Abstract
The cyclic voltammetric behavior of [Fe(CN)6]3− was investigated under homogeneous magnetic fields perpendicular to the electrode surface in order to determine the effects of magnetic fields on the distribution of an Fe2+/Fe3+ redox couple. The cathodic current was enhanced much more than the anodic current by a homogeneous magnetic field, suggesting that the concentration gradient of paramagnetic [Fe(CN)6]3− and diamagnetic [Fe(CN)6]4− formed at an electrode surface may also contribute to the asymmetric current. The apparent diffusion coefficient of the redox couple increased by over 30% in both cathodic and anodic processes upon applying a magnetic field. For a gold electrode coated with dioctadecyldimethylammonium, the application of a magnetic field perpendicular to the surface increased the peak-to-peak separation, and enhanced the asymmetric current. It is inferred that the application of a magnetic field promotes the electron-tunneling process by tilting chain molecules in the barrier membrane.
Introduction
The effects of magnetic fields on biological systems such as biomembranes and cytoplasms are focused on, because many types of electric devices produce electromagnetic waves, and processes based on magnetism such as MRI as well as linear-motor cars operate under very high magnetic fields. However, little is understood about the mechanisms by which a magnetic field affects biological systems because of the very complex structures of organisms and the correlation among elementary processes. Mass-transport phenomena [Citation1, Citation2], catalytic behavior [Citation3, Citation4], and electrochemical processes [Citation5–7] as model elementary processes in biological systems have been widely investigated under steady magnetic fields. We also found that very low magnetic fields (<0.5 T) markedly changed the potential and resistance of black lipid membranes, because the magnetic orientation of lipid molecules brought about changes in the apparent fixed charge density of the membranes [Citation8, Citation9].
Cyclic voltammetry (CV) is one of the most important techniques for investigating the effect of magnetic fields on redox reactions and mass-transport processes such as convection, diffusion, and migration. When magnetic fields are externally imposed on an electric field, the mass transportation at the electrode–solution interface is altered, which changes the electrical double layer around the electrode. The changes in the electrochemical processes have mainly been attributed to the magnetohydrodynamic effect (MHD), which leads to an increase in the limiting current and rate of the electrochemical reaction [Citation10–14]. The electrolytic current depends on the electrode arrangement against the direction of the magnetic field. The diffusion current in electrolysis may be controlled by three types of MHD effects on the convective diffusion structure [Citation15]. Aogaki et al studied the MHD effect quantitatively by analyzing the flow of electrolyte solutions between electrodes [Citation16]. The increase in electrolytic current under a steady magnetic field, however, arises from not only MHD effects but also magneto-convection, which may arise from the concentration gradient of the magnetic susceptibility under an inhomogeneous magnetic field or magnetic field gradient [Citation17–21]. The magneto-convection affects the diffusion processes of redox species when an inhomogeneous magnetic field is applied, even when it is parallel to the electrolytic current. Sugiyama et al detected the spin effect using a local magnetic field gradient and a special electrode, and carried out a quantitative analysis to obtain the magnetic parameters [Citation21].
Magnetic fields affect electrochemical processes such as those of ferrocene and K3[Fe(CN)6] [Citation5–7], [Citation22, Citation23]. The peak current of the [Fe(CN)6]3− redox reaction depends strongly on the direction and polarity of the magnetic field [Citation5–7]. The [Fe(CN)6]3− redox process is typically reversible, in which paramagnetic [Fe(CN)6]3− (magnetic susceptibility χ/cm3 mol−1=+2.29×10−3) is converted into diamagnetic [Fe(CN)6]4− (χ/cm3 mol−1=−1.72×10−4) [Citation24]. As the electrolysis progresses, a gradient of the magnetic susceptibility of the solution around the electrode develops. Then, the cyclic voltammogram (CVM) may change if the distribution of the redox couple is affected by a homogeneous magnetic field via the χ-gradient.
