Abstract
The clinical demand for cartilage tissue engineering is potentially large for reconstruction defects resulting from congenital deformities or degenerative disease due to limited donor sites for autologous tissue and donor site morbidities. Cartilage tissue engineering has been successfully applied to the medical field: a scaffold pre-cultured with chondrocytes was used prior to implantation in an animal model. We have developed a surgical approach in which tissues are engineered by implantation with a vascular pedicle as an in vivo bioreactor in bone and adipose tissue engineering. Collagen type II, chitosan, poly(lactic-co-glycolic acid) (PLGA) and polycaprolactone (PCL) were four commonly applied scaffolds in cartilage tissue engineering. To expand the application of the same animal model in cartilage tissue engineering, these four scaffolds were selected and compared for their ability to generate cartilage with chondrocytes in the same model with an in vivo bioreactor. Gene expression and immunohistochemistry staining methods were used to evaluate the chondrogenesis and osteogenesis of specimens. The result showed that the PLGA and PCL scaffolds exhibited better chondrogenesis than chitosan and type II collagen in the in vivo bioreactor. Among these four scaffolds, the PCL scaffold presented the most significant result of chondrogenesis embedded around the vascular pedicle in the long-term culture incubation phase.
1. Introduction
Cartilaginous defects are particularly challenging for reconstructive surgeons. The regeneration potential of damaged articular cartilage has been limited and it is difficult to regain the original hyaline structures of the articular cartilage using conventional treatments including subchondral abrasion, Pridie perforations, microfracture, transplantation of osteochondral plugs and autologous chondrocyte implantation [Citation1–Citation4]. Cartilage grafts have been used in nasal and ear reconstructions and augmentation rhinoplasty with satisfactory results. In some cases, the donor sources of cartilage can be limited due to the large amount of cartilage graft required. Cartilage tissue engineering potentially provides a strategy to repair the cartilage defects or reconstruction without the requirement of autologous cartilage graft. Currently, one of the major challenges in tissue engineering is the identification of the optimal scaffolds. A good scaffold in tissue engineering contains several qualities; it should: (i) consist of a three-dimensional highly porous structure with an interconnected pore network and (ii) be biocompatible and bioresorbable in vivo. Both natural and synthetic polymers have been used in cartilage tissue engineering with varying results. Chitosan is a naturally derived polysaccharide and its structure is similar to some glycosaminoglycans found in cartilages [Citation5]. Chitosan has been applied as a scaffold in cartilage engineering and supports chondrogenic activity. The expression of cartilage extracellular matrix (ECM) proteins has been reported after chondrocyte seeding and culture on chitosan [Citation6, Citation7]. Collagen type II occupies 70% of the cartilage collagen ECM; it has been used as a scaffold to mimic the microenvironment of cartilage for supporting the chondrocyte adhesion, proliferation and synthesis of new and functional tissue [Citation8, Citation9].
Polycaprolactone (PCL) and poly(lactic-co-glycolic acid) (PLGA) are two of the most widely used synthetic biodegradable polymers in tissue engineering. They have received approval from the Food and Drug Administration (FDA) for various clinical and research applications, such as suture materials, wound dressings and stents. Mesenchymal stem cells differentiated into chondrocytes and form cartilage after being seeded in PCL porous scaffolds in response to mechanical stimulation [Citation10]. PLGA has been used as a scaffold in cartilage engineering with promising results, supporting chondrocyte proliferation and differentiation in a concentric cylinder bioreactor under low oxygen tension [Citation10, Citation11].
Bioreactor systems enhance cell seeding on porous scaffolds, the nutrition of cells in the resulting constructs and mechanical stimulation of the developing tissue. An in vivo bioreactor generally produces an adequate tissue substitute, better than an in vitro bioreactor [Citation12]. Engineering tissues in an in vivo bioreactor has been demonstrated to promote cellular colonization, enhance vascularization and the regeneration of a range of musculoskeletal tissue including bone, cartilage, fat and muscle [Citation12–Citation14]. The authors have also successfully developed an animal model with an in vivo bioreactor in adipose and bone tissue engineering [Citation15]. Besides the aforementioned advantages, an animal-based study is closer to the clinical situation and our animal model with an in vivo bioreactor also provides a vascular pedicle, which could be applied for vascularized tissue transfer in a clinical application (under submission).
Several scaffolds have been widely applied in cartilage tissue engineering. Which scaffold has more efficacy on cartilage tissue engineering remains unknown. In this study, we compared the performance of various tissue engineering scaffolds implanted in a silicon chamber around a vascular pedicle to serve as an in vivo bioreactor. The two natural polymers (chitosan and collagen type II) and two synthetic polymers (PCL and PLGA) usually used as scaffolds for cartilage tissue engineering are selected in this study. These scaffolds were seeded with chondrocytes and applied to the bioreactor. Chondrogenesis was evaluated in the scaffolds at various time points after implantation. The optimal scaffold chosen from this study can be used in further clinical applications.
