Abstract
The magnetic field effect (MFE) in conjunction with laser flash photolysis has been used for the study of the interaction of one of the small drug like quinone molecules, 2-methyl, 1,4-naphthoquinone, commonly known as menadione (MQ), with one of the DNA bases, thymine (THN), and its corresponding nucleoside, thymidine (THDN), in acetonitrile (ACN) and sodium dodecylsulfate (SDS) micelles. It has been observed that THN undergoes electron transfer (ET) and hydrogen (H) abstraction with MQ, while THDN undergoes only H abstraction in both the media. However, our earlier studies showed that a purine base, adenine (ADN), and its nucleoside, 2′-deoxyadenosine (ADS), undergo ET in ACN and H abstraction in SDS. Here we have attempted to explain the differences in the reactions of these DNA bases with MQ. We also reveal the crucial role of a sugar unit in altering the behavior of purine and pyrimidine bases with respect to ET and H abstraction.
Introduction
In biological systems the quinones are extremely important compounds, because when attached to proteins they can act as prosthetic groups and participate in electron and hydrogen (H) transfer reactions [Citation1–3]. In the present study, we have chosen one such quinone, menadione (MQ, 2-methyl, 1,4-naphthoquinone), which is well known for its anticancer activity [Citation4], to study its interaction with one of the DNA bases, thymine (THN), a pyrimidine base, and its nucleoside, thymidine (THDN). We have focused our experiments on two very different reactions, electron transfer (ET) and H abstraction between MQ and THN and THDN using laser flash photolysis coupled with an ac magnetic field. Quinones can accept a hydrogen atom or electron during H abstraction or ET producing radical pairs (RPs) or radical ion pairs (RIPs), respectively [Citation5, Citation6]. The H abstraction and ET involve a change in the number of unpaired spins, and this makes it possible to manipulate the reaction dynamics in the presence of a magnetic field (MF) [Citation5–8]. The electron spin multiplicity of the excited state (singlet (S) or triplet (T)) is generally preserved during H abstraction or ET, and a memory of the initial spin multiplicity is retained in the resulting RP or RIP until the latter undergoes further reactions. The nature of the products formed is spin-dependent. A singlet-born RP or RIP favors recombination, whereas a triplet-born RP/RIP favors the formation of a free-radical or radical ion. In solution, when the partners of a geminate RP/RIP are separated by diffusion to such an extent that the exchange interaction becomes zero, an intersystem crossing occurs between S and T±, T0 states by an electron-nuclear hyperfine interaction acting as an internal MF. An external MF removes the degeneracy of T± with S and T0, thereby reducing the intersystem crossing (ISC). The overall effect is an increase in the population of the initial spin state and its corresponding products, as mentioned before.
Since in living systems, one of the fundamental molecules is DNA, it is very important to elucidate a possible mechanism for the interaction between this model drug and any nucleoside or DNA base. In our earlier communication we reported, for the first time, a laser flash photolysis study and the effect of a magnetic field on the interaction between one of the DNA bases, adenine (ADN), and its nucleoside, 2′-deoxyadenosine (ADS), where H transfer is competitive with ET, particularly in a sodium dodecyl sulfate (SDS) micellar medium [Citation4]. Here we have chosen another base and its corresponding nucleoside, THN and THDN, respectively, because these have considerably different structures from ADS and ADN, which contain a purine moiety rather than the pyrimidine moiety found in THN and THDN. Our main objective is to show the effect of the structure and size of the DNA bases on their reaction with quinone drugs; here we consider MQ in homogeneous acetonitrile (ACN) and a SDS micellar medium.
Experimental
Materials
Menadione (MQ), THN, THDN and SDS were purchased from Sigma. UV-spectroscopy-grade ACN was obtained from Spectrochem and used without further purification. Water used for the preparation of solutions was triply distilled. The chemical structures of the compounds used in this work are shown in scheme 1.
