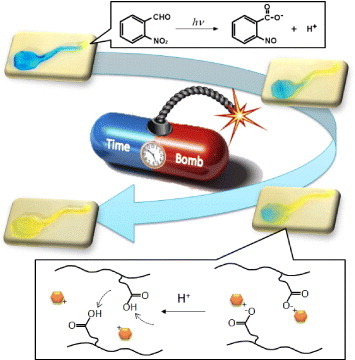
Abstract
We demonstrate a timed explosive drug release from smart pH-responsive hydrogels by utilizing a phototriggered spatial pH-jump reaction. A photoinitiated proton-releasing reaction of o-nitrobenzaldehyde (o-NBA) was integrated into poly(N-isopropylacrylamide-co-2-carboxyisopropylacrylamide) (P(NIPAAm-co-CIPAAm)) hydrogels. o-NBA-hydrogels demonstrated the rapid release of protons upon UV irradiation, allowing the pH inside the gel to decrease to below the pKa value of P(NIPAAm-co-CIPAAm). The generated protons diffused gradually toward the non-illuminated area, and the diffusion kinetics could be controlled by adjusting the UV irradiation time and intensity. After irradiation, we observed the enhanced release of entrapped L-3,4-dihydroxyphenylalanine (DOPA) from the gels, which was driven by the dissociation of DOPA from CIPAAm. Local UV irradiation also triggered the release of DOPA from the non-illuminated area in the gel via the diffusion of protons. Conventional systems can activate only the illuminated region, and their response is discontinuous when the light is turned off. The ability of the proposed pH-jump system to permit gradual activation via proton diffusion may be beneficial for the design of predictive and programmable devices for drug delivery.
1. Introduction
Polymers that can change some of their properties upon the application of external stimuli are called ‘smart’ polymers. The last two to three decades have witnessed explosive growth in the applications of smart polymers. They have been used in bioseparation [Citation1, Citation2], actuators [Citation3], drug delivery [Citation4], diagnosis [Citation5, Citation6] and regenerative medicine [Citation7, Citation8]. The uniqueness of smart polymers lies not only in the rapid macroscopic changes occurring in their structure but also in the reversible nature of these transitions. The affected factors include shape [Citation9], surface characteristics [Citation10, Citation11], solubility [Citation12], molecular affinity [Citation13, Citation14], and sol–gel transition [Citation15], etc., whereas the external triggers can be temperature [Citation2, Citation5, Citation16], pH [Citation4, Citation17], ionic strength [Citation18], or certain chemicals [Citation19, Citation20]. More recently, electric fields [Citation21], magnetic fields [Citation22], and light [Citation23] have been used as additional stimuli for these polymers. Stimuli-responsive polymers have several promising applications because the stimuli can be applied or removed instantaneously, avoiding the delay associated with the diffusion of the stimulus in many other cases. For example, Shimoboji et al developed light-responsive polymer-enzyme switches and demonstrated ‘on-off’ control of the catalytic activity by alternating irradiation with UV and visible light [Citation24, Citation25]. Ikeda and Tsutsumi demonstrated the repeated and precise light-induced bending of a film of a liquid-crystal network containing an azobenzene chromophore [Citation26]. The reversible bending of a polymer film was also demonstrated by Hosono et al. They used polymer brushes that consisted of a polymethacrylate backbone densely grafted to paraffinic side chains containing azobenzene [Citation27].
While field stimuli are advantageous because they allow local and remote control, they result in a discontinuous response when the stimulus is turned ‘off’. In other words, only the illuminated region is active, and continuous illumination is necessary. In the human body, however, the appearance of numerous bioactive molecules is tightly controlled to maintain a normal metabolic balance via the feedback system called homeostasis. For example, hormones or cytokines not only act locally (local signals), but also travel to other locations in the body via blood circulation (remote signals) [Citation28]. These signals are sometimes amplified or transferred to another signal by sequentially interacting with many other different molecules. A representative process is blood coagulation, which is a complex sequence involving numerous clotting factors [Citation29]. The concentration gradients of protons (pH), ions, and oxidizing or reducing agents are also important characteristics observed in living systems. The human body exhibits variations of pH along the gastrointestinal tract, tumoral areas, inflamed or infected tissues, and the endosomal lumen [Citation30]. Therefore, it would be highly beneficial if drugs could be released by a system that senses particular signals, evaluates their magnitude, amplifies or transfers them, and then acts to release the correct amount of the drug.