2. Materials and methods
2.1. Materials
Chitosan (Mw = 55000), PLGA (lactide:glycolide = 85:15), PCL (Mn = 45000), acetic acid, sodium chloride, chloroform, phosphate-buffered saline (PBS), collagenase I, insulin-transferrin-sodium selenite media supplement (ITS) and Ficoll® were purchased from Sigma-Aldrich (St Louis, MO, USA). Dulbecco's modified Eagle medium (DMEM) and fetal bovine serum (FBS) were purchased from Gibco-BRL (Gaithersburg, MD). Anti-aggrecan, anti-collagen type II and anti-collagen type I antibodies were obtained from Acris Antibodies GmbH (Hiddenhausen, Germany). Genipin was purchased from Challenge Bioproducts (Taiwan).
2.2. Collagen type II and chitosan sponge fabrication and crosslinking with genipin
Collagen type II was isolated from bovine trachea using the method previously described in [Citation8]. In order to prepare scaffolds, 0.5% collagen type II and 1% chitosan were dissolved in 0.05 M acetic acid, respectively. A 100 μl solution was placed in a chamber, with a diameter of 8 mm, to form in sponge using a freeze-drying method. Collagen type II and chitosan sponges were washed with 70% alcohol three times and put into 100% alcohol with 0.25% genipin for 24 h in 37 °C incubator. These sponges were washed with deionized water three times to remove excess genipin.
2.3. PLGA and PCL scaffolds fabrication
PLGA and PCL scaffolds were fabricated using a solvent casting/particulate leaching method [Citation16]. Sodium chloride particles, 170–300 μm in diameter, were mixed with a 66.7% (w/v) chloroform solution of PLGA or PCL, with the mass ratio of sodium chloride/polymer at 8:1 (w/w). The polymer/sodium chloride mixture was poured into a ring-shape mold (height = 5 mm, inner diameter = 7 mm and outer diameter = 14 mm) and dried in a vacuum for 24 h to remove the solvent. Then salt was leached from the scaffolds into deionized water during 3 days immersion, changing the water three times a day. After being washed with deionized water, the scaffolds were dried under air and a vacuum. The porous scaffolds were obtained and sterilized with UV irradiation for 1 h.
2.4. Scaffold characterization
The scaffold morphology was observed by scanning electron microscopy (SEM). Both surface and cross-sections were examined. The cross-sections were obtained by fracturing after freezing in liquid nitrogen. The samples were gold-coated and observed at an accelerating voltage of 6 kV. Pore sizes were measured in SEM images through the NIH Image J software [Citation17, Citation18]. Scaffold porosity was measured using the liquid displacement method with absolute ethanol in compliance with the method published in [Citation19].
2.5. Cell isolation and culture
Chondrocytes were isolated from the ear cartilage of Sprague–Dawley rats and digested by collagenase using procedures described in [Citation29]. In brief, ear cartilage was harvested from Sprague–Dawley rats (weighing 150 g) and rinsed with PBS. The perichondrium was removed using sterile scalpels. Then the cartilage was finely minced, transferred into a 2 ml tube with an enzyme solution of 2.5 mg ml−1 collagenase I and 1% penicillin–streptomycin. The diced cartilage was digested for 4 h at 37 °C. The digests were filtered through a 70 μm nylon mesh (Falcon: Franklin Lake, NJ), centrifuged at 750 g for 5 min and washed twice with PBS (pH = 7.4). The cell pellets were re-suspended in an expansion medium (DMEM containing 10% FBS and 1% penicillin) and plated in 10 cm tissue culture dishes. The cells were incubated at 37 °C in a 5% CO2 atmosphere; each dish was replenished with 10 ml of medium every 3 days. After 1 week of primary culture, each dish of cells was subcultured into five dishes. Passage 3 chondrocytes were used in this study.
2.6. Cell-scaffold construct preparation
The chondrocytes were seeded onto the four types of porous scaffolds (107 cells per scaffolds). After cell embedding, porous scaffolds were incubated with the chondrogenic defined medium, DMEM with ITS: 6.25 μg ml−1 insulin, 6.25 μg ml−1 transferring, 5.33 μg ml−1 linoleic acid, 1.25 mg ml−1 bovine serum albumin, 100 nM dexamethasone, 100 μg ml−1 sodium pyruvate, 50 μg ml−1 ascorbic acid, 40 μg ml−1 l-proline, penicillin (100 U ml−1) and streptomycin (100 U ml−1). Non-adherent cells were removed by changing the media after 2 days. Cell-constructs were incubated for 1 week in vitro as a pre-culture.
2.7. Animal model
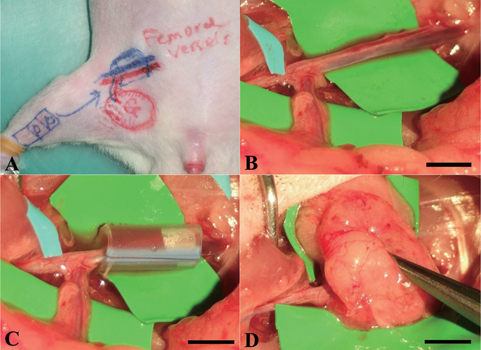
All animal procedures complied with the Chang Gung Memorial Hospital animal research guidelines. Sprague–Dawley rats weighing 250–400 g were used for in vivo studies using a similar animal model based on implantation around an avascular pedicle, as published in [Citation20, Citation21]. Rats were anesthetized with isoflurane and the groin area was shaved and sterilized with 10% beta iodine. A 3 cm incision was made along the groin region and, by careful dissection and hemostasis, a pedicled groin cutaneous flap was elevated based on the inferior epigastric vessels. The femoral artery and veins, 1.5 cm in length, were dissected and the small side branches were ligated. The femoral nerve was carefully dissected away from the vessels and preserved.