Spectral methods
The excitation light used was the third harmonic (355 nm) of a Nd:YAG laser (DCR – 11, Spectra Physics) with a duration of 8 ns. The analyzing light was obtained from a 250 W xenon lamp. The laser and analyzing light beams, intersecting at right angles, passed through a quartz cell with 1 cm2 cross section. A monochromator equipped with an IP28 photomultiplier was used to analyze transient absorption (Applied Photophysics). The signals from the photomultiplier were displayed and recorded as a function of time on a Tektronix 500 MHz oscilloscope (1 Gs s−1 sampling rate). Each data point was obtained by averaging multiple results to improve the signal-to-noise ratio. The transient absorption was obtained from a series of oscilloscope traces measured using the same solution in a point-by-point manner with respect to the wavelength using the Origin 5.0 software. The samples were deaerated by passing pure argon gas for 20 min through the solutions prior to each experiment. No degradation of the samples was observed during the experiments. The strength of the MF used was 0.08 T [Citation9].
Results and discussion
Transient absorption spectra of triplet and RP/RIP by laser flash photolysis
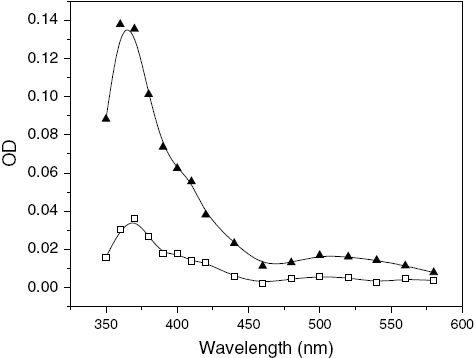
The transient absorption spectra of argon-saturated solutions of MQ and a mixture of MQ and THN in ACN irradiated separately by a 355 nm laser pulse after 1 μ s time delay are shown in figure . The spectrum obtained from the excitation of MQ solution represents the T–T absorption of MQ with a maximum at 370 nm and a hump around 510 nm. The transient absorption spectrum obtained from the flash photolysis of MQ in the presence of THN exhibits maximum absorption at 370 nm with a distinct hump at 420 nm. The region above 500 nm also exhibits significant absorption. In our earlier work on the interaction of photoexcited MQ with organic amines, we reported that the maxima at 370 nm and 420 nm are due to the simultaneous formation of MQ•− and MQH• [Citation10]. MQ•− also exhibits a hump around 490 nm. This hump around 500 nm can also be assigned to the radical cation generated upon ET from THN, i.e., THN•+, respectively [Citation11, Citation12]. Geimer et al reported the formation of a thymine-1-yl radical due to the rapid deprotonation of the initially formed THN• + [Citation13]. The distinct hump at 420 nm can also be attributed to facile H abstraction from THN. Therefore, the spectrum indicates the possibility of both ET and H abstraction from THN to MQ molecules in ACN.
Figure 1 Transient absorption spectra of (1) MQ (0.2 mM) (□) and (2) MQ (0.2 mM)-THN (2.0 mM) (▴) in ACN after 1.0 μs time delay after laser pulse with excitation wavelength of 355 nm.
The following are the reaction steps involved:
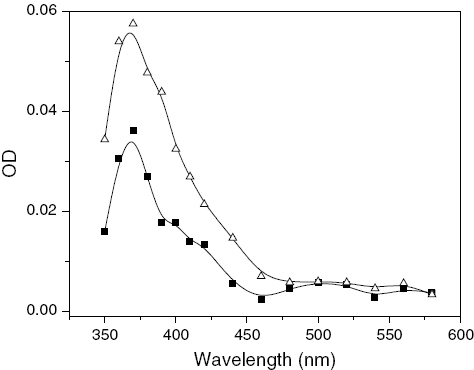
Figure depicts the transient spectra of MQ and MQ with THDN in ACN 1 μs after a laser pulse with an excitement wavelength of 355 nm. In the presence of THDN, a peak is observed around 370 nm; however, there is no peak or hump around 500 nm, which indicates the nonexistence of radical ions. Therefore, the peak at 370 nm is due to MQH• only. These observations can be explained if we consider that only a H atom is transferred from THDN to MQ as follows without any ET.
