Abstract
Objective
Betel nut is a common stimulant and addictive substance in Asian countries. We wondered whether chewing betel nut affects sustained attention and inhibitory control after sleep deprivation.
Method
After one night of deprived or normal sleep, habitual chewers and non‐chewers were asked to complete the sustained attention to response task and the stop‐signal task. Before each task, participants chewed one gum or betel nut. The gum was to control for the effect of mere chewing. In the sustained attention to response task, participants responded to a sequence of numbers on a screen, but to withhold their response whenever they saw the number 3. In the stop‐signal task, they responded to a square or a circle, but withheld their response when a stop signal appeared.
Results
In the sustained attention to response task, the habitual chewers had lower false alarm rate and longer RT prior to false alarm (particularly when they had normal sleep) than the non‐chewers. In the stop‐signal task, deprived‐sleep participants had longer stop signal reaction time than normal‐sleep participants.
Conclusion
We reported that the habitual chewers have better sustained attention than the non‐chewers. Also, sleep deprivation weakens inhibitory control. Betel nut chewing does not have an acute effect on either sustained attention or inhibitory control.
Conflict of interest: None.
Betel nut chewing is a common practice across various Asian‐Pacific areas and among a small number of migrant communities in western countries. A betel nut consists of three major ingredients: a raw areca nut, slaked lime, and piper betel flower. Participants place a whole betel nut into their mouths and macerate it by biting for approximately 3-min and then spit out. It is ranked fourth among the most prevalent abuse substances worldwide after caffeine, tobacco, and alcohol (IARC, Citation2004). Although, betel nut is regarded as a human carcinogen by the World Health Organization (IARC, Citation2004), chewing betel nut remains popular.
One important, yet unexplored, issue is whether chewing betel nut can affect sustained attention and inhibitory control after sleep deprivation. There are two reasons why this issue is important. First, impaired sustained attention and inhibitory control are closely related to increased impulsivity (de Wit, Citation2009), increasing the probability of risky behaviours. Second, many habitual chewers have long working hours, e.g., as long‐distance bus and truck drivers (Department of Statistics, Citation2003). Thus, it is of practical importance to examine whether chewing betel nut could influence sustained attention and inhibitory control when users are deprived of sleep.
Different substance types can have various effects on sustained attention and inhibitory control. For example, nicotine can enhance the smokers' sustained attention acutely (Heishman, Kleykamp, & Singleton, Citation2010) and chronically (Rusted, Caulfield, King, & Goode, Citation2000) but does not affect inhibitory control in both smokers (Bekker, Bocker, Van Hunsel, van den Berg, & Kenemans, Citation2005) and non‐smokers (Wignall & de Wit, Citation2011). Alternatively, alcohol can impair both sustained attention (Finnigan, Schulze, & Smallwood, Citation2007) and inhibitory control (Li, Luo, Yan, Bergquist, & Sinha, Citation2009) both acutely (Fillmore, Citation2007) and chronically (Anstey, Mack, & Cherbuin, Citation2009).
As for betel nut, arecoline (the primary alkaloid in the betel nut) has been shown to act on the muscarinic and nicotinic acetylcholine receptors (Chu, Citation2001) and affect cognitive functions (Freo, Pizzolato, Dam, Ori, & Battistin, Citation2002; Ho & Wang, Citation2010, Citation2011). Stimulation of these receptors can improve sustained attention (Sarter, Givens, & Bruno, Citation2001). Thus, we hypothesize that betel nut may improve sustained attention both acutely and chronically. Contrary to the specific arecoline effects on the receptors, widespread arecoline effects on the central and autonomic nervous systems have been reported (Chu, Citation2001; Freo et al., Citation2002). Animal studies showed that these areas included frontal cortex that is related to attention (Freo et al., Citation2002); therefore, possibly both sustained attention and inhibitory control can be improved by arecoline. However, because sustained attention and inhibitory control are associated with different parts of frontal cortex (e.g., the right prefrontal area for sustained attention and the inferior frontal gyrus for inhibitory control), it remains possible that only one of them is affected.
Sleep deprivation can impair many cognitive functions that are dependent on the frontal cortex (Alhola & Polo‐Kantola, Citation2007), including sustained attention (Blatter et al., Citation2006) and inhibitory control (Drummond, Paulus, & Tapert, Citation2006). Therefore, we hypothesize that both sustained attention and inhibitory control can be impaired by deprived sleep.