A silicone tube (3.3 mm inner diameter and 8 mm long) was wrapped around the pedicle. The cell-scaffold constructs were placed inside the chamber, in which the femoral vessels were already included; then the chamber was closed with two stitches of 6–0 prolene. A pedicled groin flap was harvested based on the inferior epigastric vessels and was used to wrap around the chamber to secure and seal-off the chamber from the adjacent tissues. The incision was closed using 4–0 nylon (figure ). Three milliliters of normal saline was given by intraperitoneal injection for each rat after the surgery. The rats were warmed with heating pads and a heating lamp during the recovery period. Animals were monitored daily for post-operative complications.
Figure 1. Surgical procedures of the in vivo bioreactor animal model. (A) Design of recipient site and pedicle groin flap. (B) The femoral artery and vein were exposed and other small branches were ligated. (C) A silicon tube contained cell-construct was wrapped around the pedicle. (D) The flap was sutured to the abdominal muscle to minimize chamber movement. Scale bars = 5 mm.
2.8. Histological evaluation
After being harvested, the cell-scaffold constructs were rinsed with PBS and fixed in 10% formalin for 24 h. The samples were then embedded in paraffin and sectioned into 4 μm-thick sections. Later, the sections were stained with hematoxylin and eosin (H&E) and Alcian blue using standard protocols.
2.9. Immunohistochemistry staining
Collagen type II and aggrecan were selected as biomarkers for chondrogenesis, whereas collagen type I was used as an osteogenesis marker. Briefly, deparaffinized sections were incubated in 10 mM sodium citrate (pH = 6) at 121 °C for 3 min and then cooled down to room temperature. The sections were incubated in 0.3% hydrogen peroxide with methanol at room temperature for 10 min to remove endogenous peroxidase. After the PBS wash step, non-specific antibody binding was blocked by incubating sections in protein blocking buffer from the supersensitive polymer-HRP IHC detection system (Biogenex, Fremont, CA) at room temperature for 10 min. For immunohistochemistry, slides were incubated overnight at 4 °C with either aggrecan (1:200), collagen type II (1:200) or collagen type I (1:200) in PBS buffer (pH = 7.6). Sections were incubated with the Super Enhancer™ for 30 min, followed by treatment with the Supersensitive Polymer-HRP IHC detection system. Finally, the sections were counterstained with hematoxylin for 3 min, washed with deionized water and mounted with a mounting medium. Microscopic images were photographed with a microscopy Olympus BX51 system and analyzed with Abode Photoshop CS5.
2.10. cDNA synthesis and quantitative real time-PCR (Q-PCR) analysis
Aggrecan and collagen type II were selected as biomarkers of chondrogenesis; collagen type I and osteocalcin were used for osteogenesis evaluation. The cell-scaffold constructs were cut into fragments and total RNA was extracted with a 1 ml TRIzol reagent. The TRIzol mixtures were centrifuged and the supernatant was precipitated by a series of solvents before being quantified using the Quant-iT™ RNA Assay Kit, following the manufacturer's instructions. The total RNA was then reverse-transcribed to cDNA using the MMLV High-Performance Reverse Transcriptase (Epicentre, USA), according to the manufacturer's instructions. Real-time PCR assays were performed using the SYBR® Green PCR Master Mix (Applied Biosystems, USA) according to the manufacturer's instructions; they were monitored using an ABI Prism 7300 real-time PCR system (Applied Biosystems, USA). The mRNA expression levels of the target genes were normalized by dividing their values with the mRNA levels of glyceraldehyde-3-phosphate dehydrogenase (GAPDH). The thermal cycling conditions were 95 °C for 15 min, followed by 40 cycles at 95 °C for 15 s and at 60 °C for 1 min. The primers used were as follows. Primer sequence GAPDH forward primer: 5′-AATGTATCCGTTGTGGATCTGACA-3′, reverse primer: 5′-AGCCCAGGATGCCCTTTAGT-3′; aggrecan forward primer: 5′-CTAGCTGCTTAGCAGGGATAACG-3′, reverse primer: 5′-TGACCCGCAGAGTCACAAAG-3′; collagen type II forward primer: 5′-GAGTGGAAGAGCGGAGACTACTG-3′, reverse primer: 5′-CTCCATGTTGCAGAAGACTTTCA-3′; collagen type I forward primer: 5′-CGATTCACCTACAGCACGCT-3′, reverse primer: 5′-TCCATTCCGAATTCCTGGTCT-3′; osteocalcin forward primer: 5′-GAGCTAGCGGACCACATTGG-3′, reverse primer: 5′-TCGAGTCCTGGAGAGTAGCCA-3′.
2.11. Statistical analysis
All the data are presented as mean values ± the standard deviation in each independent experiment. Differences were considered significant when the p value <0.05, based on the Bonferroni t-test for a comparison of two samples. All the analyses were performed with SPSS ver. 11.0 (SPSS Inc., Chicago, IL, USA).
