Abstract
Objective
Adaptive human behaviour requires cognitive control ‐ the monitoring of actions and performance, to regulate and coordinate ongoing behaviour. Major depression is associated with neuropsychological differences in cognitive control, however behavioural experiments have failed to consistently reflect this. We explore this discrepancy.
Method
Two experiments were conducted, in which participants completed an Emotional Stroop task, and the Beck Depression Inventory‐II. In Experiment 1, participants were instructed to judge the font colour of a printed word. The word could have emotional or non‐emotional meaning. In Experiment 2, participants judged the font colour of the word, and also identified whether any letter was italicised. This manipulation was designed to induce errors to facilitate analysis.
Results
Depression symptoms are linked to severe deficits in cognitive control following errors. In Experiment 1, for emotional words, major depression symptoms were associated with a failure to instigate behavioural adjustments following errors, leading to reduced performance (F(1,25) = 4.61, p = .042). For non‐emotional content, we found major depression symptoms were associated with substantial adjustments following errors, mitigating reduced performance. These findings were replicated in Experiment 2 using a more robust analysis (F(1,30) = 6.45, p = .017).
Conclusions
These findings suggest that under emotional priming, major depression is marked by a failure to adapt behaviour in response to relevant environmental feedback. This work has implications for interpreting prior and future scientific findings, and may also inform clinical applications for depression treatment.
What is already known about this topic
Post‐error slowing is a benchmark of reactive cognitive control.
Depression is associated with deficits in reactive cognitive control when emotional regulation is required.
Despite the links between depression and reactive cognitive control, and the strong links between depression and neurophysiology of errors and error awareness, post‐error slowing has not been reliably linked to depression.
What this topic adds
For participants with depression symptoms, reliable and severe impairments in reactive control were identified when post‐error slowing was calculated separately for emotional and non‐emotional (neutral) stimuli.
When participants with depression symptoms were primed with task‐irrelevant emotional content, reactive cognitive control was compromised; this failure to slow down after errors resulted in a substantial reduction in accuracy.
In the case of a major depressive episode—where task‐irrelevant emotional thoughts are frequent, automatic, and ruminated upon—an inability to monitor environmental feedback may severely disrupt adaptive and goal‐driven behaviour.
INTRODUCTION
Major Depressive Disorder (depression) accounted for 4.3% of disability adjusted life years worldwide in 2004, and is expected to be the leading cause of disability adjusted life years by 2030 (Mathers & Ma Fat, Citation2008). Cognitive theories of depression have spawned well‐supported psychotherapeutic treatments (e.g., Beck, Rush, Shaw, & Emery, Citation1979) that target the core symptoms of the disorder—emotional dysregulation, cognitive or information processing deficits, and behavioural deficits. To improve treatment outcomes, it is imperative to better understand the relationship between these core symptoms (Gotlib & Joormann, Citation2010). Here we help clarify these relationships by documenting the impact of emotional priming on cognitive control—the ability to regulate information processing and maintain goal‐directed behaviour under varying environmental demands.
Cognitive control has been conveniently dichotomised as either proactive or reactive (Braver, Citation2012). Proactive control describes the preparatory cognitive adjustments required to successfully adapt behaviour for a known environment. For example, it is common to minimise emotional behaviour (e.g., fear, crying) in professional settings as compared to other settings. Reactive control describes the post‐hoc cognitive adjustments required to adapt behaviour in response to an unplanned or unexpected challenge. For example, additional and specific adjustments are sometimes required to minimise emotional behaviour when an unplanned event occurs (e.g., conflict, a demotion). Recent evidence hints that depression might be associated with a specific deficit in reactive control when emotional regulation is required (Holmes & Pizzagalli, Citation2007; Saunders & Jentzsch, Citation2014).
Two long‐standing behavioural markers of reactive control are post‐error adjustment of response time, and post‐error adjustment of response accuracy. In cognitive tasks participants typically slow down following errors (Laming, Citation1979; Rabbitt, Citation1966), an adjustment commonly argued to aide performance, in terms of accuracy, on subsequent attempts (Botvinick, Braver, Barch, Carter, & Cohen, Citation2001; Laming, Citation1979). In tasks where accuracy is high this post‐error slowing is believed to be ubiquitous and is considered a benchmark of reactive cognitive control (Botvinick et al., Citation2001).
Depression and reactive cognitive control are linked by well‐established findings in neurophysiological tasks. For example, Pizzagalli, Peccoralo, Davidson, and Cohen (Citation2006) note that structural (Ballmaier et al., Citation2004), neurochemical (Auer et al., Citation2000), and functional (Kumari et al., Citation2003) differences have been documented for depressed populations in the anterior cingulate cortex, a region central to the network implicated in error monitoring and correction. In addition, EEG studies have demonstrated that changes in the error‐related negativity (ERN) (Olvet & Hajcak, Citation2008; West, Choi, & Travers, Citation2010) and error‐positivity (PE) (Holmes & Pizzagalli, Citation2010) are associated with depression.