Further, betel nut chewing may lead to an immediate improvement in habitual chewers' sustained attention and inhibitory control that have been impaired after one night of deprived sleep. After sleep deprivation, chewing betel nut can immediately improve habitual chewers' selective attention (Ho & Wang, Citation2010). Nevertheless, it remains possible that chewing betel nut is unable to improve sustained attention and inhibitory control, because these two aspects of attention and selective attention may differ in regard to their neural circuitry (Cabeza & Nyberg, Citation2000). Alternatively, chewing betel nut may acutely impair non‐chewers' sustained attention and inhibitory control. Previous studies (Chu, Citation2001) showed that betel nut can acutely induce uncomfortable feelings (e.g., dizziness and palpitation) for the non‐chewers. These uncomfortable feelings may worsen non‐chewers' sustained attention and inhibitory control.
In the current study, we adopted the sustained attention to response task (SART) and stop‐signal task for measuring sustained attention and inhibitory control, respectively. We tested whether chewing betel nut could influence participants' sustained attention and inhibitory control after one night of sleep deprivation. We hypothesize that (1) betel nut may have chronic effects on habitual chewers' sustained attention and inhibitory control, (2) sleep deprivation may impair sustained attention and inhibitory control, and (3) betel nut may have acute effects on habitual chewers' and non‐chewer's sustained attention and inhibitory control.
Method
Participants
Betel nut chewing participants were eligible if they were (1) current betel nut chewers, (2) at least 18 years of age, and (3) free from current major medical or vision problems that could interfere with the experiment protocol. We recruited the participants from Taichung city via three methods. First, the agency from human resource companies introduced day labours. Second, we posted the recruitment advertisement on the largest bulletin board system in Taiwan. Third, we asked former participants to introduce others. There were four different groups of participants (N = 16 per group): the habitual chewers with deprived sleep (deprived chewers), the non‐chewers with deprived sleep (deprived non‐chewers), the habitual chewers with normal sleep (normal chewers), and the non‐chewers with normal sleep (normal non‐chewers). Participants had a low level of drowsiness in daily life on the Epworth sleepiness scale (ESS; Johns, Citation1992), and were morning or intermediate types on the Morning–Evening Questionnaire (MEQ; Horne & Ostberg, Citation1976), ruling out the possibility that different everyday sleepiness status affected sleep deprivation (Table ). Each participant had normal or corrected‐to‐normal vision. None of them worked night shifts. All participants gave their informed consent, and the current study was approved by the Central Regional Research Ethics Center on human research.
Table 1. The means and standard deviation (SD; in parentheses) for the four groups
Apparatus
Both tasks were programmed with E‐prime software (Psychology Software Tools, Inc., Sharpsburg, PA, USA), and presented on a 17 inch CRT (cathode ray tube) desktop monitor (refresh rate = 85-Hz). The viewing distance was 50-cm. During the tasks in the morning, heart rate (HR) was monitored with an 8‐channel biofeedback system (ProComp Infiniti, model SA7500, Thought Technology, Montreal West, Quebec, Canada).
Design
SART
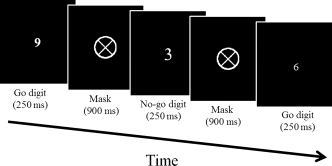
Participants watch a sequence of numbers generated randomly from 1 to 9 and are required to press a left mouse button as accurately and quickly as possible for each number they see (Fig. 1). The catch is that if they see a number 3, they need to withhold their response. Each digit appeared for 250-ms, followed by a 900‐ms mask composed of an X presented within a 3.3° ring with a diagonal cross in the middle. Participants made a response during the response period (1,150-ms) from the digit onset to the mask offset. The digit and mask were white and located centrally on a black display. The digit size (width × height) varied randomly between 0.3° × 0.5°, 0.8° × 1.3°, 1.0° × 1.6°, 1.3° × 2.1°, and 1.4° × 2.4°. The font name was ‘symbol’. There were 27 (3 trials per digit) practice trials, and 225 (25 trials per digit) formal trials.