3. Results
3.1. Cell-scaffold constructs
The images in figure show the structure of the four different scaffolds under SEM. A highly porous structure of interconnected pores can be seen in all four scaffolds. In synthetic scaffolds, the use of a freeze drying procedure resulted in smaller pores than the use of salt leaching methods. The mean pore size of chitosan and collagen II scaffolds was 80 ± 24 μm, while the mean pore size of the PCL and PLGA scaffolds was 150 ± 43 μm.
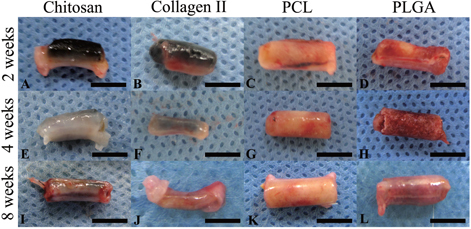
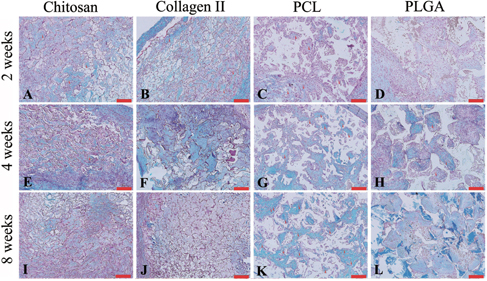
After in vivo implantation, the scaffolds degraded with time. The images in figure revealed the gross appearance of the cell-scaffold constructs harvested at different time points. The natural scaffolds were almost completely degraded after 8 weeks, while the degradation of the synthetic materials (PCL and PLGA) was incomplete; the construct maintained the original chamber shape and size even 8 weeks after implantation.
Figure 3. Chondrocytes were seeded onto chitosan (A), (E), (I), collagen type II (B), (F), (J), PCL (C), (G), (K) and PLGA (D), (H), (L), respectively and cultured in the in vivo bioreactor for 2 (A)–(D), 4 (E)–(H) and 8 (I)–(L) weeks. The gross examination pictures showed degradation of materials at different time points. Scale bars = 5 mm.
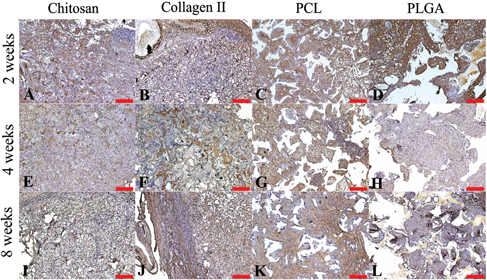
3.2. Histology and immunohistochemistry staining
The H&E staining of each group are shown in figure . Cartilage was observed at 2 weeks in each group. Cartilaginous structures observed in chitosan and collagen II groups decreased after 2 weeks resulting in few or no cartilaginous structures left at 8 weeks. On the contrary, the cartilaginous structure was maintained in the PCL and PLGA groups at 8 weeks.
Figure 4. H&E staining of chitosan (A), (E), (I), collagen type II (B), (F), (J), PCL (C), (G), (K), and PLGA (D), (H), (L) showed cell morphology at 2 (A)–(D), 4 (E)–(H) and 8 (I)–(L) weeks. Scale bars = 50 μm.
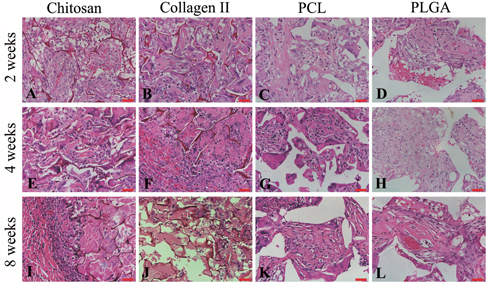
These findings were further supported by Alcian blue staining. Considerable amounts of glycosaminoglycan and proteoglycans had shown in all specimens (figure ). The Alcian blue staining was shown earlier in the natural scaffold groups than in the synthetic scaffold groups. At 2 weeks, glycosaminoglycan was shown more in the chitosan and collage type II groups but not in the PCL or PLGA groups. At the 4-week time point, positive staining was present in each group. However, the positive staining area decreased gradually and was rarely detected at 8 weeks. In contrast to the natural scaffolds, the area of Alcian blue staining increased at later time points in the synthetic scaffold groups. Hardly any staining was observed in the 2-week group and later the staining increased in 4 weeks in both the PCL and PLGA groups. The staining area consistently increased and remained present in the 8-week group in both the PCL and PLGA groups. The results indicate that more glycosaminoglycan was detected at the later time point in the synthetic scaffolds.
Figure 5. Alcian blue staining showed proteoglycan accumulation (blue color) in chitosan (A), (E), (I), collagen type II (B), (F), (J), PCL (C), (G), (K) and PLGA (D), (H), (L) at 2 (A)–(D), 4 (E)–(H) and 8 (I)–(L) weeks. Scale bars = 100 μm.