The CVMs of redox couples at a gold electrode coated with a self-assembled monolayer acting as an electric barrier (thickness d) have been studied by many researchers [Citation25–30]. The modification of an electrode by the organic membrane changes the distribution of redox couples in a diffuse layer at the membrane–water interface, which modifies the effect of the magnetic field on the CVM. Homogeneous magnetic fields should also affect the CVM such as through the electron-tunneling process, because the magnetic orientation of molecular chains may occur.
In this paper, we examined the effect of homogeneous magnetic fields on a diffuse layer at a bare electrode surface comprising species of different spin states associated with the redox reaction of [Fe(CN)6]3−, and also on electron-tunneling process in a lipid-membrane-coated electrode. The magnetic fields were expected to affect the diffusion processes and interfacial phenomena of the complex ions, which have different magnetic properties at the bare and membrane-coated electrodes.
Experimental
K3[Fe(CN)6] (Sigma-Aldrich, Ltd), dioctadecyldimethyl-ammonium chloride (DODAC; TCI, Ltd), and KNO3 (Wako Pure Chemicals, Ltd) were used without further purification. Aqueous K3[Fe(CN)6] solutions of various concentrations were prepared in 1 M aqueous KNO3 solution. Pure water was prepared using a MilliQ-labo system.
A conventional three-electrode arrangement was used for CV, comprising an HAB-151 electrochemical instrument (Hokuto Denko Co Ltd), a homemade Ag/AgCl electrode as a reference electrode, Pt and membrane-coated Au disk electrodes (cross-sectional area=3.14 mm2) as working electrodes, and a Pt disk electrode as a counter electrode. Each working electrode was polished with alumina slurry, and then underwent sonication in pure water for 20 min. Au electrodes were activated by applying a scanning electric potential using the CV equipment in 1 M aqueous KNO3 solutions containing K3[Fe(CN)6] ([Fe(CN)6]3− solutions), followed by washing with water and drying under N2. The Au electrode was covered with a 5-mm-long Teflon sleeve to avoid MHD convection [Citation21]. Several μl of an aliquot of 1000 ppm DODAC in CHCl3 was added dropwise to the Au electrode surface, and the electrode was then placed in 0.2 M KCl to form a DODAC monolayer on the electrode surface. CV was performed at various potential scan rates ν between 5 and 50 mV s−1 in the bore of a Sumitomo Heavy Industries superconducting magnet (10 T) at 298±0.1 K.
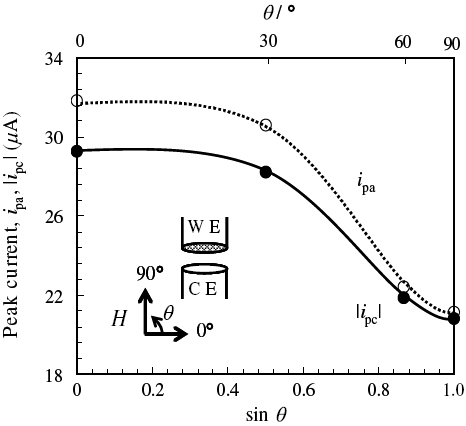
Homogeneous magnetic fields (10T; ± 0.1%) in the center of the superconducting magnet were applied perpendicular to the working and counter electrode surfaces to avoid the MHD effect, because the peak current ip in CVMs increased upon decreasing the angle θ between the direction of the magnetic field and the electrode surface, indicating the occurrence of the MHD effect (figure ).