Given these links between depression and the brain regions implicated in reactive cognitive control, it is extremely surprising that the behavioural signatures of reactive cognitive control—post‐error adjustments—have not been reliably linked to depression. Rather, post‐error slowing and adjustments of post‐error accuracy have been described as inconsistent or non‐specific for depressed populations (Pizzagalli et al., Citation2006; Saunders & Jentzsch, Citation2014). Some evidence hints that reactive cognitive control, and therefore post‐error adjustments, may be compromised for depressed individuals when emotional regulation is required. For example, Holmes and Pizzagalli (Citation2007) found when spurious negative performance feedback was given to participants to begin a block of trials in a cognitive task; those with elevated depression scores exhibited impaired post‐error slowing. Compared to subjects with low depression scores, those with elevated depression showed faster RT (accompanied by a lower accuracy) following errors. Similarly, Saunders and Jentzsch (Citation2014) found subjects with high depression scores had disrupted reactive control mechanisms, as evidenced by an impaired congruency sequence effect,Footnote1 in an emotional Stroop task, but not in a classic Stroop task. Importantly, the emotional Stroop task requires emotional regulation, whereas the classic Stroop task does not. We aimed to extend upon these findings for depressed participants by analysing post‐error adjustments (or their absence) in the emotional Stroop task (Ben‐Haim et al., Citation2016; Williams, Mathews, & MacLeod, Citation1996).
Interestingly, Compton et al. (Citation2008) performed a similar study, using a modified Emotional Stroop task to explore the effect of emotional stimuli on post‐error slowing in a population with depression symptoms. Participants were asked to indicate the number of words presented on a monitor. Up to four emotionally charged words, or emotionally neutral words, were presented on each trial. As depression symptoms increased, post‐error slowing increased and post‐error accuracy decreased. Unfortunately, these findings are difficult to meaningfully interpret because post‐error adjustments were averaged across emotional and neutral trials. Any effect of emotional stimuli could have been contaminated by neutral stimuli. The experiment also featured performance feedback after each trial, which may give rise to post‐error slowing independently of erroneous performance (Saunders & Jentzsch, Citation2012).
We remedied these issues in two experiments by using a classic emotional Stroop task (Williams et al., Citation1996) with no feedback on performance, and by considering emotional and non‐emotional content separately. Depression symptoms were assessed using the Beck Depression Inventory–II (Beck, Steer, & Brown, Citation1996). We suspected that participants with depression symptoms would show impaired post‐error slowing following emotionally charged stimuli. Our findings demonstrate that post‐error adjustments may be reliably associated with depression symptoms—when emotional regulation is experimentally controlled.
EXPERIMENT 1
Method
Participants
Thirty‐three undergraduate psychology students (24 females) volunteered through an online experimental management system. Subject ages ranged from 18 to 43 (M = 24.6, SD = 8.0). Participants had normal or corrected to normal vision, English as first language, and were compensated with course credits.
Stimuli and apparatus
Stimuli were presented using Python which also recorded response times accurate to 1 ms. Button responses were made on a Cedrus response pad and button locations were counterbalanced across participants.
Table shows the complete stimulus set of 24 words, six positive emotional words (e.g., BETTER, GLAD), six negative emotional words (BITTER, SAD) and 12 uncategorised non‐emotional words (BUTTER, PAD). Non‐emotional and emotional words were matched on frequency, length, and were orthographic neighbours where possible (BITTER, BUTTER). Items were piloted at an earlier stage to ensure accurate categorisation into emotionally positive, emotionally negative and non‐emotional conditions. Post‐experiment, participants completed a word identification questionnaire to further ensure word conditions were correctly categorised, revealing 98% correctly identified, with no participants excluded for poor accuracy. Words were printed in red or green colour (RGB values of 220, 0, 0, and 0, 170, 0, for red and green, respectively). Words appeared in uppercase Arial font, bold and size 30.
Table 1. List of stimulus items for Experiments 1 and 2
Procedure
The experiment was conducted in a quiet, dimly lit room with air‐conditioning. Participants sat 60 cm from the 17″ monitor so stimuli occupied a visual angle of up to 4.77°. Participants were given task instructions followed by 24 demonstration trials in which correct responses were automatically displayed on the screen. This was followed by 24 practice trials with participant responses and feedback, and then 24 practice trials without feedback. Data were collected in the subsequent eight experimental blocks with a forced 1 min break between blocks. On each trial, a single word was presented in the centre of a white background. To avoid adaptation and minimise reliance on local cues we introduced a trial‐to‐trial spatial uncertainty of up to 40 pixels around the target location. Participants were asked to identify the colour of the word while refraining from reading the word, by pressing either the red‐ or green‐marked button. On each trial, presentation of a fixation cross for 500 ms was followed by a blank screen for 500 ms, then followed by the stimulus for a maximum of 4,000 ms. The presentations of stimuli were response terminated.
The words used are listed in Table ; within a block each word appeared two times printed in red and two times in green, totalling 96 trials per block, with order of presentation randomised. At the end of the experiment participants were presented with a list of all stimulus words (Table ) and indicated how they responded to each word during the experiment: non‐emotional, emotionally positive or emotionally negative. Participants overwhelmingly concurred with our emotional categorisation of items (98% agreement). Participants then completed the Beck Depression Inventory–II (Beck et al., Citation1996) which has demonstrated strong validity and reliability in nonclinical and clinical populations.