Stop‐signal task
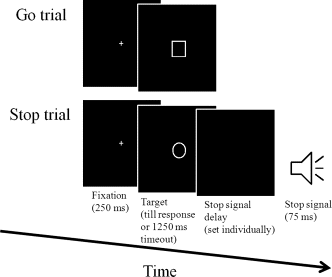
Each trial began with a fixation for 250-ms, followed by a target shape. The primary task was to identify a square (length = 1.9°) or a circle (diameter = 2.1°) (Fig. 2). Participants pressed the left mouse button to respond to a square and right mouse button to a circle as accurately and quickly as possible. The target remained on the screen until response, or until 1,250-ms had elapsed. Occasionally (25% of total trials), a stop signal (75-ms) was presented shortly after the target onset in the primary task, when participants withheld their responses (a stop trial). In the stop trials, the temporal delay between the target onset and the stop‐signal onset (stop‐signal delay, SSD) was initially set at 250-ms. When response inhibition was successful, SSD increased by 50-ms; when it was unsuccessful, SSD decreased by 50-ms. There were 32 practice trials and 3 blocks of 64 formal trials (16 stop trials and 48 go trials). Between blocks, participants had a 10‐s break where their performances on the last block would be displayed. The inter‐trial interval was 2,000-ms.
Procedure
Participants were first required to complete a questionnaire, including demographic background, the Verran and Snyder‐Halpern sleep scale (VSS; Snyder‐Halpern & Verran, Citation1987), betel nut usage history, and betel nut dependency scale (BNDS; Li, Ho, Tang, & Chang, Citation2012). The VSS was to evaluate their sleep quality the night before the experiment, and the BNDS was to assess the habitual chewers' dependency on betel nut. The non‐chewers did not need to complete the betel nut usage history and BNDS.
Each of the four groups underwent 2 (chewing gum or betel nut) × 2 (SART or stop‐signal task) conditions, counterbalanced across participants. Each participant came to the laboratory twice. Specifically, half of the participants took part in the chewing gum condition first, and the remaining half took part in the betel nut condition first. Within the gum‐first participants, half of them completed the SART first, and the other half completed the stop‐signal task first. The same rule applied to the betel nut‐first participants.
The gum we used had a different taste, texture, and color from the betel nuts, and was a small white cube of a size similar to that of a betel nut (about 3-cm × 3-cm × 2-cm). The gum was to control for the effect of mere chewing. We recognize that one cannot control for the effects based on anticipation. To estimate this chewing effect, a ‘no‐chewing control’ can be added. But because the betel nut and gum we used were similar in size, we simply assume that gum can control for the mere chewing. The two conditions were separated by about 6 days in average (deprived chewers: mean = 5.8 days, standard deviation (SD) = 1.4; deprived non‐chewers: mean = 6.4 days, SD = 1.3; normal chewers: mean = 5.1 days, SD = 2.5; and normal non‐chewers: mean = 6.6 days, SD = 1.1). There was no interval difference between these four groups (F(3, 60) = 2.64, p > .06). The laboratory prepared the betel nuts (bought from the nearby betel nut stalls) and the gums so that participants chewed the same type of substances.
The deprived‐sleep groups needed to stay awake all night in the company of the experimenter. Each participant arrived at the laboratory at 10.00pm, the night before the SART and stop‐signal task. The order of these two tasks was counterbalanced across participants. Participants could carry out quiet activities, including music listening, reading, watching TV, or video, playing video games, and so on. We did not allow activities that were too exciting (e.g., gambling games). To evaluate participants' sleepiness overnight, the Stanford sleepiness scale (SSS; Hoddes, Zarcone, Smythe, Phillips, & Dement, Citation1973) was administered every hour from 11.00pm to 7.00am. The following morning at 7.00am, each participant chewed either the betel nut or chewing gum before the SART and stop‐signal task. That is, for each task, after practice and before the formal trials, participants were required to chew the substance for 3-min. Immediately after they spat out the substance, the formal trials began. Heart‐rate was monitored before participants chewed the substance until the end of formal trials.
For the normal chewers and non‐chewers, the procedure was similar to that for the deprived‐sleep groups except that they had normal sleep at home before they began the tasks at 7.00am. The monitoring of SSS overnight was not required for the normal‐sleep groups.
Results and Discussion
Participant characteristics
Both normal chewers and deprived chewers had significantly higher BNDS than the cut point of 24 (all ps < .05) suggested by Li et al. (Citation2012) (Table ). This indicated that both chewer groups were betel nut dependent. The groups did not differ in gender ratio (χ2 = 3.85, df = 3, p = .28).
