Abstract
The chiral electrode behaviors of magneto-electrodeposited (MED) Ag and Cu films were examined for the electrochemical reactions of D-glucose, L-glucose and L-cysteine. The Ag and Cu films were electrodeposited under a magnetic field of 2 T parallel (+2 T) or antiparallel (-2 T) to the faradaic current. For MED films of both Ag and Cu, the oxidation current of L-glucose was larger than that of D-glucose on the +2 T-film electrodes, and the results were opposite on the - 2 T-film electrodes. These facts demonstrate that the MED metal films possess the ability of chiral recognition for D- and L-glucoses. The MED Ag film electrodes also exhibited chiral behavior for the oxidation of L-cysteine.
Introduction
The magnetic field control of chiral chemical reactions is one of the goals in magnetoscience. Many scientists have studied this subject, however, very few have succeeded in achieving such control. Rikken and Raupach found an enantioselective magnetic field effect in a photochemical reaction and obtained the enantiomeric excess of 0.01% under a magnetic field of 10 T [Citation1]. Their result is significant as a breakthrough in magnetochiral chemistry, and simultaneously the quantity of the obtained enantiomeric excess implies that the direct magnetic field control of chiral reactions is difficult.
We have attempted chirality control by magneto-electrochemical procedures. The Lorentz force induces the formation of spiral structures in the two-dimensional growth of magneto-electrodeposited metals [Citation2–5] and conducting polymers [Citation6], and the formation of three-dimensional helical structures in silicate membrane growth [Citation7]. Chiral structures can be used for the enantioselective recognition of chiral molecules, which is one of the most important processes in biochemistry. If chiral structures can be induced at the nanometer scale on the magneto-electrodeposited (MED) films, such film surfaces would serve as an enantioselective catalyst.
As a first attempt, we attempted to prepare chiral conducting polymer films by magneto-electropolymerization and found that such polyaniline films exhibited chiral electrode behavior for the oxidation of ascorbic acid [Citation8, Citation9]. We next attempted to prepare chiral film surfaces by the magneto-electrodeposition of metals [Citation10]. When a magnetic field is applied to an electrochemical cell, the Lorentz force induces convection in the electrolytic solution, resulting in an increase in the faradaic current. This is well known as the magnetohydrodynamic (MHD) effect [Citation11, Citation12]. Aogaki pointed out that the micro-MHD effect plays an important role in electrodeposition when the magnetic field is parallel to the faradaic current [Citation13]. In electrodeposition processes, nonequilibrium fluctuation produces a large number of humps on the deposited surface. The faradaic current around such humps is not parallel to the magnetic field; thus, a Lorentz force may cause vortexlike convection in the local areas around the humps. Such local convection is expected to induce chiral structures on the deposited surface.
Here, we report the chiral electrode behaviors of MED films of Ag and Cu for the oxidation of D-glucose, L-glucose and L-cysteine.
Experimental
All chemicals were reagent grade and were used as received. All aqueous solutions were prepared with distilled water. For electrochemical experiments, a conventional system with the following three electrodes was employed: a platinum disc working electrode with a diameter of 3 mm, a platinum plate counter electrode, and a Ag|Ag+ (50 mM/(M= mol dm−3)) reference electrode for the Ag electrodeposition or a Ag|AgCl|NaCl(sat) reference electrode for the Cu electrodeposition and the voltammetric measurements. A potentiostat (Princeton Applied Research model 263A) was used for all the electrochemical experiments.
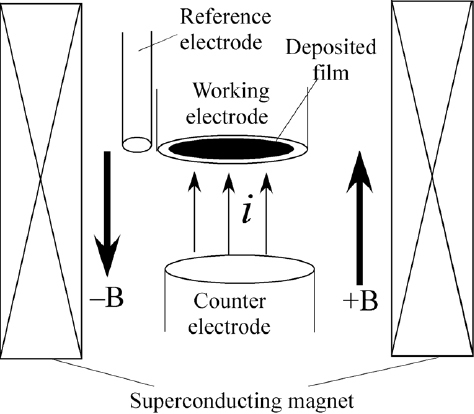
Ag electrodeposition was conducted at a constant potential of −100 mV (versus Ag|Ag+) in 50 mM AgNO3 aqueous solution containing 0.1 M NaClO4 as a supporting electrolyte. The Ag films were deposited by passing a charge of 0.2 C cm−2 through the working electrode. Cu electrodeposition was conducted at 0 mV (versus Ag|AgCl) in 50 mM CuSO4 aqueous solution containing 0.1 M Na2SO4. The Cu films were deposited by passing a charge of 0.15 C cm−2. A schematic illustration of the magneto-electrodeposition process is shown in figure . The electrochemical cell was placed at the bore center in a cryocooled superconducting magnet (Sumitomo Heavy Industries Ltd.), which can produce magnetic fields of up to 5 T. A magnetic field B was applied perpendicular to the working electrode surface and parallel (+B) or antiparallel (-B) to the faradaic current. The MED films prepared at +2 T and −2 T are referred to as the +2 T-film and −2 T-film, respectively. The temperature within the magnet bore was controlled at 25 °C by circulating thermoregulated water.