From these perspectives, we propose a new system that exhibits the programmable release of a drug by utilizing a spatio-temporally controllable ‘physical’ signal and a gradually diffusible ‘chemical’ signal. We have recently reported the spatio-temporal control of the proton concentration in pH-responsive hydrogels by utilizing a photoinitiated proton-releasing reaction of photoacid generators (PAGs) [Citation31]. The PAG-integrated pH-responsive hydrogels demonstrated a rapid pH-jump reaction within the gel upon UV irradiation, resulting in the gel shrinking in response to a decrease in pH. Most recently, we have developed reversible self-bending actuators based on the pH-jump reaction [Citation32]. Importantly, these results can be used in spatially and temporary programmable devices because PAG-integrated gels permit not only local activation in the illuminated area but also gradual activation in the non-illuminated area as protons diffuse. In this study, PAG was integrated into pH-responsive poly(N-isopropylacrylamide-co-2-carboxyisopropylacrylamide) (P(NIPAAm-co-CIPAAm)) hydrogels to demonstrate a timed explosive release of entrapped L-3,4-dihydroxyphenylalanine (DOPA) (figure ). First, to determine the proton releasing kinetics of the PAG, we measured the pH of the solution containing PAGs as a function of UV intensity. Next, we investigated the proton generation and diffusion kinetics inside the gels, both experimentally and theoretically. We also monitored the release profiles of DOPA from the gels after local UV irradiation. Since the electrostatic interactions between CIPAAm and DOPA were reduced to below the value of the acid dissociation constant, pKa, local UV irradiation successfully triggered the release of DOPA from not only the irradiated region but also the non-irradiated region via the gradual diffusion of protons.
2. Materials and methods
2.1. Materials
N-Isopropylacrylamide was kindly supplied by Kohjin Co. Ltd (Tokyo, Japan) and purified by recrystallization with n-hexane. The carboxylate monomer 2-carboxyisopropylacrylamide (CIPAAm) was synthesized according to the protocol published previously [Citation33]. All other reagents are commercially available and were used without further purification. N,N′-methylene-bisacrylamide (MBAAm), N,N,N′,N′-tetramethylenediamine (TEMED), ammonium persulfate (APS), o-Nitrobenzaldehyde (o-NBA), m-nitrobenzaldehyde (m-NBA), p-nitrobenzaldehyde (p-NBA), 5-hydroxy-o-nitrobenzaldehyde (5-hydroxy-o-NBA), and 4-hydroxy-m-nitrobenzaldehyde (4-hydroxy-m-NBA) were purchased from Tokyo Chemical Industry Co. Ltd (Tokyo, Japan). Bromocresol green, L-3,4-dihydroxyphenylalanine (DOPA), and Milli-Q water were supplied by Sigma-Aldrich (St. Louis, MO, USA), Alfa Aesar (Ward Hill, MA, USA), and Millipore (Tokyo, Japan), respectively.
2.2. Photoinduced pH-jump reaction
Photoinduced changes in the pH in water were detected by monitoring the solution pH during room-temperature irradiation with UV light (250 SX-UI2150HQ lamp, Ushio, Tokyo, Japan, equipped with a heat absorbing filter, HA-50 Hoya, Tokyo, Japan). The intensity and wavelength of the UV light were 19.90 mW cm−2 and 365 nm, respectively. Solutions containing PAG were prepared by dissolving o-NBA, m-NBA, p-NBA, 5-hydroxy-o-NBA, or 4-hydroxy-m-NBA in water at a concentration of 10 mM.