Results
The CVMs of [Fe(CN)6]3− solutions of various concentrations were measured at various magnetic fields. Figure shows the CVM of an 8 mM [Fe(CN)6]3− solution at 6 T as an example. Both the cathodic (Epc) and anodic peak potentials (Epa) shifted by less than 0.02 V in the magnetic field of 6 T. Thus, the peak-to-peak separation potential (ΔEp) and formal potential (E0=(Epc+Epa)/2) were almost unchanged, suggesting that magnetic fields do not affect the solution around the redox couples. On the other hand, the cathodic (|ipc|) and anodic peak currents (ipa) increased with increasing magnetic field at all concentrations of [Fe(CN)6]3−. Note that the cathodic current was enhanced much more than the anodic current by the magnetic fields. This tendency was obtained in an equimolar mixture of [Fe(CN)6]3− and [Fe(CN)6]4−, as shown in figure (A). Moreover, we carried out CV using the Au electrode with a Teflon sleeve to confirm that the MHD effect was not significant in our setup. Figure (B) shows that an asymmetric trend was observed, although the current enhancement due to a 6 T magnetic field was rather small. The ratio of the cathodic to anodic peak current |ipc|/ipa, obtained by scanning at 20 mV s−1 in the range −0.2 to 0.7 V, was plotted against magnetic field intensity at various [Fe(CN)6]3− concentrations in figure . The |ipc|/ipa ratio increased from 1.01 (unity for a reversible redox couple) at a zero field to 1.1 at 10 T with increasing magnetic field. This suggests that the magnetic properties of a solution containing Fe2+/Fe3+ complex ions around an electrode should bring about such asymmetric behavior under homogeneous magnetic fields.
Figure 2 Effect of a 6 T magnetic field on CVMs of 1 M aqueous KNO3 solution with 8 mM K3[Fe(CN)6] at ν/mV s−1=50 and 308±0.1 K. Magnetic field, H: solid line, 0 T; broken line, 6 T.
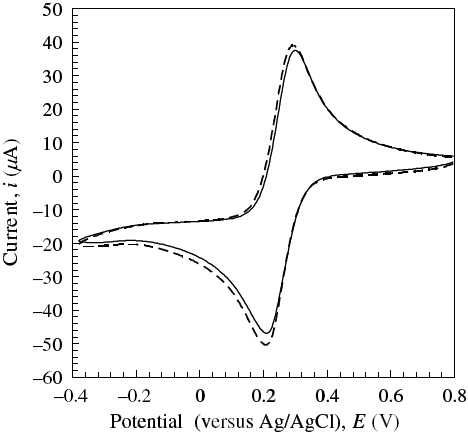
Figure 3 Effects of a 6 T magnetic field on CVMs of 1 M aqueous KNO3 solution with a mixture of 4 mM K3[Fe(CN)6] and 4 mM K4[Fe(CN)6] using Au electrodes without (A) and with (B) a 5-mm-long sleeve at ν/mV s−1=50 and 308±0.1 K. Magnetic field, H: solid line, 0 T; broken line, 6 T.
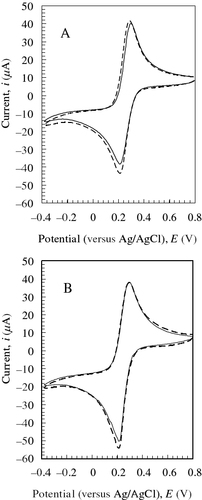
Figure 4 Values of |ipc|/ipa in CVMs of K3[Fe(CN)6] solutions at ν/mV s−1=20 and 308±0.1 K as a function of magnetic field intensity.
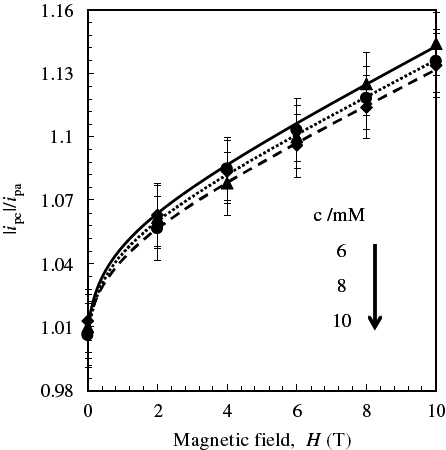
The redox current ipαH under a homogeneous magnetic field H may mainly arise from redox reactions under a zero field (ipα0) and the effect of inhomogeneous magnetization (ΔipαH), since MHD and probably magnetoconvection effects may be neglected in our setup.