Manipulation check
Sleep deprivation on SSS
To assess sleepiness change during sleep deprivation, we conducted a 2 (group: deprived chewers and deprived non‐chewers) × 2 (substance: betel nut or gum) × 9 (time: each hour from 11.00pm to 7.00am) analysis of variance (ANOVA) on SSS. The first was a between‐participant factor, and the latter two were the repeated‐measure factors. Only the main effect of time was significant (F(8, 240) = 50.149, p < .0001, ηp2 = .626). Further analysis showed that SSS scores increased as the hours that participants stayed awake in the laboratory increased. The mean SSS score was 2 (‘Functioning at high levels, but not at peak; able to concentrate’) at 11.00pm and was 4 (‘Somewhat foggy, let down’) at 7.00am (p < .0001). The elevated SSS scores revealed an increasing fatigue during sleep deprivation.
Betel nut chewing on HR
To assess betel nut's acute effect on HR, we analysed HR measured by the biofeedback system at the three time points: immediately before participants chewed substance (‘BEFORE’), immediately after they spat out substance (‘AFTER’), and immediately after they completed the task (‘END’). We conducted a 2 (sleep: deprived or normal sleep) × 2 (group: chewers or non‐chewers) × 2 (substance: betel nut or gum) × 2 (task: SART or stop‐signal task) × 3 (time: BEFORE, AFTER, or END) ANOVA on HR. The first two were the between‐participant factors, and the latter three were the repeated‐measure factors.
A significant interaction effect of time × substance (F(2, 120) = 20.345, p < .0001, ηp2 = .253) indicated that chewing betel nut, but not gum, immediately heightened HR. There were no other significant interaction effects. Further analysis showed that HR increased immediately after chewing betel nut (BEFORE < AFTER) and decreased by the end of experiment (AFTER > END) (all ps < .0001) (BEFORE: mean = 78.5-bpm; AFTER: mean = 88.4-bpm; END: mean = 79.4-bpm). There was no difference between HRs before chewing betel nut and at the end of experiment (BEFORE = END, p = .426).
On the other hand, there was no HR difference between HRs before (mean = 79.3-bpm) and after chewing gum (mean = 79.3-bpm) (BEFORE = AFTER, p = .999). HR at the end of experiment (mean = 77.0-bpm) was smaller than those before and after chewing gum (BEFORE > END, AFTER > END, all ps < .05).
SART
The false alarm rates in No‐go trials and response times (RTs) preceding a false alarm and a successful response withholding were analysed to assess sustained attention (Cheyne, Carriere, & Smilek, Citation2006; Robertson, Manly, Andrade, Baddeley, & Yiend, Citation1997).
SART—False alarm rate
We conducted a 2 (sleep: deprived or normal sleep) × 2 (group: chewers or non‐chewers) × 2 (substance: betel nut or gum) ANOVA on the false alarm rate (Table ). The first two were the between‐participant factors, and the last was a repeated‐measure factor. The main effect of group was significant (F(1, 60) = 4.455, p < .05, ηp2 = .069), indicating a lower false alarm rate among the habitual chewers (mean = .468) than the non‐chewers (mean = .595). The main effect of sleep was marginally significantFootnote1 (F(1, 60) = 3.136, p = .07, ηp2 = .052), showing that the deprived‐sleep group (mean = .586) produced more false alarms than the normal‐sleep group (mean = .476). All the non‐significant F values were smaller than 1.98 (all ps > .17).
Table 2. The means and SD (in parentheses) in the SART
SART—RT prior to false alarm and successful withholding of response
We assessed whether speeding of responses preceding a false alarm (erroneously respond to digit 3) is faster than preceding a successful withholding of response. We computed the mean RTs in the four go trials preceding a false alarm error and preceding a successful withholding (Manly, Robertson, Galloway, & Hawkins, Citation1999; Robertson et al., Citation1997). Further, we conducted a 2 (sleep: deprived or normal sleep) × 2 (group: chewers or non‐chewers) × 2 (substance: betel nut or gum) × 2 (response: false alarm error or correct withholding) ANOVA on the preceding go RTs. The first two were the between‐participant factors, and the latter two were the repeated‐measure factor. The main effect of response was significant (F(1, 54) = 61.702, p < .0001, ηp2 = .533), showing shorter RTs prior to a false alarm error (mean = 333.8-ms) than a successful withholding (mean = 368.8-ms). This supported that the speeding of responses preceding a false alarm is faster than preceding a successful response withholding.
Further, the interaction of sleep × group was significant (F(1, 54) = 4.634, p < .05, ηp2 = .079). The normal habitual chewers (mean = 381.9-ms) had longer RT than the normal non‐chewers (mean = 296.6-ms) (p < .010). The deprived habitual chewers (mean = 352.1-ms) had similar RT as the deprived non‐chewers (mean = 359.1-ms) (p > .8). The longer RT in the habitual chewers (particularly when they had normal sleep) means that they had less prepotent tendency to respond than the non‐chewers. All the non‐significant F values were smaller than 2.197 (all ps > .14).