2.3. Preparation of P(NIPAAm-co-CIPAAm) hydrogels
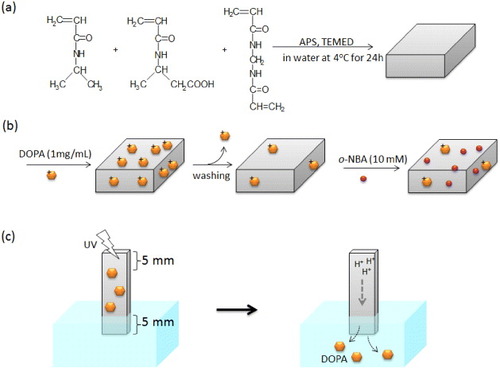
P(NIPAAm-co-CIPAAm) hydrogels were prepared by redox polymerization (scheme (a)) [Citation34, Citation35]. Briefly, NIPAAm (95 mol%), CIPAAm (5 mol%), MBAAm (2 mol%), and TEMED (19 mol%) were dissolved in water. The pre-gel solution was bubbled with nitrogen gas at 4 °C to remove oxygen. After APS was added, spontaneous gelation was initiated between glass slides separated by a 1 mm spacer. The solution was kept at 4 °C for 24 h. The hydrogels were removed from the molds and washed with fresh water for 24 h to remove unreacted compounds.
Scheme 1 Preparation scheme of P(NIPAAm-co-CIPAAm) gels by a redox polymerization (a). For drug loading, P(NIPAAm-co-CIPAAm) gels were first swollen at room temperature in a solution containing 1 mg ml−1 of DOPA. After 2 days, the gels were rinsed in water to remove non-interacted DOPA. The gels were then immersed in a solution containing 10 mM of o-NBA for 2days (b). For the drug release experiment, one part of the gel (5 × 5 mm2) was immersed in water and another part (5 × 5 mm2) was exposed to UV irradiation (c).
2.4. Photoirradiation of PAG-loaded hydrogels
P(NIPAAm-co-CIPAAm) gel samples measuring 20 × 5 × 1 mm−3 were swollen at room temperature in pH 7 solution containing 10 mM of o-NBA as a PAG and 1 mg ml−1 of bromocresol green as a pH-indicator. Parts of the samples were then exposed to UV light through a photomask. The color change of the pH-indicator inside the gels was continuously monitored in air, with the humidity adjusted to ∼88%.
2.5. Simulations of proton diffusion kinetics
A simple one-dimensional (1D) model has been developed for predicting the interface movement of protons in a pH-responsive hydrogel by the finite-difference method [Citation36]. The Fick's second law was used to describe the proton diffusion: where D is the proton diffusion coefficient in the gel [Citation37]. The continuous partial differential equation (PDE) of the Fick's second law was replaced with a discrete approximation. The second derivative at the point (ri, tj) was approximated with the equation
which was evaluated using the spatial derivatives at points (ri+1, ri) and (ri, ri−1):
Then, the proton concentration at (ri, tj+1) can be obtained as
In this model, the initial concentrations of UV-induced protons in the hydrogels at different UV exposure times were determined from the results shown in figure (b).
2.6. Drug release
For drug release experiments, P(NIPAAm-co-CIPAAm) hydrogels were first swollen in a solution containing 1 mg ml−1 of DOPA at room temperature. After 2 days, the gels were rinsed in water to remove non-interacted DOPA for 24 h. The gels were then immersed in a solution containing 10 mM of o-NBA for 2 days (scheme (b)). As illustrated in scheme (c), one part of the gel was immersed in water and another part was exposed to UV irradiation (humidity was adjusted to ∼88%). The released DOPA was collected, and the absorbance at 280 nm was measured with a UV-visible spectrometer (V-550, JASCO International, Tokyo, Japan).