Figure 1 Electrode configuration in the magneto-electrodeposition process in a superconducting magnet. A magnetic field B is applied parallel (+) or antiparallel (−) to the faradaic current, and perpendicular to the electrode surface.
The MED films were used as modified electrodes, and their chiral properties were examined by measuring the voltammograms of 20 mM D-glucose, L-glucose and L-cysteine in 0.1 M NaOH aqueous solutions. Before the voltammetric measurements, both metal film electrodes underwent potential sweeps in 0.1 M NaOH aqueous solution as a pretreatment, and the background current was measured for each electrode. The voltammograms of glucose and cysteine were measured at a potential sweep rate of 50 mV s−1 in the absence of a magnetic field, and correction of the background was carried out for each voltammogram.
Results and discussion
MED Ag film electrodes
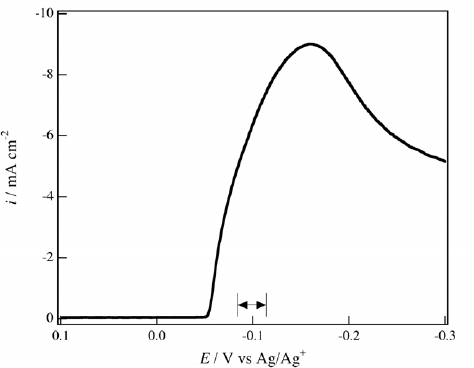
Figure shows a voltammogram of the reduction of Ag+ ions in 50 mM AgNO3 aqueous solution, and it shows that Ag deposition occurs at potentials of less than −60 mV. Potentiostatic electrodeposition was conducted at various potentials from −60 to −200 mV under a magnetic field of 2 T, and the voltammograms of D- and L-glucoses were obtained for each film electrode. It was found that the chiral electrode behavior was only observed in the films electrodeposited at around −100 mV, as indicated by arrows in figure .
Figure 2 Voltammogram of 50 mM AgNO3 in 0.1 M NaClO4 aqueous solution. The potential sweep rate was 10 mV s−1. The chiral Ag films were magneto-electrodeposited in the potential region indicated by arrows.
The chiral electrode behavior of the MED films was examined by measuring voltammograms of the chiral species. Figure shows voltammograms of 20 mM D- and L-glucoses in 0.1 M NaOH aqueous solution on the +2 T-film and −2 T-film Ag electrodes. The oxidation peak of glucose appears at 220 mV for the Ag electrode, where silver oxide is simultaneously formed [Citation10]. It is known that silver oxide induces electrocatalytic reactions on the Ag electrode through specific adsorption [Citation14] that are sensitive to the surface structure of the electrode.
Figure 3 Voltammograms of 20 mM D- and L-glucoses in 0.1 M NaOH aqueous solution on the Ag electrodes of (a) the +2 T-film and (b) the -2 T-film. The potential sweep rate was 50 mV s−1.
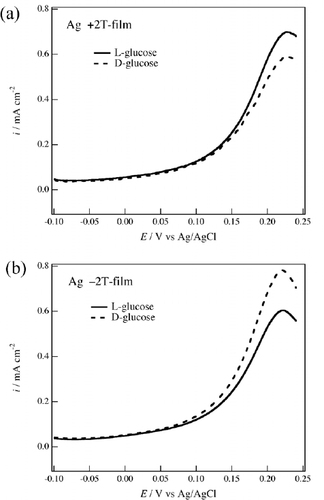
On the 0 T-film electrode, the voltammograms of D- and L-glucoses were nearly coincident with each other [Citation10]. On the +2 T-film electrode, a difference between the two voltammograms can be seen in the peak currents (figure (a)). The oxidation current of L-glucose is larger than that of D-glucose. However, the result is opposite for the MED film prepared under the reverse magnetic field. The oxidation current of D-glucose is larger than that of L-glucose on the −2 T-film electrode (figure (b)). These results indicate that the MED process induces chirality in the Ag electrodeposited film and that the origin of this chirality is the Lorentz force because other magnetic field effects are independent of the polarity of the magnetic field.