(1)
The apparent diffusion coefficient Dappα/cm2 s−1 under steady magnetic fields was estimated assuming the Randles–Sevick equation [Citation31] for the experimental peak current under a magnetic field:
(2)
where n is the number of electrons transferred in the redox reaction, A is the surface area of the electrode, c is the [Fe(CN)6]3− concentration, and v is the scan rate. The estimated value of Dappα increased with increasing magnetic field at various [Fe(CN)6]3− concentrations at 20 mV s−1, and the values of Dappc in the cathodic process were larger than those of Dappa in the anodic process, as shown in figure . The value of Dappα at a zero field (D0) agreed with the reported results [Citation32]. Since Dappα/10−5 cm2 s−1 was proportional to H1/2/T1/2, the following experimental equation was obtained:
(3)
After the slopes at various concentrations [c/mM] were averaged, A, B, and C were estimated, which were 1.114, −0.0278, and 0.0950 for Dappc and 1.153, −0.0330, and 0.0783 for Dappa, respectively.
Figure 5 Dapp for cathodic (solid line) and anodic processes (broken line) in CVMs of K3[Fe(CN)6] solutions as a function of magnetic field intensity.
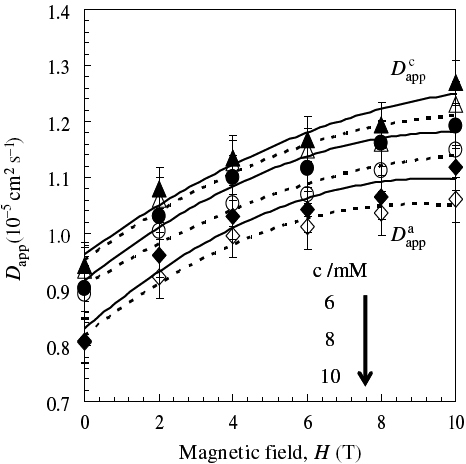
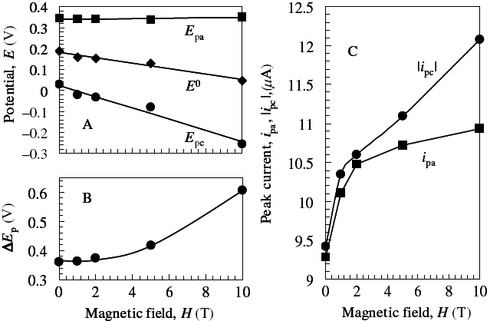
Figure shows the change in the CVMs upon coating the Au electrode with a DODAC membrane. The reversible redox process was affected by the electric barrier; the peak-to-peak separation ΔEp increased and the peak current decreased, indicating that electron-tunneling occurred through the organic barrier. Upon applying a magnetic field perpendicular to the electrode surface, both ΔEp and ip increased with increasing magnetic field, as shown in figure , although the increase in ΔEp due to the increase in barrier thickness usually brings about a decrease in peak current. Figure shows plots of ΔEpα ΔEp, E0, and ip as a function of H. In contrast with the absence of magnetic field dependence of ΔEpα, ΔEp, and E0 in the bare electrode (figure ), significant potential shifts were observed in the tunneling system. Considering that the current was reduced approximately four fold by the barrier, ΔipαH in the membrane system was much larger than that in the bare system. The asymmetric enhancement of the peak current was also observed at the soft interface, and moreover, the anodic current was markedly increased by the effect of the magnetic field on the tunneling process.
Figure 6 CVMs of bare Au (broken line) and DODAC/Au electrodes (solid line) in 1 M aqueous KNO3 solution with 10 mM K3[Fe(CN)6] at ν/mV s−1=10 and 308±0.1 K.
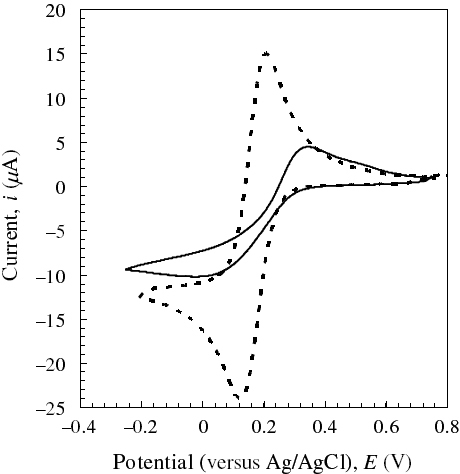
Figure 7 CVMs of DODAC/Au system in 1 M aqueous KNO3 solution with 10 mM K3[Fe(CN)6] under steady magnetic fields at ν/mV s−1=10 and 308±0.1 K. The magnetic fields were applied perpendicular to the electrode surface.