3. Results and discussion
3.1. Photoinduced pH-jump reaction of PAGs
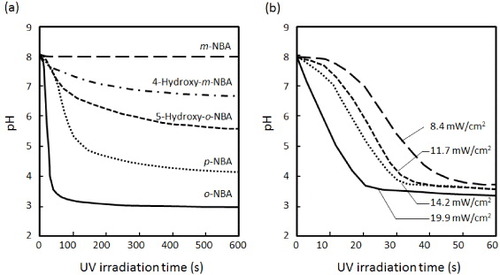
Photoinitiated proton-releasing reactions are important not only for investigating the dynamics of pH-dependent phenomena such as protein folding kinetics, but also for rapidly changing the proton concentration in solutions [Citation38]. In particular, it would be useful to change the solution pH inside intact materials such as hydrogels. Figure (a) shows the substituent effect of various NBA derivatives on the proton releasing kinetics in water. Five different derivatives, o-NBA, m-NBA, p-NBA, 5-hydroxy-o-NBA, and 4-hydroxy-m-NBA, were examined in this study. The initial pH of the solution was adjusted to 8.0. A solution containing 10 mM of an NBA derivative was irradiated by UV light (19.90 mW cm−2) and its pH was continuously measured. Interestingly, although the pH of solutions containing o-NBA or p-NBA decreased dramatically after UV irradiation, the pH was stable for the m-NBA solution. In general, electron-donating groups with lone pairs on the atoms adjacent to the π system activate the aromatic ring by increasing the electron density on the ring through a resonance donating effect [Citation39, Citation40]. Therefore, the resonance localizes the electron density at the o- and p-positions. The concentration of released protons increased in the order m-NBA < 4-hydroxy-m-NBA < 5-hydroxy-o-NBA < p-NBA < o-NBA. The proton releasing efficiencies were 1.0 × 10−4, 2.0 × 10−3, 2.0 × 10−2, 5.9 × 10−1 and 9.5% for m-NBA, 4-hydroxy-m-NBA, 5-hydroxy-o-NBA, p-NBA, and o-NBA, respectively. These results suggest that the addition of substituents at different positions leads to different deprotonation yields, and o-NBA demonstrated the highest proton releasing efficiency among the PAGs tested in this study. Figure (b) shows the effect of UV irradiation time on the decrease in pH in water. When o-NBA solution was irradiated by UV light, its pH decreased more rapidly at higher UV intensities. On the basis of these results, 10 mM of o-NBA solution and a UV intensity of 19.90 mW cm−2 of UV intensity were used in subsequent experiments.
3.2. Spatial control of pH-jump reaction inside the gels
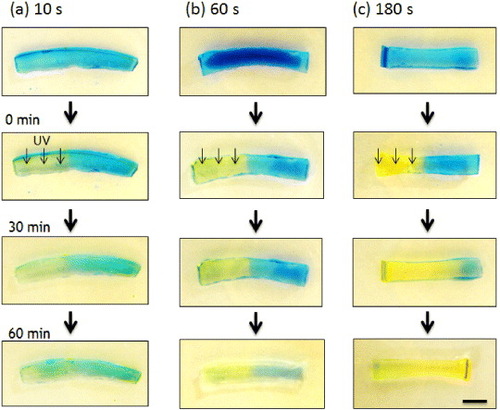
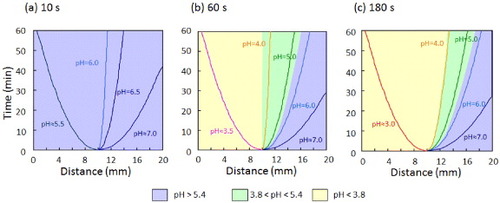
The o-NBA-integrated P(NIPAAm-co-CIPAAm) hydrogel was previously shown to shrink rapidly upon UV irradiation at 37 °C without forming a polymer ‘skin layer’ because the pH inside the gel decreased uniformly. In this study, all experiments were carried out at room temperature to eliminate the effects of the change in volume of the gel on the proton release and drug release kinetics. The spatial control of the pH jump in the gel was demonstrated by irradiating a mask-covered gel with UV light. Swollen hydrogel samples were locally irradiated by UV light for 10, 60, or 180 s in air (humidity 88%). To visualize the pH profile inside the gels, we used an indicator whose color varies with the pH level (yellow: < pH 3.8, blue: > pH 5.4). Figure shows the time evolution of the gel color. After UV irradiation, the color started to change from blue to yellow, but only in the irradiated region. The yellow color became brighter with longer UV exposure. A sharp pH gradient at the interface was revealed by image analysis using ImageJ software (data not shown). The boundary between the yellow and blue regions gradually moved with time; it shifted to the left after 10 s irradiation (figure (a)), whereas it moved to the right after 180 s irradiation (figure (c)), but remained almost unchanged after 60 s exposure (figure (b)). These observations are clearly related to the concentrations of protons released from o-NBA via the pH-jump reaction.