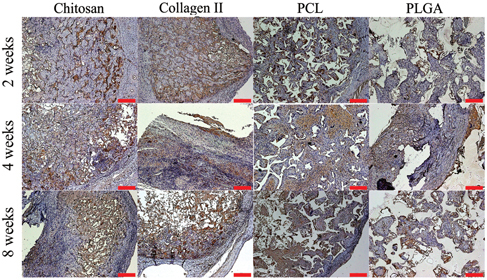
Aggrecan is a critical component for cartilage structure and the function of joints; it plays an important role in mediating chondrocyte–chondrocyte and chondrocyte–matrix interactions through its ability to bind hyaluronan [Citation22]. The images in figure present the immunohistochemistry staining for aggrecan in each group at each time point. The aggrecan started to appear in each group at 2 weeks. The amount of aggrecan in the collagen type II scaffold was the highest one among these four scaffolds. In 8 weeks, aggrecan still remained present in each group; yet the amount of aggrecan was increased in the synthetic scaffold groups at later time points. This phenomenon was even more dramatic in the PCL group at the 8-week time point, in which more stained areas were found compared to the other groups.
Figure 6. Representative images of chitosan (A), (E), (I), collagen type II (B), (F), (J), PCL (C), (G), (K) and PLGA (D), (H), (L) stained for aggrecan (brown color) after harvest from an in vivo bioreactor at 2 (A)–(D), 4 (E)–(H) and 8 (I)–(L) weeks. Scale bars = 100 μm.
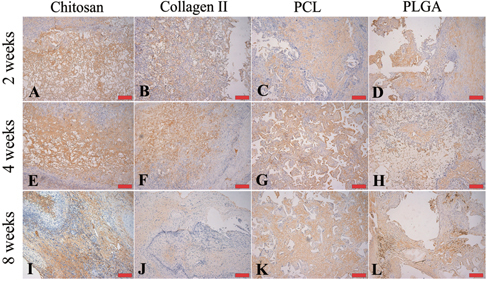
Collagen type II protein is the basis of articular and hyaline cartilage. Collagen type II protein forming fibrils in natural tissue was selected as a marker for chondrogenesis in this study. The images in figure reveal the staining of collagen type II in each group at different time points. The expression of collagen type II protein, which was stained a brown color, appeared in each specimen and time point. At 2 weeks, collagen type II protein was found more in the natural scaffold groups, but collagen type II protein was not observed in the natural scaffold groups at 8 weeks. In the groups with synthetic scaffolds, the presence of collagen type II increased with time. In the 4-week group, fibrillar networks were observed. This phenomenon was also observed in the PCL and PLGA groups; however, the content of the brown-colored fibrillar networks was higher in the PCL than in the PLGA groups.
Figure 7. Representative images of chitosan (A), (E), (I), collagen type II (B), (F), (J), PCL (C), (G), (K) and PLGA (D), (H), (L) stained for collagen type II (brown color) after harvest from an in vivo bioreactor at 2 (A)–(D), 4 (E)–(H) and 8 (I)–(L) weeks. Scale bars = 100 μm.
Collagen type I protein, which is usually found in fibrocartilage and bone, was detected in all the groups at 2 weeks. The collagen type I protein remained at 8 weeks, except in the PLGA scaffold group. Collagen type I was almost gone in the PLGA group at 8 weeks. The presence of collagen type I deceased with time and showed a higher level in the collagen type II group compared to other groups at 8 weeks (figure ).
3.3. Gene expression
Aggrecan and collagen type II were used as markers for chondrogenesis (figures (A) and (B)). The aggrecan gene expression was higher in PCL and PLGA scaffolds at the 2-week time point in comparison to the natural scaffold groups (p < 0.05). The gene expression of aggrecan in PCL and PLGA scaffolds down-regulated with time, from 4.2 ± 1.3 and 3.9 ± 1.2 to 1.4 ± 1.2 and 1.3 ± 1.2 (p < 0.001). However, the gene expression had no statistical differences between the synthetic scaffold and the natural scaffold after 4 weeks.
Figure 9. The gene expression of cell-constructs harvested from an in vivo bioreactor at 2, 4 and 8 weeks. The mRNA level of cell-constructs was analyzed with a chondrogenic gene marker (aggrecan (A) and collagen type II (B)) and osteogenic gene marker (collagen type I (C) and osteocalcin (D)), n = 5. ∗p < 0.05, ∗∗p < 0.01, ∗∗∗p < 0.001.
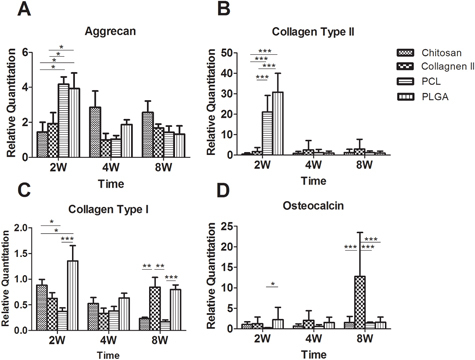
The gene expression of collagen type II was similar with that of aggrecan but was significantly different between chondrocytes in natural and synthesis scaffolds at 2 weeks. The collagen type II expressed 21 ± 8 in PCL groups and 30 ± 9 in PLGA groups at 2 weeks and reduced to 1.2 ± 1.6 in PCL groups and to 0.9 ± 0.9 in PLGA groups at 4 weeks, respectively. The collagen type II gene expression appeared in other groups with time.