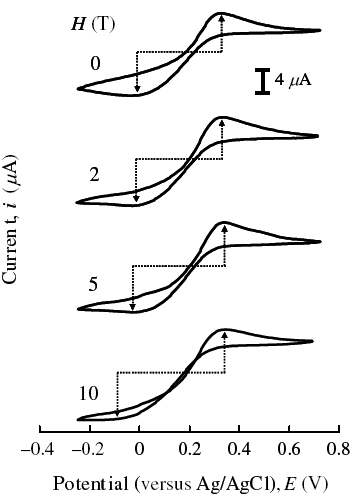
Figure 8 Peak data of DODAC/Au systems obtained from the CVMs in figure as a function of magnetic field intensity. (A) Potential, E(V): anodic, ▪; cathodic, •; formal, ♦. (B) Peak-to-peak separation potential, ΔEp (V). (C) Peak current, ip (μA): anodic, ▪; cathodic, •.
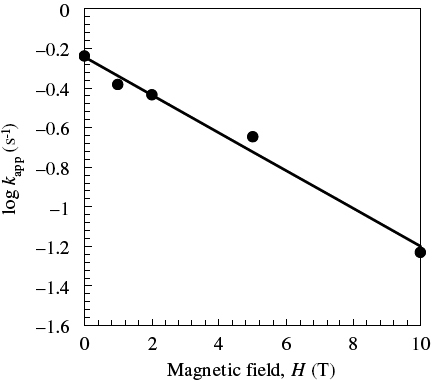
The apparent electron transfer rate constant kapp of the DODAC/Au systems under magnetic fields was estimated from the potential data, using Laviron's formula [Citation33]. Figure shows that log kapp decreased linearly with increasing H; i.e.
(4)
where γ is the electron-tunneling constant. The values of the electron transfer coefficients α and 1−α were 0.41 and 0.65, respectively. This suggests that the tunneling process in the DODAC membrane systems should be retarded under a steady magnetic field. Comparing equation (4) with Marcus theory [Citation34], the d-dependence of the electron transfer rate constant, exp(−βd), is thought to be replaced by pathway dependence under a magnetic field.
Discussion
In the reversible redox couple system, the anodic current should be equal to the cathodic current. The asymmetric peak current with ipa<|ipc| suggests that the inhomogeneity of the magnetization of the solution also should be taken into account under homogeneous magnetic fields, in addition to the contribution of the MHD effect, which appears even under the perpendicular magnetic field [Citation21], and the magneto-convection due to the local magnetic field gradient around the electrode.
The chemical potential gradient in a solution causes the diffusion of redox species. Under a homogeneous magnetic field H, the gradient of the magnetochemical potential of a unit volume having molar magnetization may produce a magnetic force FHα−H2(∂χ/∂x)α−H2(χIII−χII)(∂CIII/∂x) in addition to the usual diffusion −D(∂CIII/∂x). This relation suggests that the solution at position x will flow to the electrode under magnetic field H, when [Fe(CN)6]3− has a high concentration far from the surface. During the forward scan, a diamagnetic diffuse layer is expected to be formed around the electrode surface. Then, the [Fe(CN)6]3− flow toward the electrode will promote the cathodic reaction under a homogeneous magnetic field. In the reverse scan, the oxidation process should relax the χ gradient, thus depressing the current enhancement.