Figure 3 Time-dependent color changes of o-NBA-integrated P(NIPAAm-co-CIPAAm) gels after UV irradiation. The gels were swollen in pH 7 solution containing 10 mM of o-NBA and 1 mg ml−1 of bromocresol green as a pH-indicator (yellow: < pH 3.8, blue: > pH 5.4). The left half of the sample was exposed to UV light through a photomask for 10 s (a), 60 s (b) and 180 s (c) (bar = 5 mm).
To elucidate the proton diffusion kinetics, we developed a 1D model for predicting the interface movement of protons in the hydrogels by the finite-difference method. The generated proton concentrations after UV irradiation for 10, 60, and 180 s were calculated from the proton release kinetics in figure (b) to be 3.2 × 10−6, 3.2 × 10−4, and 1.0 × 10−3 M, respectively. Figure presents time-dependent profiles of the proton concentrations inside the hydrogels. Because a longer irradiation time generates a higher proton concentration, protons diffuse more rapidly toward the periphery of the gel after longer irradiation. To verify our experimental results, we also plotted the simulated pH distribution within the gel in figure . The iso-pH points are shown in different colors. The simulated pH distribution predicts that the time-dependent color change (yellow < pH 3.8 < green < pH 5.4 < blue) depends on the initial proton concentration. The isosbestic points of proton concentration (the average value between the UV-irradiated and non-irradiated areas) were observed in all the simulation results in figure . For the case of 60 s irradiation, the position of the border between the yellow and blue regions was constant. This result is consistent with the observed color changes of bromocresol green (pH 3.8) in figure (b). The predicted pH distributions do not perfectly match our experimental results, because the proton-releasing kinetics of o-NBA in the solution may be different from that inside the hydrogel. Nevertheless, we can predict the diffusion of protons inside the gel for the subsequent drug release experiments using our simulation.
Figure 4 Time-dependent profiles of proton concentrations inside the hydrogels calculated by a simple 1D model. The initial proton concentrations were calculated from the proton release kinetics (figure (b)) to be 3.2 × 10−6 M (a), 3.2 × 10−4 M (b) and 1.0 × 10−3 M (c) for 10, 60 and 180 s UV irradiation, respectively.
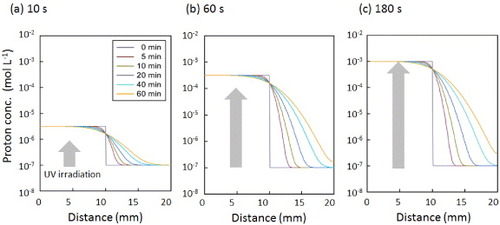
Figure 5 Distributions of pH within the gel calculated by a simple 1D model. The iso-pH points are shown in different colors: yellow < pH 3.8 < green < pH 5.4 < blue. The initial proton concentrations were calculated from the proton release kinetics (figure (b)) to be 3.2 × 10−6 (a), 3.2 × 10−4 (b) and 1.0 × 10−3 M (c) for 10, 60, and 180 s UV irradiation, respectively.