Figure 2 Transient absorption spectra of (1) MQ (0.2 mM) (▪) and (2) MQ (0.2 mM)-THDN (2.0 mM) (utri;) in ACN after 1.0 μs time delay after laser pulse with excitation wavelength of 355 nm.
Therefore, it can be concluded that in the presence of THN, both ET and H abstraction may occur with MQ while the latter preferentially undergoes H abstraction from THDN in a homogeneous ACN medium.
After assessing the interaction pathways between MQ and THN and THDN in ACN, we attempted to determine those in a micellar medium. Micellar media are unique in the sense that they provide a cage environment for RPs and RIPs so that their separation into free ions is restricted. We carried out the experiments in 5% SDS. Figure shows the transient absorption spectra of MQ and a mixture of MQ and THN in SDS obtained by a 355 nm laser pulse. In the absence of THN, the spectrum indicates the formation of MQH• with a peak at 370 nm and a hump around 400 nm due to H abstraction from SDS by MQ. In the presence of THN, the maximum around 370 nm broadens with appreciable absorption around 400 nm. The peak at 370 nm indicates the presence of both MQ•− and MQH•, while the hump around 400 nm implies the formation of MQH•. Again, the small hump above 500 nm indicates the presence of THN•+. Therefore, we conclude that both ET and H atom transfer occur between MQ and THN in the SDS micellar medium with the following reaction steps.
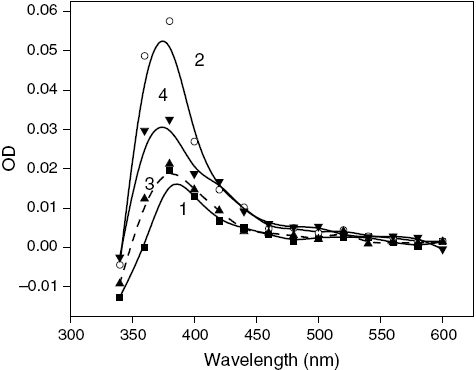
Figure 3 Transient absorption spectra of MQ (0.4 mM) in (1) absence (▪) and (2) presence of magnetic field (○), and MQ (0.4 mM) – THN (5.0 mM) in (3) absence (✦) and (4) presence of magnetic field (▴) in SDS micelles after a delay of 1.0 μs.
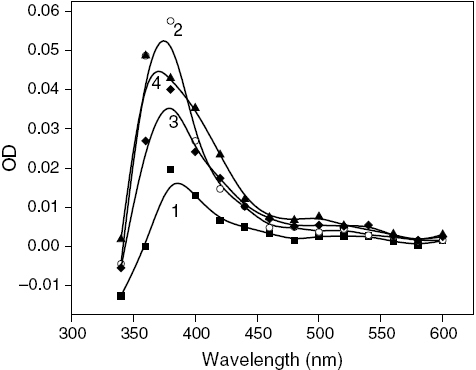
Figure displays similar transient spectra to those in figure with THDN instead of THN in the micellar medium. Peaks generated upon the interaction of MQ with THDN have a maximum at 380 nm with a small hump around 400 nm; however, no hump around 500 nm could be discerned. This result may be attributed to the occurrence of only H atom transfer, because if ET had been present, the hump around 500 nm should have existed. Thus, irrespective of the medium, i.e., ACN or SDS micelles, THN undergoes both ET and H abstraction while its nucleoside, THDN only undergoes H abstraction with MQ as follows:
Magnetic field effect
We studied the effect of an MF on the formation of RPs or RIPs in a SDS micellar medium. Micelles provide a suitable medium for compartmentalizing molecules in separate microcages, which leads to an increase in the lifetime of the transients by slowing down random collisions, as well as sufficient volume is maintained so that the component radicals of a pair can separate to a distance at which the exchange interaction becomes negligible but the spin correlation is still retained; therefore, spin flipping and subsequent geminate recombination or the formation of free ions are possible, fulfilling the essential criteria of the MFE. However, in homogeneous ACN media, MFE can hardly be observed except for a few cases due to the very short lifetime of the geminate RPs or RIPs, which cannot satisfy the above criteria of spin correlation.
