements of mechanical properties of composite materials
The mechanical properties of the composite samples (diameter: 14 mm, thickness: 20 mm) were measured as a function of PLGA content using a biaxial tensile test setup, in an Instron machine with a 490-Newton load cell (AG-5000G, Shimadzu, Japan). A rod (diameter: 20 mm) with a flat tip was hammered vertically at a crosshead speed of 1 mm min−1 on the specimen placed on a grip, and the stress–compression curves were obtained.
Preparation of paclitaxel-eluting stents
A solution of PLGA/PEVA composite (1 wt%) with PTX was sprayed onto a clean stent surface. The spraying system is described elsewhere [Citation14]. It combines an ultrasonic atomizing nozzle with low-pressure air to produce a soft, highly focused stream of small drops. The fine focusing is achieved by using a small nozzle tip and air orifice. An isolated hypotube delivers liquid to the nozzle tip, while compressed air, delivered through the nozzle orifice at a fixed low pressure, shapes the atomized droplets into a well-defined spray. The stent was traversed and rotated during the spraying process. All the stents were dried in a vacuum oven at 37 °C for 48 h to evaporate the residual THF, and the absence of THF in stents was confirmed by gas chromatography. The coating thickness of the 5 wt% PTX-loaded stent was about 15 μm.
In vitro release studies
The release kinetics of PTX was investigated in vitro by incubating a PTX-eluting stent in 5 ml of medium at pH 7.4. The PEVA/PLGA composite with a weight ratio of 85:10 was used as a coating material, and the loading amount was 5 wt%. The incubation medium consisted of 0.01 M phosphate-buffered saline (PBS) containing 0.05 wt% Tween 80. The agitation rate and temperature were maintained constant at 120 rpm and 37 °C, respectively. At selected time intervals, the incubation medium was completely removed for analysis and replaced with fresh medium. The PTX release was measured by reverse-phase high-performance liquid chromatography (RP-HPLC) using a Capcell-pack C18 column and an acetonitrile/water (60:40 v/v) mobile phase, for 10 min, at a flow rate of 1.2 ml min−1. The eluent was monitored by UV absorption at 227 nm.
Results
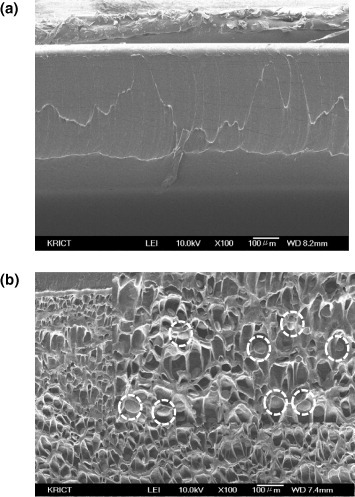
As shown in figure , the mixture of PLGA and PEVA forms a homogeneous liquid phase in THF. Upon evaporation of THF from the PLGA/PEVA mixture, which contains PTX and Tween 80 as a model drug and surfactant, respectively, the phase separation is expected to form a PLGA microdomain in the PEVA matrix. To observe the formation of this PLGA/PEVA composite, 1 wt% THF solutions of PLGA/PEVA containing PTX and Tween 80 were prepared with different PLGA/PEVA ratios, and the solution mixtures were cast on glass plates. Cross sections of the PEVA matrix and composite were examined by SEM, as shown in figure . The PEVA matrix consists of a homogeneous phase; however, the formation of PLGA microparticles (microdomains) was observed in the PLGA/PEVA composite.
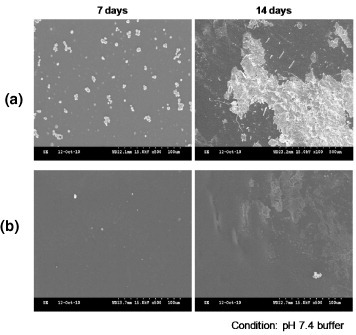
The composite consists of 4 components, PEVA, PLGA, Tween 80, and PTX, and contacts aqueous media after being coated on the stent. Therefore, stability in aqueous media is the main criterion for determining the suitability of the composite as a stent-coating material. Figure (a) shows an SEM image of PEVA containing PTX and Tween 80. After equilibration in PBS for 7 days, crystallization of PTX was observed in the PEVA matrix. Significant progress in the crystallization was observed after holding the samples in PBS for 14 days. However, very low crystallization of PTX was observed for the composite during a holding time of 7 days (see figure (b)).