RESULTS
Depression (BDI‐II) scores
Scores on the BDI‐II ranged from 0 to 44 (M = 10.09, SD = 9.71). Consistent with Compton et al. (Citation2008), participants scoring ≥20 on the BDI‐II (n = 6) were categorised as displaying depression symptoms, and participants scoring ≤12 (n = 22) were categorised as controls.
Response time and post‐error slowing
Mean scores were calculated for each participant and variable of interest using Matlab. These mean scores were then collated and analysed using JASP (Love et al., Citation2015). We note accuracy, response time (RT), and post‐error slowing showed no difference for positive and negative stimuli—hence these data were collapsed to form the stimuli category Emotional Stimuli.
For Experiment 1, mean RT across all participants ranged from 353 to 677 ms (M = 478 ms, SD = 67 ms) and showed no relationship to BDI‐II (rho = 0.11, p = .537). Mean accuracy ranged from 78.9–99.5% (M = 94.7%, SD = 4.7%) and also showed no relationship to BDI‐II (rho = 0.14, p = .436). Paired sample t‐tests revealed no differences across participants for neutral (M = 468 ms; M = 95.1%) versus emotional (M = 465 ms; M = 94.5%) words for either accuracy, t(32) = 0.76, p = .451, or correct response RT, t(32) = 1.63, p = .114. These RT results confirmed there was no emotional Stroop effect (Williams et al., Citation1996) observed in our data, which is defined as slower responding for emotional words relative to neutral words on the current trial. The lack of an emotional Stroop effect is a common finding for intermixed presentation designs (Algom, Chajut, & Lev, Citation2004).
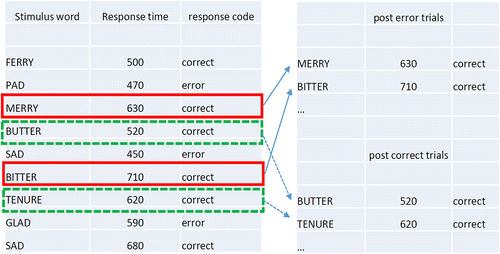
We aimed to calculate post‐error adjustments using two methods—the traditional method, and the robust method (Dutilh, van Ravenzwaaij, et al., Citation2012). The traditional method is commonly applied and involves subtracting the mean RT of a participant's post‐error trials from the mean RT of their post‐correct trials (see Fig. 1 for example calculation). Similarly, for accuracy, it subtracts the conditional probability of a hit preceded by a hit from the conditional probability of a hit preceded by a miss. A drawback of the traditional method is that it produces an average effect across the entire sequence of trials. Therefore, it cannot differentiate the impact of errors from long‐term effects like fatigue, distraction, or boredom (Dutilh, van Ravenzwaaij, et al., Citation2012). This is a potential problem when comparing depressed versus control groups, because these long‐term effects may be more prevalent in populations with depression (Paelecke‐Habermann, Pohl, & Leplow, Citation2005; Veiel, Citation1997).
The robust method overcomes this problem by pairing post‐error trials with immediately preceding pre‐error counterparts that are also post‐correct trials. Pairwise differences are then calculated (i.e., post‐error RT minus (pre‐error + post‐correct) RT), with the mean of the differences providing the robust measure of post‐error RT adjustments (Dutilh, van Ravenzwaaij, et al., Citation2012). The same type of pairing may be employed to calculate post‐error accuracy adjustments, and in both cases controls for undesired long‐term effects (undesired, as they are not indicative of reactive cognitive control). A drawback of the robust method is that some errors cannot be paired with pre‐error counterparts and are discarded from analysis. This means the robust method may require more errors to provide meaningful results.
In Experiment 1 there was a small error rate, and an uneven spread of errors across categories of word valence. For neutral stimuli, two participants made no errors, and a further nine made fewer than five errors. For emotional stimuli 13 participants made fewer than five errors. Because of this lack of errors and uneven spread, we were unable to meaningfully calculate post‐error effects using the robust method, or post‐error accuracy adjustments. We were able to calculate the traditional method of post‐error slowing, as outlined in Fig. 1. We subsequently conducted Experiment 2, which rectified the issue by also allowing the robust calculation.
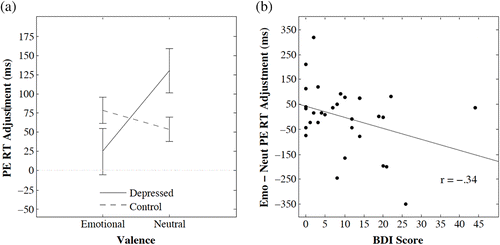
We calculated post‐error slowing separately for neutral and emotional stimuli. That is, the mean RT of trials following errors on emotional stimuli was subtracted from mean RT of trials following correct responses on emotional stimuli, and likewise for neutral stimuli. These results are presented in Fig. 2a, separately for depressed and control groups. Of paramount interest was whether participants with depression symptoms (n = 6) showed the same pattern of post‐error slowing as controls (n = 21; one control subject made no errors on neutral stimuli). Fig. 2a highlights participants with depression symptoms showed sizeable post‐error slowing following neutral words (M = 129.9 ms) but very little post‐error slowing following emotional words (M = 24.8 ms). In contrast, controls showed consistent post‐error slowing for both neutral (M = 54.0 ms) and emotional (M = 78.3 ms) words. A mixed‐model analysis of variance (ANOVA) confirmed the interaction between depression category and word valence was significant, F(1,25) = 4.61, p = .042, effect‐size partial Eta squared = 0.156. There was no main effect of depression category or word valence [F(1,25) = 1.80, p = .192; F(1,25) = 0.067, p = .798].Footnote2
Fig. 2b depicts the marginally reliable negative relationship between the difference in post‐error slowing for emotional and neutral words and BDI‐II scores for all participants (rho = −0.34, p = .065, 95% confidence interval = (−0.617, 0.021)).