In the membrane system, the magnetic orientation of the DODAC molecules in the membrane should be considered in addition to the possible mechanisms by which the magnetic field affects the bare electrode systems. The current profiles seemed to show the existence of a limiting current at a zero field, which arose from a sufficient concentration of the redox couples at the membrane surface, because the current in the membrane-coated electrode was much less than that in the bare electrode. The increase in ΔEp may arise from the tunneling process across the membrane. Since the tunneling process is usually associated with a decrease in the peak current, the increase in the peak current here seems to arise from other processes such as the supply of redox couples by MHD convection. However, the same behaviors (the increase in both ΔEp and ip) were observed in a gold electrode coated by (ferrocenylmethyl)dodecyldimethylammonium bromide (FDDA) in 1 M aqueous KNO3 solution, in which no MHD effect contributed to the current [Citation35]. Therefore, one of the possible explanations for the current increase is the enhancement of the electron-tunneling current by the magnetic field.
Upon applying steady magnetic fields perpendicular to the electrode surface, the average tilt angle ϕ(H) of the hydrocarbon chains (length lc) increased. It was suggested from the results of in situ measurements using a quartz crystal microbalance that the orientation of the alkanethiolates on the Au surface was affected by applying a magnetic field, particularly one over 4 T [Citation36]. The length Lt(H) of a tunneling pathway comprising the through-bond superexchange along a chain and the through-space superexchange due to a single interchain hop is given by the following equation [Citation35]:
(5)
where a is the distance between adsorption sites on the electrode surface and a cos ϕ(H)≡dcc is the chain-to-chain distance or the hopping distance. The increase in the path length or ϕ(H) associated with interchain superexchange hops will lead to an increase in ΔEp. The total tunneling current It under a magnetic field is given by the following equation [Citation25–29, Citation35]:
(6)
where I0 is the current anticipated in the absence of the self-assembled monolayer (SAM), ns is the number of carbons in a hydrocarbon chain, and βtb and βts(H) are the tunneling constants for through-bond and interchain tunneling, respectively. For both ΔEp (or Lt(H)) and It (or ip) to increase with increasing magnetic field, the number of hops (lc/{a sin ϕ(H)}) and the hopping probability should increase with increasing magnetic field intensity; this seems to be possible because the larger the tilt angle is, the smaller the chain-to-chain distance dcc is. It was reported theoretically and experimentally in a few tunneling systems that the total current increased rapidly as the tilt angle of the thiolates increased beyond 35° [Citation29, Citation30]. These results suggest that the parallel conduction pathways for a tunneling electron may enhance the peak current under a magnetic field.
Conclusion
The magnetoelectric behavior of [Fe(CN)6]3− was examined under steady magnetic fields. The cathodic peak currents of [Fe(CN)6]3− were larger than the anodic peak currents. The asymmetric behavior of peak currents may arise from the concentration gradient of the redox pair, which developed around the electrode surface with a Teflon sleeve under magnetic fields, because paramagnetic [Fe(CN)6]3− ions were converted into diamagnetic [Fe(CN)6]4− ions in the electrochemical reaction. The apparent diffusion coefficient of Fe2+/Fe3+ complexes increased by over 30% in both the cathodic and anodic processes upon applying a magnetic field of 10 T. Moreover, the enhancement of the asymmetric peak current and the peak-to-peak separation potential of the redox probe at the surface of a gold electrode coated with a dioctadecyldimethylammonium membrane increased with increasing magnetic field applied perpendicular to the electrode. The magnetic orientation of alkyl chains in the membrane brought about enhanced electron tunneling.
Acknowledgment
This work was supported by a Grant-in-Aid for Scientific Research no 16655004 from MEXT of Japan.