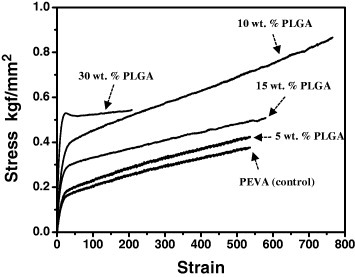
For stent applications, the composite must be elastic to allow for the elongation of the coating during stent deployment without distorting or cracking. The relative tensile strength of the composite was measured to evaluate the coating's integrity at different PLGA contents in the composite, and representative stress-strain curves of solution-cast films are shown in figure . With the formation of PLGA microparticles in the composite, a significant improvement in the elasticity was observed. The best results were obtained for 10 wt% PLGA, and this composition was used for other experiments.
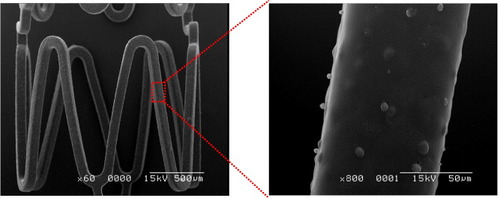
Figure shows SEM images of the produced PTX-eluting stent. After coating with 5 layers, the formation of PLGA microparticles was observed, indicating that the composite was formed on the stent.
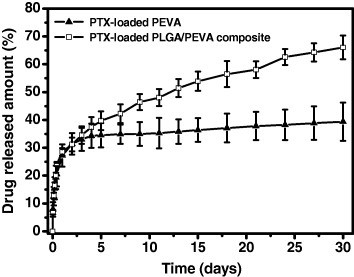
The in vitro release pattern of PTX from the stent is shown in figure , which reveals a two-phase release profile. Both systems showed an initial burst within 2 days because of the immediate dissolution of the PTX dispersed in the PEVA domain. In the case of a stent coated with the control system, the release was minimal after the burst release phase. However, almost zero-order release kinetics was observed for the stent coated with the PTX-loaded PLGA/PEVA composite after the initial burst.
Discussion
Temperature-induced phase transitions were reported for Pluronic/PLGA and Pluronic/PEO mixtures, where Pluronic is a poly(ethylene oxide)-poly(propylene oxide)-poly(ethylene oxide) triblock copolymer and PEO stands for poly(ethylene oxide) [Citation15, Citation16]. The polymer mixtures formed a homogeneous phase upon heating above the melting temperature. The phase separation was induced by cooling, which led to the formation of spherical nano/microparticles in the mixture. Based on this phenomenon, the phase transition was induced by evaporating the solvent from the polymer mixture solution. As shown in figure , the PLGA microparticles were formed upon evaporation of THF from the PLGA/PEVA/THF solution mixture. PLGA is a hydrophobic material and PTX has a limited solubility in water (5.11 μg ml−1). A hydrophobic interaction between PLGA and PTX is expected with the efficient loading of PTX in the PLGA microparticles during the formation of the composite. As shown in figure , PLGA microparticles were formed, and this is a result of the phase separation of PLGA and PEVA upon the evaporation of THF. With the presence of PLGA microparticles in the PEVA matrix, a significant amount of PTX was loaded into the PLGA microparticles, and the content of PTX in the PEVA matrix was decreased. This hindered the crystallization of PTX, as shown in figure .
It was reported that two factors contribute significantly to the mechanical properties of the composite material: the molecular level of blending between polyurethane (PU) and amorphous ultrahigh-molecular-weight polyethylene (UHMWPE), and the formation of a nanoscale composite between crystalline UHMWPE and PU or PU/amorphous UHMWPE blend [Citation17]. Although PLGA formed the microparticles, the improvement in the elasticity of the composite (see figure ) can be explained in terms of the molecular level of blending and the formation of a nanoscale composite between two immiscible polymeric components, as described previously.