INTERIM DISCUSSION
Word valence (emotional or neutral) substantially impacted the behavioural benchmark of reactive cognitive control in participants with depression symptoms, but not controls. For participants with depression symptoms, post‐error slowing was absent for errors made on emotional words. Furthermore, the difference between post‐error slowing on neutral words and emotional words was associated with the degree of depression symptoms across the full range of BDI‐II scores, suggesting the degree of cognitive control impairment was associated with the degree of depression symptoms.
The results of Experiment 1 were thus encouraging, but rested on only a few participants with depression symptoms and a marginally reliable correlation. We were also unable to meaningfully calculate post‐error accuracy adjustments, or to compare the traditional and robust methods of measuring post‐error slowing (see Williams, Heathcote, Nesbitt, & Eidels, Citation2016) because of the lack of errors. We therefore introduced a second decision in Experiment 2 to add cognitive load and therefore increase the number of errors (Heathcote et al., Citation2015; Strayer & Johnston, Citation2001). We hoped this would allow post‐error accuracy adjustments and additional post‐error slowing measures to be meaningfully calculated.
EXPERIMENT 2
Method
Participants
Thirty‐seven undergraduate psychology students (29 females) volunteered through an online experimental management system. Subject ages ranged from 18 to 48 (M = 23.3, SD = 6.1). To recruit a greater number of participants with depression symptoms, our recruitment poster indicated a preference for very happy or very sad volunteers. Exclusion criteria were as per Experiment 1.
Stimuli and apparatus
The word set was the same as Experiment 1 (see Table again for the complete list). In Experiment 2, each word could be presented with and without italic letters. When italicised, a single letter in the string—either at the beginning, middle, or end of each word stimuli—would be italic. Participants were instructed to respond differently depending on whether the word contained an italicised letter. Thus, the task required scanning of the letters but did not require processing of the emotional content. Each trial had an equal likelihood of sampling an italicised or non‐italicised word.
Procedures
Participants were given task instructions followed by 12 example trials with automated responses. This was followed by 20 practice trials with participant participation and feedback and then 20 practice trials without feedback. Data were collected in the subsequent eight experimental blocks. The words listed in Table were presented on average three times per block, and there were a total of 72 trials per block. Participants were asked to identify the colour of the word and whether or not it contained an italic letter. The four response options were “italic red”, “italic green”, “non‐italic red”, and “non‐italic green”.
To differentiate the impact of errors from long‐term effects like fatigue, distraction, or boredom, we employed both the traditional and robust (Dutilh, van Ravenzwaaij, et al., Citation2012; more details below) methods of calculating post‐error slowing. The procedure was otherwise the same as Experiment 1.
RESULTS
Accuracy, response time (RT), and post‐error slowing again showed no difference for positive and negative stimuli, thus we again combined these data to form an Emotional Content condition. Scores on the BDI‐II ranged from 0 to 40 (M = 12.95, SD = 10.47). Participants with depression symptoms (n = 9) and controls (n = 23) were classified as per Experiment 1.
Mean RT ranged from 684 ms to 1,587 ms (M = 998 ms, SD = 211 ms) and showed no relationship to BDI‐II (rho = 0.17, p = .315). Accuracy ranged from 85.2–98.9% (M = 94.0%, SD = 3.2%) and showed a marginal tendency to increase as BDI‐II scores increased (rho = 0.31, p = .064). A paired sample t‐test revealed no difference in accuracy for neutral (M = 93.6%) versus emotional (M = 94.3%) words, t(36) = 0.98, p = .334. However, a similar t‐test revealed a positive emotional Stroop effect where RTs were slower for emotional (M = 979 ms) than neutral (M = 959 ms) words, t(36) = 3.57, p = .001. This emotional Stroop effect showed no relationship to BDI‐II (rho = −0.24, p = .15).
The mean proportion of errors increased only marginally for Experiment 2 (6% vs. 5.2%), however the spread of errors across participants and categories of word valence was more favourable. For neutral stimuli, just one subject made fewer than five errors. For emotional stimuli, just two participants made fewer than five errors. This allowed us to calculate post‐error accuracy adjustments and employ the robust method.