References
- FujiwaraSHaraguchiHUmezawaY 1968 Anal. Chem. 40 249 http://dx.doi.org/10.1021/ac60257a023
- FujiwaraSUmezawaYKuboT 1968 Anal. Chem. 40 2186 http://dx.doi.org/10.1021/ac50158a046
- HaberditzlW 1967 Nature 213 72 http://dx.doi.org/10.1038/213072a0
- UenoSIwasakaM 1996 J. Appl. Phys. 79 4705 http://dx.doi.org/10.1063/1.361646
- ZheleztsovA V Zh 1976 Analit. Khim. 31 629
- ZheleztsovA V Zh 1976 Chem. Abstr. 85 13278v
- IwakuraCEdamotoTTamuraH 1984 Denki Kagaku 52 596
- OzekiSKurashimaHMiyanagaMNozawaC 2000 Langmuir 16 1478 http://dx.doi.org/10.1021/la9912085
- KurashimaHOzekiS 2002 Encyclopedia of Surface and Colloid Science AHubbard New York Marcel Dekker p 3109
- KellyE J 1977 J. Electrochem. Soc. 124 987 http://dx.doi.org/10.1149/1.2133514
- Guerin-OulerDNicollinC 1982 Electrochim. Acta 27 909 http://dx.doi.org/10.1016/0013-4686(82)80215-6
- QuraishiM SFahidyT Z 1978 Electrochim. Acta 23 963 http://dx.doi.org/10.1016/0013-4686(78)87022-4
- QuraishiM SFahidyT Z 1980 Electrochim. Acta 25 591 http://dx.doi.org/10.1016/0013-4686(80)87062-9
- OliverAFahidyT Z 1982 J. Appl. Electrochem. 12 417 http://dx.doi.org/10.1007/BF00610483
- FahidyT Z 1983 J. Appl. Electrochem. B 13 553 http://dx.doi.org/10.1007/BF00617811
- AogakiRFuekiKMukaiboT 1975 Denki Kagaku 43 505 509
- O'BrienR NSanthanamK S V 1997 J. Appl. Electrochem. 27 573 http://dx.doi.org/10.1023/A:1018402813235
- RagsdaleS RGrantK MWhiteH S 1998 J. Am. Chem. Soc. 120 13461 http://dx.doi.org/10.1021/ja982540q
- PullinsM DGrantK MWhiteH S 2001 J. Phys. Chem. B 105 51 http://dx.doi.org/10.1021/jp012093s
- WaskaasMKharkatsY I 2001 J. Electroanal. Chem. 502 51 http://dx.doi.org/10.1016/S0022-0728(00)00528-3
- SugiyamaAHashirideMMorimotoRNagaiYAogakiR 2004 Electrochim. Acta. 49 5115 http://dx.doi.org/10.1016/j.electacta.2004.06.024
- MogiIKindoGNakagawaY 1990 Bull. Chem. Soc. Japan. 63 1871 http://dx.doi.org/10.1246/bcsj.63.1871
- TakahashiFSakaiYTamuraT 1983 Electrochim. Acta 28 1147 http://dx.doi.org/10.1016/0013-4686(83)80020-6
- 2006–2007 CRC Handbook of Chemistry and Physics 87th edn Boca Raton, FL CRC Press p 4
- KhoshtariyaD EDolidzeT DZusmanL DWaldeckD H 2001 J. Phys. Chem. A 105 1818 http://dx.doi.org/10.1021/jp0041095
- NapperA MLiuHWaldeckD H 2001 J. Phys. Chem. B 105 7699 http://dx.doi.org/10.1021/jp0105140
- SekSBilewiczR 2001 J. Electroanal. Chem. 509 11 http://dx.doi.org/10.1016/S0022-0728(01)00353-9
- FinkleaH OLiuLRavenscraftM SOunturiS 1996 J. Phys. Chem. 100 18852 http://dx.doi.org/10.1021/jp962831q
- SlowinskiKChamberlainR VMillerC JMajdaM 1997 J. Am. Chem. Soc. 119 11910 http://dx.doi.org/10.1021/ja971921l
- YamamotoHWaldeckD H 2002 J. Phys. Chem. B 106 7469 http://dx.doi.org/10.1021/jp014612x
- BardA JFaulknerL R 1980 Electrochemical Methods: Fundamentals and Applications New York Wiley p 218
- 2006–2007 CRC Handbook of Chemistry and Physics 87th edn Boca Raton, FL CRC Press p 5
- LavironE 1979 J. Electroanal. Chem. 101 19 http://dx.doi.org/10.1016/S0022-0728(79)80075-3
- MarcusR ASutinN 1985 Biochim. Biophys. Acta 811 265
- SaravananGOzekiS 2008 J. Phys. Chem. B 112 3 http://dx.doi.org/10.1021/jp076663l
- SaravananGNomuraTOzekiS , in preparation