SART—Correct rate
We applied the 2 × 2 × 2 ANOVA on the correct rate and found that only the main effect of sleep was significant (F(1, 60) = 9.691, p < .005, ηp2 = .139), indicating a larger correct rate in the normal sleep condition (mean = .987) than the deprived sleep condition (mean = .947). All the non‐significant F values were smaller than 1.979 (all ps > .165).
Stop‐signal task
The stop‐signal reaction time (SSRT) was primarily analysed to assess inhibitory control ability (Table ). Presumably, the finishing time of stop processes falls somewhere along the go RT distribution. In stop trials, the go RTs faster than the finishing time of stop processes lead to response execution (the probability is P(response|stop signal)). Obversely, go RTs slower than the stop processes finishing time leads to successful response inhibition. Therefore, the finishing time of stop processes would be equal to the ranked RT determined by multiplying number of go trials by P(response|stop signal) (Schachar et al., Citation2007; van den Wildenberg, van der Molen, & Logan, Citation2002; Verbruggen, Liefooghe, & Vandierendonck, Citation2004). For example, if P(response|stop signal) is .45 and the number of go trials is 100, the finishing time of stop processes would be the 45th percentile (.45 × 100) of go RT distribution. Because the stop processes would not begin until the onset of the stop signal, the SSRT is obtained by subtracting the SSD from the finishing time of stop processes. The SSRT for each individual were accordingly computed and analysed.
Table 3. The means and SD (in parentheses) in the stop‐signal task
Stop‐signal task—SSRT
We conducted a 2 (sleep: deprived or normal sleep) × 2 (group: chewers or non‐chewers) × 2 (substance: betel nut or gum) ANOVA on the SSRT. The first two were the between‐participant factors, and the last was a repeated‐measure factor. Only the main effect of sleep was significant (F(1, 60) = 5.611, p < .05, ηp2 = .086), showing a longer SSRT in deprived sleep condition (mean = 252.6-ms) than normal sleep condition (mean = 222.4-ms). This indicates that sleep deprivation weakens inhibitory control. All the non‐significant F values were smaller than 2.481 (all ps > .120).
Stop‐signal task—Miss rate, incorrect rate, and go RT
The same 2 × 2 × 2 ANOVA was conducted on the miss rate, incorrect rate, and go RT. For the miss rate, only the main effect of sleep was significant (F(1, 60) = 12.007, p < .005, ηp2 = .167), showing larger miss rate when sleep was deprived (mean = .084) than when sleep was normal (mean = .035). All the non‐significant F values were smaller than 2.881 (all ps > .10). For the incorrect rate, there were no any significant effects (all Fs < 1.326; all ps > .25).
For the go RT, there was a marginally significant sleep × group effect (F(1, 60) = 3.167, p = .08, ηp2 = .05), showing that in the non‐chewers, deprived‐sleep participants (mean = 842.7) had longer RT than the normal‐sleep participants (mean = 736.2) (p < .05). In the habitual chewers, deprived‐sleep participants (mean = 765.4) had similar RT as the normal‐sleep participants (mean = 780.5) (p = .771). All the non‐significant F values were smaller than 2.544 (all ps > .12).
General Discussion
We asked whether chewing betel nut could influence the habitual chewers' and non‐chewers' sustained attention and inhibitory control after one night of sleep deprivation. Our hypotheses were partially supported. Betel nut had chronic effects on habitual chewers' sustained attention. Sleep deprivation can impair inhibitory control. Betel nut did not have acute effects on sustained attention and inhibitory control.
In the SART, the habitual chewers had lower false alarm rates in the No‐go trials. This indicates that the habitual chewers are more likely (relative to the non‐chewers) to execute a mindful and conscious withholding of response in a monotonous task that requires only repetitive key tapping response. We also found that errors in SART can be predicted by participants' response speed on go trials prior to errors (Cheyne et al., Citation2006; Manly et al., Citation1999; Robertson et al., Citation1997). We found that RTs prior to a false alarm error were faster than those prior to a successful inhibition. This reveals a prepotent response tendency prior to a false alarm and a slower RT that may be beneficial for development of a successful withholding response (Manly et al., Citation1999). Further, the sleep × group interaction on the preceding go RTs reveals that the habitual chewers had less of a prepotent tendency to respond than the non‐chewers when they had normal sleep.