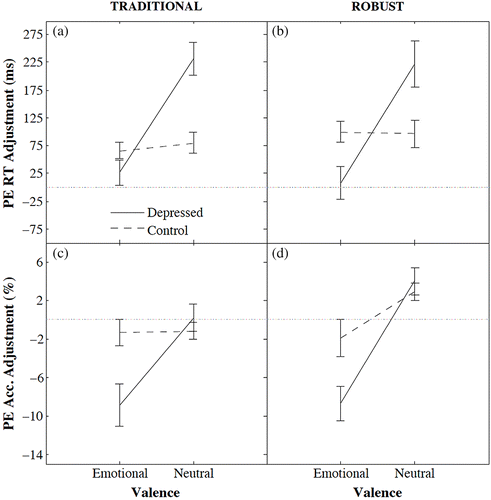
Fig. 3, Panels a and b indicate Experiment 2 produced a near perfect replication of the pattern of post‐error RT adjustments found in Experiment 1. We use the subscripts T and R to refer to the Traditional and Robust methods, respectively. Participants with depression symptoms showed substantial post‐error slowing (M T = 231.2 ms; M R = 221.7 ms) for neutral words and no post‐error slowing for emotional words (M T = 26.9 ms; M R = 7.7 ms), whereas controls showed consistent post‐error slowing for both neutral (M T = 80.0 ms; M R = 96.1 ms) and emotional (M T = 65.8 ms; M R = 99.5 ms) words. A mixed‐model ANOVA confirmed a significant interaction between depression group and word valence on post‐error slowing (F T(1,30) = 10.88, p = .003, effect‐size partial Eta squared = 0.266; F R(1,30) = 6.45, p = .017, effect‐size partial Eta squared = 0.177), and a significant main effect of valence (F T(1,30) = 14.38, p < .001, effect‐size partial Eta squared = 0.324; F R(1,30) = 6.05, p = .020, effect‐size partial Eta squared = 0.168), but a non‐significant main effect of depression group [F T(1,30) = 2.28, p = .141; F R(1,30) = .13, p = .721].Footnote3 Fig. 3, Panels c and d show the post‐error accuracy adjustments, and confirm the inability of depression participants to implement cognitive control following errors on emotional stimuli. The depression group members were not less accurate following errors on neutral stimuli when they slowed (M T = 0.2%; M R = 4.0%), but were far less accurate when they did not slow following errors on emotional stimuli (M T = −8.9%; M R = −8.7%). Controls showed no significant decrease or increase in accuracy following errors on neutral (M T = −1.2%; M R = 2.9%) or emotional (M T = −1.3%; M R = −1.9%) stimuli. Mixed‐model ANOVAs showed marginally significant interactions between depression group and valence on accuracy for the traditional but not robust method (F T(1,30) = 3.84, p = .059, effect‐size partial Eta squared = 0.113; F R(1,30) = 2.67, p = .112, effect‐size partial Eta squared = 0.082), and a marginal and significant main effect of valence (F T(1,30) = 4.05, p = .053, effect‐size partial Eta squared = 0.119; FR(1,30) = 13.29, p = .001, effect‐size partial Eta squared = 0.307). The between‐subject main effects of depression versus control were non‐significant and marginal (F T(1,30) = 1.75, p = .195; F R(1,30) = 3.99, p = .055), respectively.
Simple‐effects testsFootnote4 suggested the depression symptom group showed lower post‐error accuracy following errors on emotional words when compared to the control group, (TT(30) = 1.94, p = .062; TR(30) = 2.25, p = .032). Similar tests suggested that, for neutral stimuli, post‐error slowing was greater for the depression symptom group than controls, (TT(30) = 2.90, p = .007; TR(30) = 1.74, p = .093), but there was no difference in post‐error accuracy for the depression symptom group and controls, (TT(30) = 0.564, p = .577; TR(30) = 0.415, p = .681).
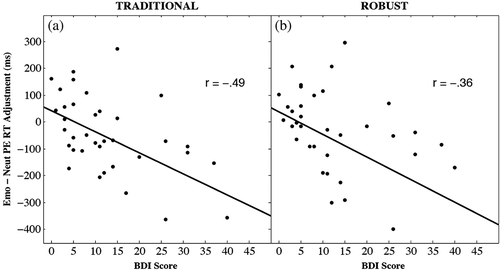
Figure 4, Panels a and b depicts the negative relationship between the difference in post‐error slowing for emotional and neutral words and BDI‐II scores for all participants for both the traditional (r = −0.487, p = .002, 95% confidence interval = (−0.701, −0.194)) and robust (r = −0.360, p = .029, 95% confidence interval = (−0.613, −0.041]) calculation methods. This completes the replication of Experiment 1 results and indicates that the difference in post‐error slowing for emotional words relative to neutral words could account for up to 25% of the variation in depression symptoms.
GENERAL DISCUSSION
Post‐error slowing is a benchmark effect of reactive cognitive control. We aimed to explore this effect in a group with depression symptoms, and controls, with emotional and non‐emotional priming. Our results indicated that errors to non‐emotional stimuli impaired subsequent trial performance by slowing responding without a compensatory increase in accuracy. This effect held for both the depression symptom group and controls, although the magnitude of post‐error slowing was greater for the depression group. We also documented a specific deficit in reactive cognitive control for participants with depression symptoms when exposed to task‐irrelevant emotional content. Participants with depression symptoms failed to slow down after errors to emotionally valanced stimuli, resulting in a substantial reduction in post‐error accuracy (~9%) compared to controls (who exhibited the expected post‐error slowing).