Figure shows that in the presence of an external MF, the rate of decay of MQ in SDS micelles decreases at 380 nm on application of an external MF accompanied by enhanced absorption in the spectrum (figures and ). The MFE in the absence of a base is due to the formation of a geminate spin-correlated RP3 (MQH•R•), which is formed by H abstraction by MQ from SDS micelles. Upon the addition of THN, the nature of the decay profiles shows a significant change in the presence of magnetic field, as shown in curve 4 of figure . This implies that the behavior of the transients formed is different in the presence of THN because of the presence of more than one species other than MQH•. This is also observed in the transient absorption spectra. The peak around 380 nm in figure , which broadens upon the addition of THN, is significantly enhanced in the presence of a magnetic field, which indicates the presence of both the semiquinone, MQH•, and the radical ion, MQ•−. The increase in the absorbance at the hump around 400 nm is a clear indication of the formation of MQH•. Again, the increase in the absorbance above 500 nm also points to the enhancement of ET in the presence of an MF since the formation of MQ•− increases. Therefore, MFE studies support the occurrence of both H abstraction and ET between MQ and THN in the triplet state in a micellar environment. However, for the reaction with THDN in figure , although the peak around 380 nm is affected by the presence of a MF, there is practically no MFE around 500 nm, which signifies that MQ undergoes only H abstraction with THDN without ET in a micellar medium.
Figure 5 Normalized OD traces at 380 nm obtained by laser flash photolysis (λ=355 nm) of MQ (0.2 mM) in SDS in (1) absence and (2) presence of magnetic field, MQ (0.2 mM) and THN (2.0 mM) in (3) absence and (4) presence of magnetic field, and MQ (0.2 mM) and THDN (2.0 mM) in (5) absence and (6) presence of magnetic field.
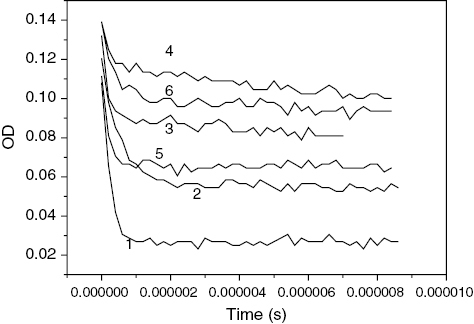
We analyzed the decay profiles shown in figure . In the presence of an external magnetic field, the decay of the RIP is expected to be biexponential[Citation14], i.e., the following equation is satisfied for the absorbance A(t):
where kf and ks are the respective rate constants for the fast and slow components of the decay profiles. The fast component of this equation corresponds to the RP decay in the micellar cage, while the slow component is due to the reaction of the escaped radicals. Upon the biexponential fitting of curves 1–6 (figure ) the values of kf obtained were 4.0×106, 1.9×106, 6.9×106, 5.4×106, 5.5×106 and 2.1×106 s−1, respectively. It is thus evident that the application of an MF resulted in a decrease in the decay rate constant, i.e., an increase in the lifetime of the RPs and RIPs for both THN and THDN. This is due to the triplet geminate RP/RIP precursor involved in these reactions. Therefore, the MFE allows us to confirm that both ET and H abstraction occur between MQ and the two bases in the triplet state. Moreover, the yield of radical ions in the bulk of the solvent may be obtained from the ratio of absorption due to the free-radical ions to that of the initial absorption immediately after the pulse. The relative escape yields of MQ-THN and MQ-THDN after 1 μs are 1.24 and 1.47, respectively, in the presence of an MF if we consider the value of escape yield to be 1.00 in the absence of an MF in each case.