We reported that sleep deprivation led to a deterioration in inhibitory control. In the stop‐signal task, the deprived‐sleep participants had longer SSRT than the normal‐sleep participants did. Finally, betel nut did not have acute effects on sustained attention and inhibitory control for both the habitual chewers and the non‐chewers.
Arecoline has been shown to act on the muscarinic and nicotinic acetylcholine receptors; these play important roles in sustained attention (Sarter et al., Citation2001). Repeated administration of arecoline may also cause up‐regulation of muscarinic and nicotinic acetylcholine receptors, resulting in better sustained attention. Physiological studies are required to explore whether repeated administration of arecoline can cause up‐regulation.
We did not find a chronic chewing effect on inhibitory control measured by the stop‐signal task. It remains possible that betel nut chewing can affect other forms of inhibition measured by other tasks. There are at least three forms of inhibition: inhibition of prepotent response, inhibition of competing distractors, and inhibition of proactive interference (Nigg, Citation2000). The stop‐signal task measures the inhibition of a prepotent response (Wignall & de Wit, Citation2011), but not the other two forms. Therefore, it remains possible that betel nut chewing can affect other forms of inhibition. Wignall and de Wit (Citation2011) reported that nicotine can improve the ability to suppress the irrelevant, competing distractor (i.e., colours in the Stroop task), but may not improve the ability to suppress the intended response in the stop‐signal task.
We reported that sleep deprivation impaired inhibitory control, providing convergent evidence that sleep deprivation impairs various aspects of cognition (Alhola & Polo‐Kantola, Citation2007). Sleep deprivation selectively affects cognitive tasks that are dependent on the frontal cortex (e.g., Alhola & Polo‐Kantola, Citation2007). Because inhibitory control requires the involvement of frontal cortex (e.g., the fronto‐basal‐ganglia circuit), it is likely to be weakened by sleep deprivation. However, although sustained attention also depends on the frontal cortex (e.g., the right fronto‐parietal circuit), sleep deprivation seems not to impair it (marginal significance). Possibly, since arecoline can stimulate the cholinergic system and improve sustained attention, chronic betel nut chewing may overcome the disadvantage of sleep deprivation for the habitual chewers. It is also possible that a short‐term sleep deprivation in the current study (9-hr) is insufficient to impair sustained attention. Indeed, Doran, Van Dongen, and Dinges (Citation2001) reported that longer sleep deprivation (e.g., over 36-hr) can impair sustained attention.
In the go trials, sleep deprivation increased the miss rate in the stop‐signal task and lowered the correct rate in the SART. This omission due to sleep deprivation is consistent with previous studies (e.g., Williamson & Feyer, Citation2000). Given deprived sleep, lapses in attention can take place when a manual response is required (e.g., go trials). Sleep deprivation may decrease performance efficacy to an extent similar to that of alcohol intoxication (Williamson & Feyer, Citation2000).
Betel nut did not produce acute effects on sustained attention and inhibitory control. Immediate elevation of HR after chewing betel nut (but not gum) ruled out the possibility that betel nut and gum have comparable physiological effects. One possibility to explain this null result is that one betel nut consumed before each task may be insufficient to affect sustained attention and inhibitory control. One betel nut would contain only about 1.3-mg arecoline (Ho & Wang, Citation2011).Footnote2 However, this amount of arecoline may be too little for an adult chewer, given that some animal studies (e.g., Freo et al., Citation2002) required 15-mg/kg to observe a widespread effect of arecoline on many brain areas. Accordingly, a 60‐kg adult chewer needs to consume 900-mg of arecoline (692 betel nuts) at a time to acutely affect relevant brain areas, which is highly impossible in real life and laboratory. Although a huge amount of betel nuts may be needed for acute effects, we did not exclude the possibility that chewing one betel nut can immediately enhance the chewers' positive expectations and feelings.
A raw areca nut has acidity so that repeated use can cause stomach problems. With high alkalinity, the slaked lime can neutralize this acidity. It is unclear whether this neutralization affects arecoline absorption. But since the HR elevation caused by betel nut was observed, arecoline should be absorbed to some degree sufficient to influence the chewers.
It may be the case that the null acute effects (betel nut vs gum) may be caused by insufficient statistical power. However, because the chewing substance was a repeated‐measure factor, it can increase the statistical power, since the individual difference was ruled out.