The results suggest that depression symptoms might be linked with specific deficits in reactive cognitive control that are both qualitatively and quantitatively distinct for emotional and non‐emotional stimuli. Our results, therefore, may help reconcile previously ambiguous evidence for behavioural deficits in depressed participants. Participants with depression symptoms differentially exhibited both substantial increases and decreases in post‐error slowing corresponding to specific experimental conditions. Averaging across emotional and non‐emotional stimuli (e.g., Compton et al., Citation2008) would combine these two opposing effects and provide inconsistent results.
The failure for participants with depression symptoms to engage cognitive control following errors on emotional stimuli builds upon previous work, reviewed above, that indicates a cognitive control impairment in depression when emotional regulation is required (Holmes & Pizzagalli, Citation2007; Saunders & Jentzsch, Citation2014). It is also consistent with several other lines of research that describe an association between error detection and correction, and emotional regulation. For example, Pizzagalli et al. (Citation2006) linked error detection and correction to the rostral anterior cingulate cortex (ACC), the brain region associated with evaluating the emotional significance of events. Activation of the emotional region of ACC via task‐irrelevant emotional content has also been implicated in the impairment of proactive cognitive control (Wyble, Sharma, & Bowman, Citation2008). Furthermore, studies in perception (Surguladze et al., Citation2004), memory (Mathews & MacLeod, Citation2005), and attention (Eizenman et al., Citation2003; Joormann, Citation2004) have shown that depression is associated with a specific impairment in the ability to disengage from emotional content (Gotlib & Joormann, Citation2010). Within the context of this prior work, our results are consistent with the view that for participants with depression symptoms, task‐irrelevant emotional content may disrupt normal function of the ACC.
The consistency of our results across experiments, and also across both the traditional and robust methods of measurement, helpfully informs our understanding of both the regular and irregular function of reactive cognitive control. Consider that when post‐error slowing was observed—for controls on all stimuli, and for participants with depression symptoms on neutral stimuli—we found no increase or decrease in accuracy following errors. Yet for participants with depression symptoms on emotional stimuli, we found no post‐error slowing and a large decrease in accuracy. This suggests recruitment of the cognitive control system, and hence post‐error slowing, may help buffer against the substantial decrease in accuracy that might otherwise occur.
A possible theoretical account of these results associates errors with a disturbance in processing (e.g., see Gehring, Goss, Coles, Meyer, & Donchin, Citation1993). An example of such a disturbance might be a lapse in attention, resulting in decreased sensory sensitivity (Purcell & Kiani, Citation2016). When an error is registered in awareness—as is typical in regular function—the reactive control system is recruited, and response speed is slowed on the following trial, possibly due to an increase in caution (Dutilh, van Ravenzwaaij, et al., Citation2012; Purcell & Kiani, Citation2016) and the need to reorient to the task (Notebaert et al., Citation2009). This diversion of time and resources to the reactive cognitive control system helps overcome the disturbance in processing and buffers against a substantial decrease in accuracy. Such a resolution is broadly consistent with recent approaches that view post‐error slowing as a heterogeneous effect (see Ullsperger & Danielmeier, Citation2016).Footnote5 In the case where error awareness is absent, the cognitive control system is not recruited and any processing lapse associated with an error might be expected to carry over to the next trial. As a result, a substantial decrease in accuracy and no post‐error slowing would be predicted. This was precisely the result we observed for participants with depression symptoms on emotional stimuli.
We suspect task‐irrelevant emotional stimuli may have disrupted normal function for the ACC for depressed participants and left them unaware of some or all of their errors. This would be commensurate with depressed participants being unable to disengage from irrelevant emotional content, which is well documented (as outlined above). We note the degree of this impairment was strongly associated with the degree of depression symptoms across the full range of BDI‐II scores. Future work would be required to verify this account of post‐error performance, perhaps combining brain imaging techniques and established cognitive models of decision making (e.g., Botvinick et al., Citation2001; Dutilh, Vandekerckhove, et al., Citation2012) for clinical populations. In their current form, our data helpfully constrain current models of post‐error behaviour and provide the impetus for testable predictions that will drive further experimentation.
In sum, Experiments 1 and 2 provided several novel and clinically relevant insights. For participants with depression symptoms, consistent and severe impairments in reactive control were identified. When these participants were primed with task‐irrelevant emotional content, reactive cognitive control was compromised. These data suggest that depression symptoms are associated with impairment in behavioural regulation in the face of even mild emotional exposure. In the case of a major depressive episode—where task‐irrelevant emotional thoughts are frequent, automatic, and ruminated upon—an inability to monitor environmental feedback may severely disrupt adaptive and goal‐driven behaviour. This informs our understanding of how emotional dysregulation, information processing deficits, and behavioural deficits, might interact to help maintain depression.