3.3. Programmable drug release via pH-jump reaction
In the drug release experiments, P(NIPAAm-co-CIPAAm) hydrogels were first swollen at room temperature in a solution containing 1 mg ml−1 of DOPA. After removing the free DOPA from the gels, they were immersed in a solution containing 10 mM of o-NBA. One part of the gel (approximately 5 × 5 mm2) was immersed in water and another part (approximately 5 × 5 mm2) was exposed to UV irradiation for 180 s, as illustrated in scheme (c). To visualize the proton diffusion kinetics during the drug release experiment, the time-dependent pH changes were monitored via the color of a pH indicator. Figure S1 (supporting information available from stacks.iop.org/STAM/13/064202/mmedia) shows the evolution of the gel color after UV irradiation for 180 s. The yellow region gradually spread to the right, and the color of the entire gel changed to yellow within 300 min. This result indicates that local 180 s irradiation was sufficient to decrease the pH inside the entire sample to below 3.8 within 300 min. Figures S2 and S3 (supporting information) respectively show the time-dependent profiles of the proton concentration and pH distribution inside the hydrogels calculated by the simple 1D model. From these results, the proton concentration in the non-irradiated part was expected to reach 10−5 M, which corresponds to the pKa of CIPAAm, between 180 and 300 min after the irradiation.
Figure shows the drug release profiles from the gels with and without UV irradiation. The release kinetics of DOPA is faster in the irradiated gel than in the non-irradiated gel. This result can be explained by the electrostatic interaction between DOPA and CIPAAm. At a neutral pH, CIPAAm can stably interact with DOPA because the –COOH groups are more negatively charged above the pKa of CIPAAm (approximately 5.0). Therefore, weak and slow drug diffusion out of the non-irradiated hydrogel was observed over 10 h. At an acidic pH, on the other hand, the electrostatic interaction between CIPAAm and DOPA is weaker because the –COOH groups are more protonated. Therefore, DOPA was released more rapidly upon UV irradiation. Another major difference between the irradiated and non-irradiated gels is the sudden acceleration of the drug release, i.e. the release of DOPA was suddenly accelerated 5 h after 180 s UV illumination.
Figure 6 Drug release profiles of DOPA from o-NBA-integrated P(NIPAAm-co-CIPAAm) gels with (solid circles) and without (open circles) UV irradiation for 180 s. The experimental setup is illustrated in scheme (c).
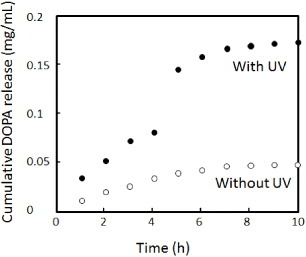
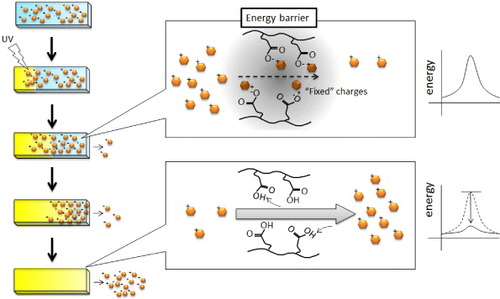
Figure outlines a mechanism for the delayed explosive drug release. As mentioned above, when the –COOH groups are protonated, the electrostatic interaction between CIPAAm and DOPA becomes weaker. Therefore, more DOPA started to dissociate from the –COOH groups as protons diffused toward the non-irradiated region. However, charge compensation within the polyelectrolytes was not achieved, and ‘fixed’ charges existed. These fixed charges reduced the permeability of DOPA via an electrostatic energy barrier [Citation41, Citation42]. In our experiment, the explosive release of DOPA was observed approximately 5 h after UV irradiation, which may correspond to the time when the pH 5.0 boundary reaches the non-irradiated end of the sample (see supporting information, figures S2 and S3 available from stacks.iop.org/STAM/13/064202/mmedia). That is, when the energy barrier completely disappeared, DOPA was able to diffuse out of the gel. In our experiment, the energy barrier was relatively low because drug release was observed from the non-irradiated gel. Also, other interactions were not considered in the discussion. These might be of importance because there may be hydrogen bonds and hydrophobic interactions between these molecules that are pH-dependent.