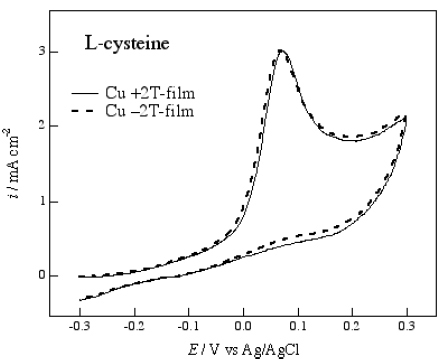
The chiral electrode behavior of the MED Ag films was also examined for the electrochemical reaction of an amino acid. Figure shows cyclic voltammograms of 20 mM L-cysteine in 0.1 M NaOH aqueous solution on the Ag film electrodes prepared at ±2 and ±0.5 T. The oxidation peak of L-cysteine appears at around 200 mV for the Ag electrodes. The +2 T-film and −2 T-film electrodes exhibit a clear difference in current (figure (a)), and this indicates that the films are able to recognize the chirality of L-cysteine.
Figure 4 Voltammograms of 20 mM L-cysteine in 0.1 M NaOH aqueous solution on the Ag electrodes of (a) the ±2 T-films and (b) the ±0.5 T-films. The potential sweep rate was 50 mV s−1.
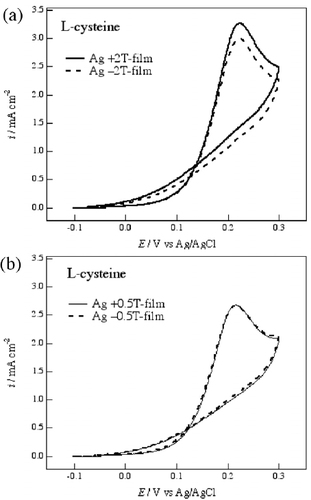
On the other hand, the voltammograms of L-cysteine on the +0.5 T-film and −0.5 T-film electrodes are coincident with each other, as shown in figure (b). Coincident voltammograms were also observed on the +4 T-film and −4 T-film electrodes. These results suggest that there is an optimal combination of the deposition current and the magnetic field for the formation of a chiral surface.
MED Cu film electrodes
Figure shows a voltammogram of the reduction of Cu2+ ion in 50 mM CuSO4 aqueous solution. A small peak at +70 mV shows the reduction from Cu2+ to Cu+ [Citation15], and Cu deposition occurs at potentials of less than 0 mV. Potentiostatic electrodeposition was conducted at various potentials from 0 to −100 mV under a magnetic field of 2 T, and chiral electrode behavior was only observed in the films electrodeposited around 0 mV, as indicated by the arrow in figure .
Figure 5 Voltammogram of 50 mM CuSO4 in 0.1 M Na2SO4 aqueous solution. The potential sweep rate was 10 mV s−1. The chiral Cu films were magneto-electrodeposited at the potential indicated by an arrow.
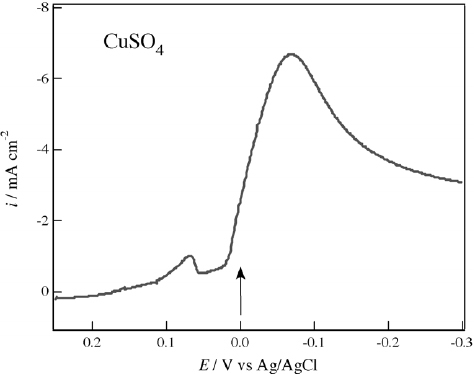
Figure shows voltammograms of 20 mM D- and L-glucoses in 0.1 M NaOH aqueous solution on the +2 T-film and −2 T-film Cu electrodes. The oxidation peak of glucose appears at around 700 mV for the Cu electrode. In the alkaline solution, Cu2O and CuO cover the surface of the Cu electrode after the pretreatment potential sweeps. Such oxides induce electrocatalytic reactions [Citation16] that are sensitive to the surface structure of the electrode.
Figure 6 Voltammograms of 20 mM D- and L-glucoses in 0.1 M NaOH aqueous solution on the Cu electrodes of (a) the +2 T-film and (b) the -2 T-film. The potential sweep rate was 50 mV s−1.