Our work was robust in that we replicated key results across two experiments. Even still, given the power limitations inherent to small and uneven samples, future work would benefit from replicating and extending on our findings with a larger clinical sample of depressed individuals. For example, exploring the boundary conditions of the deficit we have described may help determine when and how depressed individuals are able to adapt their behaviour appropriately in response to the environment. Future studies may also wish to determine the boundaries of this effect with regards to depression in the absence of anxiety and other symptoms, given that like Compton et al. (Citation2008), we used non‐controlled samples typical of those presenting for therapy.
Notes
1. Congruency sequence effects (CSEs) refer to the observation that congruency effects in conflict tasks are typically smaller following incongruent compared to following congruent trials.
2. To confirm that the absence of a post‐error slowing effect for people with depression was primarily due to an effect following error trials, rather than an effect following correct trials, we re‐ran our mixed model ANOVA model. Instead of using PES, we used the mean RT following errors as the response variable. Mean RTs were in line with expectations and we again found the critical interaction between valence and depression, F(1,25) = 4.874, p = .037. We thank an anonymous reviewer for suggesting this confirmatory analysis.
3. As per Experiment 1, we re‐ran our mixed model ANOVA model using the mean RT following errors as the response variable. Again, mean RTs were in line with expectations and we found the critical interaction between valence and depression, F(1,30) = 8.111, p = .008. This confirms our PES findings are primarily due to an effect following errors, rather than an effect following correct trials.
4. Unadjusted t‐tests.
5. We stress that this account does not necessitate nor exclude the small increases (e.g., Botvinick et al., Citation2001; Dutilh, Vandekerckhove, et al., Citation2012; Laming, Citation1979) or decreases (e.g., Notebaert et al., Citation2009) in accuracy sometimes associated with post‐error slowing.
REFERENCES
- Algom, D., Chajut, E., & Lev, S. (2004). A rational look at the emotional stroop phenomenon: A generic slowdown, not a stroop effect. Journal of Experimental Psychology: General, 133(3), 323–338.
- Auer, D. P., Pütz, B., Kraft, E., Lipinski, B., Schill, J., & Holsboer, F. (2000). Reduced glutamate in the anterior cingulate cortex in depression: An in vivo proton magnetic resonance spectroscopy study. Biological Psychiatry, 47(4), 305–313.
- Ballmaier, M., Toga, A. W., Blanton, R. E., Sowell, E. R., Lavretsky, H., Peterson, J., … Kumar, A. (2004). Anterior cingulate, gyrus rectus, and orbitofrontal abnormalities in elderly depressed patients: An MRI‐based parcellation of the prefrontal cortex. American Journal of Psychiatry, 161(1), 99–108.
- Beck, A. T., Rush, A. J., Shaw, B. F., & Emery, G. (1979). Cognitive Therapy of Depression. New York, NY: Guildford.
- Beck, A. T., Steer, R. A., & Brown, G. K. (1996). Manual for the Beck Depression Inventory‐II. San Antonio, TX: Psychological Corporation.
- Ben‐haim, M. S., Williams, P., Howard, Z., Mama, Y., Eidels, A., & Algom, D. (2016). The emotional stroop task: Assessing cognitive performance under exposure to emotional content. Journal of Visual Experimentation, 112, e53720. https://doi.org/10.3791/53720
- Botvinick, M. M., Braver, T. S., Barch, D. M., Carter, C. S., & Cohen, J. D. (2001). Conflict monitoring and cognitive control. Psychological Review, 108(3), 624–652.
- Braver, T. S. (2012). The variable nature of cognitive control: A dual mechanisms framework. Trends in Cognitive Sciences, 16(2), 106–113.
- Compton, R. J., Lin, M., Vargas, G., Carp, J., Fineman, S. L., & Quandt, L. C. (2008). Error detection and post error behavior in depressed undergraduates. Emotion, 8(1), 58–67. https://doi.org/10.1037/1528-3,542.8.1.58
- Dutilh, G., van Ravenzwaaij, D., Nieuwenhuis, S., Van der maas, H. L. J., Forstmann, B. U., & Wagenmakers, E.‐J. (2012). How to measure post‐error slowing: A confound and a simple solution. Journal of Mathematical Psychology, 56(3), 208–216. https://doi.org/10.1016/j.jmp.2012.04.001
- Dutilh, G., Vandekerckhove, J., Forstmann, B. U., Keuleers, E., Brysbaert, M., & Wagenmakers, E. J. (2012). Testing theories of post‐error slowing. Attention, Perception & Psychophysics, 74(2), 454–465. https://doi.org/10.3758/s13414-011-0243-2
- Eizenman, M., Yu, L. H., Grupp, L., Eizenman, E., Ellenbogen, M., Gemar, M., & Levitan, R. D. (2003). A naturalistic visual scanning approach to assess selective attention in major depressive disorder. Psychiatry Research, 118(2), 117–128.
- Gehring, W. J., Goss, B., Coles, M. G. H., Meyer, D. E., & Donchin, E. (1993). A neural system for error detection and compensation. Psychological Science, 4(6), 385–390. https://doi.org/10.1111/j.1467-9,280.1993.tb00586.x
- Gotlib, I. H., & Joormann, J. (2010). Cognition and depression: Current status and future directions. Annual Review of Clinical Psychology, 6, 285–312. https://doi.org/10.1146/annurev.clinpsy.121208.131305
- Heathcote, A., Coleman, J. R., Eidels, A., Watson, J. M., Houpt, J., & Strayer, D. L. (2015). Working memory's workload capacity. Memory & Cognition, 43(7), 973–989.