Collagen type I and osteocalcin were used as markers for osteogenesis. Figures (C) and (D) were the expression of collagen type I and osteocalcin in each group at each time point. The expression of collagen type I was found to be higher in the PLGA group than in the other groups, with significant differences at the 2-week time point. The expression of collagen type I was lower in the PCL group (0.4 ± 0.2) relative to the other groups initially and decreased gradually. The gene expression of collagen type I decreased with time in all groups except in the chitosan scaffold group. In the 8-week groups, the gene expression of collagen type I in the collagen type II scaffold and the PLGA scaffold were significantly higher than that in the other two groups. The osteocalcin gene expression remained low in each group at each time point. However, it increased from 1.3 ± 1.2 at 2 weeks to 13 ± 7 at 8 weeks in the group using collagen type II as the scaffold.
4. Discussion
Cartilage tissue engineering has the potential to be a good option for damaged cartilage repair or ear/nose reconstructions. Chondrogenesis has been reported using various scaffolds with chondrocytes or chondrocytes and stem cells, yet without promising results or clinical applications due to the inconsistent results and the difficulty in obtaining a controlled three-dimensional structure. To engineer the cartilage, cell sources and scaffolds are equally important. Besides, obtaining a reliable blood supply to the tissue may help to provide nutrients for tissue growth and potentially assist with safe tissue transferring. In our previous studies, we had applied an animal model with the vascular pedicle in adipose and bone engineering with promising results [Citation15, Citation21]. In this study, a similar animal model was applied to evaluate the efficacy of the inclusion of a vascular pedicle for cartilage engineering. The vascular pedicle acts as a bioreactor, which may enhance and stimulate cell migration and proliferation by supplying nutrients and the possible secretion and delivery of micro messages to the cells [Citation23]. Furthermore, the animal model was designed using a silicon chamber to wrap around the femoral vessels and a pedicled groin flap to cover the silicon chamber, to create a closed system and ensure the in vivo study is not affected by the adjacent tissues except the vascular pedicle itself. This is a new model for cartilage engineering; in this pilot study, various natural and synthetic scaffolds were included.
In this model, natural scaffolds were found to degrade faster than synthetic scaffolds in gross appearance and under the H&E staining, despite the increased crosslinking from the genipin use. This is probably due to the fact that the natural property of the collagen type II, chitosan containing protein and proteoglycans, which are easy to digest in vivo. The degradation of PLGA scaffolds was faster than that of PCL scaffolds. The structure of PLGA has more of a hydrophilic side chain compared to PLGA, potentially facilitating hydrolysis in the in vivo environment [Citation24].
In the immunohistochemistry staining, the groups with natural scaffolds had shown better chondrogenesis in the early phase (2 weeks); however, chondrogenesis was not maintained to 8 weeks. In contrast to natural scaffolds, the synthetic scaffold groups had slower chondrogenesis but maintained it up to the later time points. This may be due to the early degradation of the natural scaffolds. Early degradation may result in an inadequate structure supporting the chondrogenesis before the cells grow into the planned three-dimensional structure.
Regarding gene expressions of cartilage engineering in the in vivo study, the presence of aggrecan and collagen type II indicated chondrogenesis in all the groups. However, the gene expression was not totally in line with the immunohistochemistry staining of aggrecan and collagen type II protein. Aggrecan and collagen type II gene expression in the groups with PCL and PLGA scaffolds were significantly higher than that of chitosan and collagen II scaffold groups in the first 2 weeks. Compared to a natural scaffold, the hydrophobic character of the synthetic scaffold [Citation25, Citation26] delayed their degradation and may reduce the hormone effects or metabolism of the materials and cells/proteins from inside the host tissue. This character keeps chondrocytes in synthetic scaffolds to maintain their original property and forms cartilage tissue slowly with culturing in an in vivo bioreactor. Therefore, the aggrecan and collagen type II proteins from the natural scaffolds were metabolized faster and the protein level presented lower than that of the groups with the synthetic scaffolds at 8 weeks.