The presence of PTX-loaded PLGA microparticles in the PEVA matrix can alter the release profile from the matrix. A minimal release after the initial burst phase was observed for the stent coated using the control system. This is due to the significant crystallization of PTX in the PEVA matrix, as shown in figure . The release behavior of PTX from the stent coated with the PTX-loaded PLGA/PEVA composite showed approximately zero-order release kinetics after the initial burst release phase. The sustained release rate can be attributed to the longer diffusion route of PTX that was present deeper in the PLGA domain.
Conclusions
A PTX-loaded PLGA/PEVA composite was prepared using the phase separation of the polymer solution mixture upon evaporation of the solvent. Microscale PLGA domains were formed as a result of the phase separation. PTX was efficiently loaded into the PLGA domains owing to the hydrophobic interactions between PLGA and PTX. With the formation of PTX-loaded PLGA domains in the PEVA matrix (the composite), the mechanical property of the composite was improved owing to the homogeneous mixing of two immiscible polymers at the microscale. Our preliminary results show that the PTX-loaded PLGA/PEVA composite can be utilized as a stent coating material and the sustained release of PTX can be achieved.
Acknowledgments
This work was financially supported by the Korea Research Foundation (projects 20110027932 and 2011K000965) and a grant from the fundamental R&D program for core technology of materials funded by the Ministry of Knowledge Economy, Republic of Korea.
References
- ShinJ HSongH YChoiC GYukS HKimJ SKimY MYoonC JKimT HSuhJ YHeX 2005 Radiology 234 438 http://dx.doi.org/10.1148/radiol.2342040006
- OronsP DAmesurN BDauberJ HZajkoA BKeenanR JIaconoA T 2000 J. Vasc. Interv. Radiol. 11 89 http://dx.doi.org/10.1016/S1051-0443(07)61288-3
- SongH YJungH YParkS IKimS BLeeD HKangS GMinY I 2000 Radiology 217 551
- HongM KKornowskiRBramwellORaghebA OLeonM B 2001 Coron. Artery Dis. 12 513 http://dx.doi.org/10.1097/00019501-200109000-00011
- AcharyaGParkK 2006 Adv. Drug Deliv. Rev. 58 387 http://dx.doi.org/10.1016/j.addr.2006.01.016
- KangEVedanthamKLongXDadaraMKwonI KSturekMParkK 2009 Mol. Pharm. 6 1110 http://dx.doi.org/10.1021/mp8002623
- ChenM CLiangH FChiuY LChangYWeiH JSungH W 2005 J. Control. Release 108 178 http://dx.doi.org/10.1016/j.jconrel.2005.07.022
- DongYFengS-S 2006 J. Biomed. Mater. Res. A 78 12
- SarisozenCAricaB AHincalA ACalisS 2009 J. Microencapsulation 26 501 http://dx.doi.org/10.1080/02652040802465792
- KangEWangHKwonI KRobinsonJParkKChengJ X 2006 Anal. Chem. 78 8036 http://dx.doi.org/10.1021/ac061218s
- BalssK MLanosGPapandreouGMaryanoffC A 2008 J. Biomed. Mater. Res. A 85 258
- LevyYTalNTzemachGWeinbergerJDombA JMandlerD 2009 J. Biomed. Mater. Res. B 91 819
- TrisselL A 1997 Pharmacotherapy 17 133S
- WangG XLuoL LYinT YLiYJiangTRuanC GGuidoinRChenY PGuzmanR 2010 J. Microencapsulation 27 105 http://dx.doi.org/10.3109/02652040903046798
- LeeK EKimB KYukS H 2002 Biomacromolecules 3 1115 http://dx.doi.org/10.1021/bm020066h
- OhK SSongJ YChoS HLeeB SKimS YKimKJeonHKwonI CYukS H 2010 J. Control. Release 148 344 http://dx.doi.org/10.1016/j.jconrel.2010.08.021
- TeohS HTangZ GRamakrishnaS 1999 J. Mater. Sci. Mater. Med. 10 343 http://dx.doi.org/10.1023/A:1026421606939