Figure 7 Schematic of the release mechanism of a positively charged drug from negatively charged hydrogels. Before UV irradiation (neutral pH), CIPAAm could stably interact with DOPA because the –COOH groups were more negatively charged above the pKa of CIPAAm above. After UV irradiation (acidic pH), the electrostatic interaction between CIPAAm and DOPA weakened because the –COOH groups became more protonated. The permeation of the dissociated DOPA, however, was still reduced by the electrostatic energy barrier. Therefore, the explosive release of DOPA was observed when the energy barrier completely disappeared after the boundary of pKa reached the non-irradiated end of the gel.
4. Conclusions
We have demonstrated a predictive, timed, explosive drug release by integrating a pH-jump reaction into pH-responsive hydrogels. By utilizing systems responsive both to light and pH, local and remote signals were simultaneously induced to trigger drug release. The o-NBA-loaded hydrogels demonstrated the rapid release of protons upon UV irradiation, allowing the pH inside the gel to decrease to below the pKa value. The diffusion kinetics of the generated protons was controlled by the UV irradiation time, and the enhanced release of entrapped DOPA from the gels was observed after UV irradiation. An explosive release of DOPA was observed approximately 5 h after local UV irradiation, which corresponds to the time when the energy barrier completely disappeared within the gel. The proposed pH-jump system will provide opportunities for designing predictive and programmable drug delivery devices.
Acknowledgments
This research was partially supported by the Grants-in-Aid for Young Scientists (A) (10013481) from the Ministry of Education, Culture, Sports, Science and Technology (MEXT) Japan. The authors are grateful to Professor Allan S. Hoffman (University of Washington) and Dr. John M. Hoffman (Stratos Genomics Inc., USA) for continued and valuable discussions.
References
- LaiJ JNelsonK ENashM AHoffmanA SYagerPStaytonP S 2009 Lab Chip 9 1997 10.1039/b817754f
- EbaraMHoffmanJ MHoffmanA SStaytonP S 2006 Lab Chip 6 843 10.1039/b515128g
- LendlienALangerR 2002 Science 296 1673 10.1126/science.1066102
- ConvertineA JDiabCPrieveMPaschalAHoffmanA SJohnsonP HStaytonP S 2010 Biomacromolecules 11 2904 10.1021/bm100652w
- GoldenA LBattrellC FPennellSHoffmanA SLaiJ JStaytonP S 2010 Bioconjug. Chem. 21 1820 10.1021/bc100169y
- NashM AHoffmanJ MStevensD YHoffmanA SStaytonP SYagerP 2010 Lab Chip 10 2279 10.1039/c004991c
- KushidaAYamatoMKonnoCKikuchiASakuraiYOkanoT 1999 J. Biomed. Mater. Res. 45 355 10.1002/(SICI)1097-4636(19990615)45:4%3C355::AID-JBM10%3E3.0.CO;2-7
- ShimizuTYamatoMIsoiYAkutsuTSetomaruTAbeKKikuchiAUmezuMOkanoT 2002 Circ. Res. 90 e40 10.1161/hh0302.105722
- EbaraMUtoKIdotaNHoffmanJ MAoyagiT 2012 Adv. Mater. 24 273 10.1002/adma.201102181
- SunTWangGFengLLiuBMaYJiangLZhuD 2004 Angew. Chem. Int. Ed. 43 357 10.1002/anie.200352565