The reaction scheme used for the interpretation of the results is given below:
(1)
(2)
(3)
(4)
(5)
(6)
(7)
(8)
(9)
(10)
(11)
† The yield of escape products increases in the presence of a magnetic field (MF).
In our previous studies [Citation4], a purine base, ADN, allowed only ET in ACN, while in SDS micelles, H abstraction was found to dominate. ADS behaved in a similar manner. However, in this case, we have seen that THN allows both ET and H abstraction with MQ irrespective of the medium, while THDN allows only H abstraction. Thus, there are two main contrasting observations, which require explanations. Firstly, THN allows H abstraction and ET in ACN. We assume that H abstraction must be preceded by a H-bonding type interaction. Therefore, the THN molecules may be able to come much closer to MQ than the larger ADN, thus allowing H abstraction. In SDS, the close sequestering of the molecules allows H abstraction to a greater extent. Secondly, we found that THDN allows only H abstraction in both media. THDN possesses an extra sugar unit, and the electronegative oxygen atoms probably inhibit facile ET from THDN on account of the strong inductive effect. However, the question remains as to why ADS, which also possesses a sugar unit, participates in ET? ADS is a double-ring compound. The sugar unit deprives one ring of its electrons by the inductive effect but the second ring remains unaffected. Thus, facile ET is possible from the second ring in ADS, which is not possible in THDN on account of its single-ring structure.
Conclusion
In this work we illustrated the behavior of a quinone drug-like molecule with a DNA base, THN, and its nucleoside, THDN, in different types of media. Upon laser flash photolysis THN underwent both ET and H abstraction, while THDN only underwent the latter. In our previous studies [Citation4], we observed that ADN allowed only ET in ACN, while in SDS micelles, H abstraction was found to dominate. ADS behaved in a similar manner. Thus, we find that when switching from a purine base to a pyrimidine base, a striking difference in the reaction pattern emerges. The results of this study highlight the immense potential of a mere sugar unit in reversing the reaction course of DNA bases.
Acknowledgment
We thank Mrs Chitra Raha for her assistance in the technical part of the experiments.
References
- RichP R 1982 Faraday Discuss. Chem. Soc. 74 3183 http://dx.doi.org/10.1039/dc9827400349
- B LTrumpower 1982 Function of Quinones in Energy Conserving Systems New York Academic
- MortonR A 1965 Biochemistry of Quinones New York Academic
- SenguptaTChoudhuryS DBasuS 2004 J. Am. Chem. Soc. 126 10589 and references therein http://dx.doi.org/10.1021/ja0490976
- TanimotoYFujiwaraY 2003 Handbook of Photochemistry and Photobiology vol 1 Valencia American Scientific Publishers
- SakagachiYHayashiH 1984 J. Phys. Chem. 88 1437 http://dx.doi.org/10.1021/j150651a040
- SteinerU EUlrichT 1989 Chem. Rev. 89 51 http://dx.doi.org/10.1021/cr00091a003
- BhattacharyaKChowdhuryM 1993 Chem. Rev. 93 507 http://dx.doi.org/10.1021/cr00017a022
- AichSBasuS 1995 J. Chem. Soc.: Faraday Trans. 91 1593 http://dx.doi.org/10.1039/ft9959101593
- BoseADeyDBasuS 2007 J. Photochem. Photobiol. A: Chem. 186 130 http://dx.doi.org/10.1016/j.jphotochem.2006.07.021
- LomothRBredeO 1998 Chem. Phys. Lett. 288 47 http://dx.doi.org/10.1016/S0009-2614(98)00091-8
- SongQ LinW YaoS LinN 1998 J. Photochem. Photobiol. A: Chem. 114 181 http://dx.doi.org/10.1016/S1010-6030(98)00219-6
- GeimerJBredeOBeckertD 1997 Chem. Phys. Lett. 276 411 http://dx.doi.org/10.1016/S0009-2614(97)00824-5
- WakasaMHayashiHMikamiYTakedaT 1995 J. Phys. Chem. 99 13181 http://dx.doi.org/10.1021/j100035a022