Limitations
We matched the four groups by adopting many variables regarding betel nut use and sleep habits, believed to be important to our purpose. This matching provides our study with internal validity. However, this strict matching and the small sample size may result in poor ecological validity. In the future, more participants and variables can be collected to examine the current issue.
We recorded the chewers' betel nut history and their dependency. But without the assistance of psychiatrists, we were unable to tell whether these chewers reached the dependency diagnostic criteria (e.g., ICD‐10 or DSM V). Possibly, many chewers did not reach the criteria, reducing the performance differences between them and the non‐chewers. However, it is equally possible that many chewers reached the criteria, leading to significant differences. In the future, the psychiatrists can be included to help clarify these possibilities.
Note that sleep deprivations is not the same for all deprived participants.Footnote3 In the future, polysomnography can be employed to monitor participants' deprived sleep conditions more completely.
Acknowledgements
This study was supported by NSC‐98‐2410‐H‐040‐005‐MY3 from the National Science Council. We thank Wei‐Jyun Sie, Kun‐Bang Lin, Jun‐Yu Chen, Ya‐Ling Shih, Kai‐Hui Hsu, and Je‐Fu Deng for their assistance with the current study.
Additional information
Funding
Notes
Conflict of interest: None.
1. This and other marginal significances in the current study require about 153 participants (p = .05, power = .7) to achieve significance (Cohen, Citation1988). Since the effect sizes are rather small and these two marginal significances did not change our main conclusions, we will not discuss them further in the General Discussion.
2. In Ho and Wang (Citation2011), the arecoline was extracted from 60 betel nuts and weighed 80.2-mg. Therefore, it was estimated that each betel nut may contain about 1.3-mg arecoline. The test dose is similar to the chewing behaviour in daily life in which the chewers usually chew one betel nut at a time.
3. We thank one anonymous reviewer for this possibility.
References
- Alhola, P., & Polo‐kantola, P. (2007). Sleep deprivation: Impact on cognitive performance. Neuropsychiatric Disease and Treatment, 3(5), 553–567.
- Anstey, K. J., Mack, H. A., & Cherbuin, N. (2009). Alcohol consumption as a risk factor for dementia and cognitive decline: Meta‐analysis of prospective studies. The American Journal of Geriatric Psychiatry, 17(7), 542–555.
- Bekker, E., Bocker, K., Van hunsel, F., van den Berg, M., & Kenemans, J. (2005). Acute effects of nicotine on attention and response inhibition. Pharmacology, Biochemistry, and Behavior, 82(3), 539–548.
- Blatter, K., Graw, P., Munch, M., Knoblauch, V., Wirz‐justice, A., & Cajochen, C. (2006). Gender and age differences in psychomotor vigilance performance under differential sleep pressure conditions. Behavioural Brain Research, 168(2), 312–317.
- Cabeza, R., & Nyberg, L. (2000). Imaging cognition II: An empirical review of 275 PET and fMRI studies. Journal of Cognitive Neuroscience, 12(1), 1–47.
- Cheyne, J. A., Carriere, J. S. A., & Smilek, D. (2006). Absent‐mindedness: Lapses of conscious awareness and everyday cognitive failures. Consciousness and Cognition, 15(3), 578–592.
- Chu, N. S. (2001). Effects of betel chewing on the central and autonomic nervous system. Journal of Biomedical Science, 8, 229–236.
- Cohen, J. (1988). Statistical power analysis for the behavioral sciences. Hillsdale, NJ: Lawrence Erlbaum Associates.
- de Wit, H. (2009). Impulsivity as a determinant and consequence of drug use: A review of underlying processes. Addiction Biology, 14(1), 22–31.
- Department of Statistics (2003). Manufacture and employment of transportation, storage and communication. Taipei, Taiwan: Ministry of Transport and Communication.
- Doran, S. M., Van dongen, H. P., & Dinges, D. F. (2001). Sustained attention performance during sleep deprivation: Evidence of state instability. Archives Italiennes de Biologie, 139, 253–267.
- Drummond, S. P. A., Paulus, M. P., & Tapert, S. F. (2006). Effects of two nights sleep deprivation and two nights recovery sleep on response inhibition. Journal of Sleep Research, 15(3), 261–265.
- Fillmore, M. T. (2007). Acute alcohol‐induced impairment of cognitive functions: Past and present findings. International Journal on Disability and Human Development, 6(2), 115–125.
- Finnigan, F., Schulze, D., & Smallwood, J. (2007). Alcohol and the wandering mind: A new direction in the study of alcohol on attentional lapses. International Journal on Disability and Human Development, 6(2), 189–199.