With time, chondrocytes may turn into osteocytes in the bioreactor. This is more prominent when the cells are exposed to an environment with a rich blood supply. The osteocyte gene markers were used to determine the differentiation of osteocyte after being embedded around the vessels. The expression of the osteocyte gene marker had shown higher levels in groups with collagen type II scaffolds. Compared to other scaffolds used in this study, collagen type II scaffolds were digested with an enzyme into fibers faster than other scaffolds. After being digested into collagen fibers, the chondrocytes seeded into collagen type II scaffolds were then released from the scaffold into the silicon chamber containing the vascular pedicle. The vascular pedicle itself or the newly grown small vessels from angiogenesis may induce the ossification of the chondrocytes. Besides, there were also pellet formations of chondrocytes in the group with PCL scaffolds noted from the histology. The pellet was close to the growth plate of the articular joint and had been a good in vitro model of cartilage mineralization [Citation27]. Chondrocytes in the growth plate have a unique character: the cells migrated from cartilage into bone by calcification. Cartilages, such as hyaline cartilage, were normally not calcified [Citation28, Citation29]. Besides, the chondrocytes in the PCL scaffold group in this animal model were found to slowly transform into cartilage tissue. The formation of cartilage tissue in an in vitro environment is affected by the cell number, cell proliferation rate and matrix production. While there were too many adjacent tissues surrounding the cell-scaffold construct, the excessive tissue may preclude sufficient nutritional support coming from the outer environment into the scaffold. The relatively insufficient nutrition/blood supply then precluded cell proliferation, differentiation and the production of an extracellular matrix (ECM) [Citation30]. Compared to previous in vitro studies, our animal model with a vascular pedicle provides a better blood supply/nutrients for the cells seeded into PCL scaffolds. Further, the pedicled adipose flap wrapping around the silicon chamber prevented excessive tissue growing into the chamber and interfered with the system of tissue engineering. The PCL scaffold group presented the higher level of aggrecan and collagen type II protein at the 8 weeks time point compared to other groups. Although similar pellet formation was also found in groups with a PLGA scaffold, there was less chondrogenic protein expressed in groups with the PLGA scaffold. A possible reason may come from the metabolism products of PLGA. After degradation, the metabolite of the PLGA scaffold caused an acidic environment in the chamber, which inhibited cell viability and tissue development [Citation24]. At early time points, more aggrecan and collagen type II proteins were found in chitosan and collagen type II scaffold groups. The result may be caused due to the pre-culture with the chondrogenic medium for 1 week prior to implantation. With the faster degradation, the chondrogenic proteins were undetectable in the immunohistochemistry staining at 8 weeks.
In this study, the animal model with a bioreactor was applied using four various scaffolds. Among these four scaffolds, PCL scaffolds had the most long lasting results. This animal model with an in vivo bioreactor was developed to enhance tissue regeneration by the inclusion of a vascular pedicle. The pedicle could be further applied in clinical applications used for tissue transfer. A similar animal model has not been used in cartilage tissue engineering. The inclusion of vascular pedicle may raise the concern regarding osteogenesis in cartilage tissue engineering. However, the inclusion of a vascular pedicle may also enhance cartilage formation by providing a better blood supply/nutrients. Further study will be conducted and the transfer of cartilage tissue engineered using this animal model will be designed to repair cartilage defects in the animal model.
5. Conclusions
We developed and evaluated cartilage tissue engineering strategies in a new animal model of an in vivo bioreactor for cartilage engineering. In this scaffold comparison study, synthetic and natural matrices were applied as scaffolds. The results indicate that synthetic scaffolds could keep chondrocyte and form cartilage tissue with a more long-lasting result. PCL scaffolds with chondrocytes are more suitable in this system and further study will be conducted using PCL as scaffolds in the same animal model with an in vivo bioreactor.
Acknowledgment
This work was supported by the Taiwan National Science Council (NSC99-2314-B-182A-019-MY3) and the United States Veterans Administration.
References
- SimonT MJacksonD W 2006 Articular cartilage: injury pathways and treatment options Sports Med. Arthrosc. 14 146 154 146–54 10.1097/00132585-200609000-00006
- GilbertJ E 1998 Current treatment options for the restoration of articular cartilage Am. J. Knee Surg. 11 42 46 42–6 9533054
- O'DriscollS W 1998 The healing and regeneration of articular cartilage J. Bone Joint Surg. Am. 80 1795 1812 1795–812
- HunzikerE B 1999 Articular cartilage repair: are the intrinsic biological constraints undermining this process insuperable? Osteoarthritis Cartilage 7 15 28 15–28 10.1053/joca.1998.0159
- Di MartinoASittingerMRisbudM V 2005 Chitosan: a versatile biopolymer for orthopaedic tissue-engineering Biomaterials 26 5983 5990 5983–90 10.1016/j.biomaterials.2005.03.016