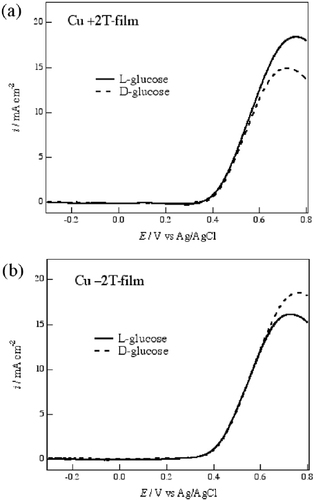
The voltammograms of D- and L-glucoses were nearly coincident with each other on the 0 T-film electrode. On the +2 T-film electrode, the oxidation current of L-glucose is larger than that of D-glucose (figure (a)). In contrast, the current of D-glucose is larger than that of L-glucose on the −2 T-film electrode (figure (b)). These facts show that the MED Cu film electrodes also possess the ability of chiral recognition for glucoses.
The comparison of the chirality between the Ag and Cu film electrodes is meaningful. As shown in figures 3 and 6, the +2 T-film electrodes of both Ag and Cu exhibit larger currents for L-glucose, and both the −2 T-film electrodes exhibit larger currents for D-glucose. These facts mean that both MED film electrodes have the same chirality, and it seems that the chirality of the film surfaces is determined by the Lorentz force.
Figure shows cyclic voltammograms of 20 mM L-cysteine in 0.1 M NaOH aqueous solution on the +2 T-film and −2 T-film Cu electrodes. The oxidation peak of L-cysteine appears at 60 mV for the Cu electrode, and both voltammograms are coincident with each other. In contrast to the case of the Ag electrodes (see figure (a)), the MED Cu electrodes do not exhibit chiral behavior for L-cysteine. The chiral recognition in electrocatalytic reactions proceeds through specific adsorption. If the specific adsorption of L-cysteine on the Cu electrode occurs at only the thiol (−SH) site of the cysteine molecule, the Cu electrode cannot recognize the chirality. It is thus considered that the chiral recognition depends on the adsorption structure.
Conclusion
We demonstrated that the magneto-electrodeposition process induces chirality in Ag and Cu films, which exhibit the chiral electrode behaviors for the oxidation reactions of D- and L-glucoses. The MED Ag films also exhibit chiral behavior for the oxidation of L-cysteine. The films electrodeposited under a reverse magnetic field possess the opposite chirality. This fact indicates that the origin of the chirality is the Lorentz force, but it remains an open issue as to how the micro-MHD effect causes the formation of the chiral structures on the MED film surfaces. Nevertheless, magneto-electrodeposition is expected to be a useful method for introducing chirality into electrodeposited films without chiral chemical species.
Acknowledgments
The MED experiments using a cryocooled superconducting magnet were performed in the High Field Laboratory for Superconducting Materials, IMR, Tohoku University. We thank Professor J-P Chopart of the University of Reims in France and Professor R Aogaki of Polytechnic University in Kanagawa for the illuminating discussion.
References
- RikkenG L J ARaupachE 2000Nature405932 http://dx.doi.org/10.1038/35016043
- MogiIOkuboSNakagawaY 1991J. Phys. Soc. Japan603200 http://dx.doi.org/10.1143/JPSJ.60.3200
- MogiIKamikoMOkuboS 1995PhysicaB 211319 http://dx.doi.org/10.1016/0921-4526(94)01055-6
- CoeyJ M DHindsGLyonsM E G 1999Europhys. Lett.47267 http://dx.doi.org/10.1209/epl/i1999-00382-3
- HeresanuVBallouRMolhoP 2003Magnetohydrodynamics39461
- MogiIKamikoM 1996Denki Kagaku64842
- UechiIKatsukiADunin-BarkovskiyLTanimotoY 2004J. Phys. Chem.B 1082527 http://dx.doi.org/10.1021/jp036018o
- MogiIWatanabeK 2005Japan J. Appl. Phys.44L199 http://dx.doi.org/10.1143/JJAP.44.L199
- MogiIWatanabeK 2006Sci. Technol. Adv. Mater.7342 http://dx.doi.org/10.1016/j.stam.2006.01.007
- MogiIWatanabeK 2007ISIJ Int.47585 http://dx.doi.org/10.2355/isijinternational.47.585
- AogakiRFuekiKMukaiboT 1975Denki Kagaku43504
- FahidyT Z 1983J. Appl. Electrochem.13553 http://dx.doi.org/10.1007/BF00617811
- AogakiR 2003Magnetohydrodynamics39453
- Avramov-IvicMJovanovicVVlajnicGPopicJ 1997J. Electroanal. Chem.423119 http://dx.doi.org/10.1016/S0022-0728(96)04787-0
- WijesunderaR PHidakaMKogaKSakaiMSiripalaW 2006Thin Solid Films500241 http://dx.doi.org/10.1016/j.tsf.2005.11.023
- TortoNRuzgasTGortonL 1999J. Electroanal. Chem.464252 http://dx.doi.org/10.1016/S0022-0728(99)00041-8