- Freo, U., Pizzolato, G., Dam, M., Ori, C., & Battistin, L. (2002). A short review of cognitive and functional neuroimaging studies of cholinergic drugs: Implications for therapeutic potentials. Journal of Neural Transmission, 109(5–6), 857–870.
- Heishman, S. J., Kleykamp, B. A., & Singleton, E. G. (2010). Meta‐analysis of the acute effects of nicotine and smoking on human performance. Psychopharmacology, 210(4), 453–469.
- Ho, M. C., & Wang, C. K. (2010). Can betel nut chewing affect the UFOV size after sleep deprivation? Chinese Journal of Psychology, 52, 445–456.
- Ho, M. C., & Wang, C. K. (2011). The effect of betel nut chewing on contour and object masking. Attention, Perception & Psychophysics, 73(8), 2583–2593.
- Hoddes, E., Zarcone, V., Smythe, H., Phillips, R., & Dement, W. C. (1973). Quantification of sleepiness: A new approach. Psychophysiology, 10(4), 431–436.
- Horne, J. A., & Ostberg, O. (1976). A self‐assessment questionnaire to determine morningness‐eveningness in human circadian rhythms. International Journal of Chronobiology, 4(2), 97–110.
- IARC (2004). Betel‐quid and areca‐nut chewing and some areca‐nut‐derived nitrosamines. IARC Monographs on the Evaluation of Carcinogenic Risks to Humans, 85, 1–334.
- Johns, M. W. (1992). Reliability and factor analysis of the Epworth Sleepiness Scale. Sleep, 15(4), 376–381.
- Li, C. S., Luo, X., Yan, P., Bergquist, K., & Sinha, R. (2009). Altered impulse control in alcohol dependence: Neural measures of stop signal performance. Alcoholism, Clinical and Experimental Research, 33(4), 740–750.
- Li, R. H., Ho, M. C., Tang, T. C., & Chang, C. F. (2012). Development of the betel nut dependency scale (BNDS). Chinese Journal of Psychology, 54, 331–348.
- Manly, T., Robertson, I. H., Galloway, M., & Hawkins, K. (1999). The absent mind: Further investigations of sustained attention to response. Neuropsychologia, 37, 661–670.
- Nigg, J. T. (2000). On inhibition/disinhibition in developmental psychopathology: Views from cognitive and personality psychology and a working inhibition taxonomy. Psychological Bulletin, 126(2), 220–246.
- Robertson, I. H., Manly, T., Andrade, J., Baddeley, B. T., & Yiend, J. (1997). ‘Oops!’: Performance correlates of everyday attentional failures in traumatic brain injured and normal subjects. Neuropsychologia, 35(6), 747–758.
- Rusted, J. M., Caulfield, D., King, L., & Goode, A. (2000). Moving out of the laboratory: Does nicotine improve everyday attention? Behavioural Pharmacology, 11(7–8), 621–629.
- Sarter, M., Givens, B., & Bruno, J. P. (2001). The cognitive neuroscience of sustained attention: Where top‐down meets bottom‐up. Brain Research Reviews, 35(2), 146–160.
- Schachar, R., Logan, G. D., Robaey, P., Chen, S., Ickowicz, A., & Barr, C. (2007). Restraint and cancellation: Multiple inhibition deficits in attention deficit hyperactivity disorder. Journal of Abnormal Child Psychology, 35(2), 229–238.
- Snyder‐halpern, R., & Verran, J. A. (1987). Instrumentation to describe subjective sleep characteristics in healthy subjects. Research in Nursing & Health, 10(3), 155–163.
- van den Wildenberg, W. P., van der Molen, M. W., & Logan, G. D. (2002). Reduced response readiness delays stop signal inhibition. Acta Psychologica, 111(2), 155–169.
- Verbruggen, F., Liefooghe, B., & Vandierendonck, A. (2004). The interaction between stop signal inhibition and distractor interference in the flanker and Stroop task. Acta Psychologica, 116(1), 21–37.
- Wignall, N. D., & de Wit, H. (2011). Effects of nicotine on attention and inhibitory control in healthy nonsmokers. Experimental and Clinical Psychopharmacology, 19(3), 183–191.
- Williamson, A. M., & Feyer, A. M. (2000). Moderate sleep deprivation produces impairments in cognitive and motor performance equivalent to legally prescribed levels of alcohol intoxication. Occupational and Environmental Medicine, 57(10), 649–655.