- LahannJMitragotriSTranT NKaidoHSundaramJChoiI SHofferSSomorjaiG ALangerR 2003 Science 299 371 10.1126/science.1078933
- ChenGHoffmanA S 1995 Nature 373 49 10.1038/373049a0
- YoshimatsuKLeselB KYonamineYBeierleJ MHoshinoYSheaK J 2012 Angew. Chem. Int. Ed. 51 2405 10.1002/anie.201107797
- MiyataTAsamiNUragamiT 1999 Nature 399 766 10.1038/21619
- JeongBKimS WBaeY H 2002 Adv. Drug Deliv. Rev. 54 37 10.1016/S0169-409X(01)00242-3
- GilE SHudsonS M 2004 Prog. Polym. Sci. 29 1173 10.1016/j.progpolymsci.2004.08.003
- ParkS YBaeY H 1999 Macromol. Rapid Commun. 20 269 10.1002/(SICI)1521-3927(19990501)20:5%3C269::AID-MARC269%3E3.0.CO;2-3
- Ramkissoon-GanorkarCBaudysMKimS W 2000 J. Biomater. Sci. Polym. Ed. 11 45 10.1163/156856200743481
- MatsumotoAIshiiTNishidaJMatsumotoHKataokaKMiyaharaY 2012 Angew. Chem. Int. Ed. 51 2124 10.1002/anie.201106252
- YoshidaRSakaiTHaraYMaedaSHashimotoSSuzukiDMuraseY 2009 J. Control. Release 140 186 10.1016/j.jconrel.2009.04.029
- SawahataKHaraMYasunagaHOsadaY 1990 J. Control. Release 14 253 10.1016/0168-3659(90)90165-P
- NamdeoMBajpaiS KKakkarS 2009 J. Biomater. Sci. Polym. Ed. 20 1747 10.1163/156856208X386372
- SugiuraSSzilagyiASumaruKHattoriKTakagiTFilipcseiGZrinyiMKanamoriT 2009 Lab Chip 9 196 10.1039/b810717c
- ShimobojiTLarenasEFowlerTKulkarniSHoffmanA SStaytonP S 2002 Proc. Natl Acad. Sci. USA 99 16592 10.1073/pnas.262427799
- ShimobojiTLarenasEFowlerTHoffmanA SStaytonP S 2003 Bioconjug. Chem. 14 517 10.1021/bc025615v
- IkedaTTsutsumiO 1995 Science 268 1873 10.1126/science.268.5219.1873
- HosonoNKajitaniTFukushimaTItoKSasakiSTakataMAidaT 2010 Science 330 808 10.1126/science.1195302
- NicholsT CFischerT HDeliargyrisE NBaldwinA Sjr 2001 Ann. Periodontol. 6 20 10.1902/annals.2001.6.1.20
- DavieE WFujikawaKKisielW 1991 Biochemistry 30 10363 10.1021/bi00107a001
- EvansD FPyeGBramleyRClarkA GDysonT JHardcastleJ D 1988 Gut 29 1035 10.1136/gut.29.8.1035
- TechawanitchaiPEbaraMIdotaNAoyagiT 2012 Colloids Surf. B 99 23 10.1016/j.colsurfb.2011.09.039
- TechawanitchaiPEbaraMIdotaNAoyagiT 2012 Soft Matter 8 2844 10.1039/c2sm07277g
- AoyagiTEbaraMSakaiKSakuraiYOkanoT 2000 J. Biomater. Sci. Polym. Ed. 11 101 10.1163/156856200743526
- EbaraMAoyagiTSakaiKOkanoT 2000 Macromolecules 33 8312 10.1021/ma000121j
- EbaraMAoyagiTSakaiKOkanoT 2001 J. Polym. Sci. Polym. Chem. 39 335 10.1002/1099-0518(20010201)39:3%3C335::AID-POLA1000%3E3.0.CO;2-H
- AmesW F 1992 Numerical Methods for Partial Differential Equations 3rd edn Boston , MA Academic
- PenkeBKinseySGibbsS JMoerlandT SLockeB R 1998 J. Magn. Reson. 132 240 10.1006/jmre.1998.1428
- AbbruzzettiSCarcelliMRogolinoDViappianiC 2003 Photochem. Photobiol. Sci. 2 796 10.1039/b301818k
- SwainC GLuptonE C 1968 J. Am. Chem. Soc. 90 4328 10.1021/ja01018a024
- PolitzerPAbrahmsenLSjobergP 1984 J. Am. Chem. Soc. 106 855 10.1021/ja00316a005
- LavallePPicartCMuttererJGergelyCReissHVoegelJ CSengerBSchaafP 2004 J. Phys. Chem. B 108 635 10.1021/jp035740j
- HodaNLarsonR G 2009 J. Phys. Chem. B 113 4232 10.1021/jp809959j