- KuoY CLinC Y 2006 Effect of genipin-crosslinked chitin-chitosan scaffolds with hydroxyapatite modifications on the cultivation of bovine knee chondrocytes Biotechnol. Bioeng. 95 132 144 132–44 10.1002/bit.21007
- KimS EParkJ HChoY WChungHJeongS YLeeE BKwonI C 2003 Porous chitosan scaffold containing microspheres loaded with transforming growth factor-beta1: implications for cartilage tissue engineering J. Control Release 91 365 374 365–74 10.1016/S0168-3659(03)00274-8
- KoC SHuangJ PHuangC WChuI M 2009 Type II collagen-chondroitin sulfate-hyaluronan scaffold cross-linked by genipin for cartilage tissue engineering J. Biosci. Bioeng. 107 177 182 177–82 10.1016/j.jbiosc.2008.09.020
- LinC HSuJ MHsuS H 2008 Evaluation of type II collagen scaffolds reinforced by poly(epsilon-caprolactone) as tissue-engineered trachea Tissue Eng. C Methods 14 69 77 69–77 10.1089/tec.2007.0336
- TortelliFCanceddaR 2009 Three-dimensional cultures of osteogenic and chondrogenic cells: a tissue engineering approach to mimic bone and cartilage in vitro Eur. Cell Mater. 17 1 14 1–14 19579210
- SainiSWickT M 2004 Effect of low oxygen tension on tissue-engineered cartilage construct development in the concentric cylinder bioreactor Tissue Eng. 10 825 832 825–32 10.1089/1076327041348545
- McCullenS DChowA GStevensM M 2011 In vivo tissue engineering of musculoskeletal tissues Curr. Opin. Biotechnol. 22 715 720 715–20 10.1016/j.copbio.2011.05.001
- StevensM MMariniR PSchaeferDAronsonJLangerRShastriV P 2005 In vivo engineering of organs: the bone bioreactor Proc. Natl Acad. Sci. USA 102 11450 11455 11450–5 10.1073/pnas.0504705102
- EmansP Jvan RhijnL WWeltingT JCremersAWijnandsNSpaapenFVonckenJ WShastriV P 2010 Autologous engineering of cartilage Proc. Natl Acad. Sci. USA 107 3418 3423 3418–23 10.1073/pnas.0907774107
- UrielSHuangJ JMoyaM LFrancisM EWangRChangS YChengM HBreyE M 2008 The role of adipose protein derived hydrogels in adipogenesis Biomaterials 29 3712 3719 3712–9 10.1016/j.biomaterials.2008.05.028
- YoshiokaTKawazoeNTateishiTChenG 2008 In vitro evaluation of biodegradation of poly(lactic-co-glycolic acid) sponges Biomaterials 29 3438 3443 3438–43 10.1016/j.biomaterials.2008.04.011
- WoodfieldT B FVan BlitterswijkC ADe WijnJSimsT JHollanderA PRiesleJ 2005 Polymer scaffolds fabricated with pore-size gradients as a model for studying the zonal organization within tissue-engineered cartilage constructs Tissue Eng. 11 1297 1311 1297–311 10.1089/ten.2005.11.1297
- GriffonD JSedighiM RSchaefferD VEurellJ AJohnsonA L 2006 Chitosan scaffolds: interconnective pore size and cartilage engineering Acta Biomater. 2 313 320 313–20 10.1016/j.actbio.2005.12.007
- GuanJFujimotoK LSacksM SWagnerW R 2005 Preparation and characterization of highly porous, biodegradable polyurethane scaffolds for soft tissue applications Biomaterials 26 3961 3971 3961–71 10.1016/j.biomaterials.2004.10.018
- CroninK JMessinaAKnightK RCooper-WhiteJ JStevensG WPeningtonA JMorrisonW A 2004 New murine model of spontaneous autologous tissue engineering, combining an arteriovenous pedicle with matrix materials Plast. Reconstr. Surg. 113 260 269 260–9 10.1097/01.PRS.0000095942.71618.9D
- MoyaM LChengM HHuangJ JFrancis-SedlakM EKaoS WOparaE CBreyE M 2010 The effect of FGF-1 loaded alginate microbeads on neovascularization and adipogenesis in a vascular pedicle model of adipose tissue engineering Biomaterials 31 2816 2826 2816–26 10.1016/j.biomaterials.2009.12.053
- KianiCChenLWuY JYeeA JYangB B 2002 Structure and function of aggrecan Cell Res. 12 19 32 19–32 10.1038/sj.cr.7290106
- PortnerRNagel-HeyerSGoepfertCAdamietzPMeenenN M 2005 Bioreactor design for tissue engineering J. Biosci. Bioeng. 100 235 245 235–45 10.1263/jbb.100.235
- SungH JMeredithCJohnsonCGalisZ S 2004 The effect of scaffold degradation rate on three-dimensional cell growth and angiogenesis Biomaterials 25 5735 5742 5735–42 10.1016/j.biomaterials.2004.01.066
- HasirciVBerthiaumeFBondreS PGresserJ DTrantoloD JTonerMWiseD L 2001 Expression of liver-specific functions by rat hepatocytes seeded in treated poly(lactic-co-glycolic) acid biodegradable foams Tissue Eng. 7 385 394 385–94 10.1089/10763270152436445
- ChenHFanXXiaJChenPZhouX JHuangJYuJGuP 2011 Electrospun chitosan-graft-poly (epsilon-caprolacton)/poly (epsilon-caprolacton) nanofibrous scaffolds for retinal tissue engineering Int. J. Nanomed. 6 453 461 453–61 10.2147/IJN.S17057
- KatoYIwamotoMKoikeTSuzukiFTakanoY 1988 Terminal differentiation and calcification in rabbit chondrocyte cultures grown in centrifuge tubes: regulation by transforming growth factor beta and serum factors Proc. Natl Acad. Sci. USA 85 9552 9556 9552–6 10.1073/pnas.85.24.9552
- ZhangZMcCafferyJ MSpencerR GFrancomanoC A 2004 Hyaline cartilage engineered by chondrocytes in pellet culture: histological, immunohistochemical and ultrastructural analysis in comparison with cartilage explants J. Anat. 205 229 237 229–37 10.1111/j.0021-8782.2004.00327.x
- PacificiMKoyamaEIwamotoMGentiliC 2000 Development of articular cartilage: what do we know about it and how may it occur? Connective Tissue Res. 41 175 184 175–84 10.3109/03008200009005288
- JeongC GHollisterS J 2010 A comparison of the influence of material on in vitro cartilage tissue engineering with PCL, PGS, and POC 3D scaffold architecture seeded with chondrocytes Biomaterials 31 4304 4312 4304–12 10.1016/j.biomaterials.2010.01.145