- Holmes, A. J., & Pizzagalli, D. A. (2007). Task feedback effects on conflict monitoring and executive control: Relationship to subclinical measures of depression. Emotion, 7(1), 68–76. https://doi.org/10.1037/1528-3,542.7.1.68
- Holmes, A. J., & Pizzagalli, D. A. (2010). Effects of task‐relevant incentives on the electrophysiological correlates of error processing in major depressive disorder. Cognitive, Affective, & Behavioural Neuroscience, 10(1), 119–128. https://doi.org/10.3758/CABN.10.1.119
- Joormann, J. (2004). Attentional bias in dysphoria: The role of inhibitory processes. Cognition and Emotion, 18(1), 125–147. https://doi.org/10.1080/02699930244000480
- Kumari, V., Mitterschiffthaler, M. T., Teasdale, J. D., Malhi, G. S., Brown, R. G., Giampietro, V., … Checkley, S. A. (2003). Neural abnormalities during cognitive generation of affect in treatment‐resistant depression. Biological Psychiatry, 54(8), 777–791.
- Laming, D. (1979). Choice reaction performance following an error. Acta Psychologica, 43, 199–224.
- Love, J., Selker, R., Marsman, M., Jamil, T., Dropmann, D., Verhagen, A. J., … Wagenmakers, E. J. (2015). JASP (Version 0.7)[Computer software]. Amsterdam: JASP Project.
- Mathers, C., & Ma fat, D. (2008). The global burden of disease 2004 update. Geneva: World Health Organisation.
- Mathews, A., & Macleod, C. (2005). Cognitive vulnerability to emotional disorders. Annual Review of Clinical Psychology, 1, 167–195.
- Morey, R. D. (2008). Confidence intervals from normalised data: A correction to Cousineau (2005). Reason, 4(2), 61–64.
- Notebaert, W., Houtman, F., Van opstal, F., Gevers, W., Fias, W., & Verguts, T. (2009). Post‐error slowing: An orienting account. Cognition, 111(2), 275–279.
- Olvet, D. M., & Hajcak, G. (2008). The error‐related negativity (ERN) and psychopathology: Toward an endophenotype. Clinical Psychology Review, 28(8), 1,343–1,354. https://doi.org/10.1016/j.cpr.2008.07.003
- Paelecke‐habermann, Y., Pohl, J., & Leplow, B. (2005). Attention and executive functions in remitted major depression patients. Journal of Affective Disorders, 89(1–3), 125–135. https://doi.org/10.1016/j.jad.2005.09.006
- Pizzagalli, D. A., Peccoralo, L. A., Davidson, R. J., & Cohen, J. D. (2006). Resting anterior cingulate activity and abnormal responses to errors in subjects with elevated depressive symptoms: a 128‐channel EEG study. Human Brain Mapping, 27(3), 185–201. https://doi.org/10.1002/hbm.20172
- Purcell, B. A., & Kiani, R. (2016). Neural mechanisms of post‐error adjustments of decision policy in parietal cortex. Neuron, 89(3), 658–671.
- Rabbitt, P. (1966). Error and error correction in choice‐response tasks. Journal of Experimental Psychology, 71(2), 264–272.
- Saunders, B., & Jentzsch, I. (2012). False external feedback modulates posterror slowing and the f‐P300: implications for theories of posterror adjustment. Psychonomic Bulletin and Review, 19(6), 1,210–1,216. https://doi.org/10.3758/s13423-012-0314-y
- Saunders, B., & Jentzsch, I. (2014). Reactive and proactive control adjustments under increased depressive symptoms: Insights from the classic and emotional‐face Stroop task. Quarterly Journal of Experimental Psychology (Hove), 67(5), 884–898. https://doi.org/10.1080/17470218.2013.836235
- Strayer, D. L., & Johnston, W. A. (2001). Driven to distraction: Dual‐task studies of simulated driving and conversing on a cellular telephone. Psychological Science, 12(6), 462–466.
- Surguladze, S. A., Young, A. W., Senior, C., Brebion, G., Travis, M. J., & Phillips, M. L. (2004). Recognition accuracy and response bias to happy and sad facial expressions in patients with major depression. Neuropsychology, 18(2), 212–218. https://doi.org/10.1037/0894-4,105.18.2.212
- Ullsperger, M., & Danielmeier, C. (2016). Reducing speed and sight: How adaptive is post‐error slowing? Neuron, 89(3), 430–432.
- Veiel, H. O. (1997). A preliminary profile of neuropsychological deficits associated with major depression. Journal of Clinical and Experimental Neuropsychology, 19(4), 587–603. https://doi.org/10.1080/01688639708403745
- West, R., Choi, P., & Travers, S. (2010). The influence of negative affect on the neural correlates of cognitive control. International Journal of Psychophysiology, 76(2), 107–117. https://doi.org/10.1016/j.ijpsycho.2010.03.002
- Williams, J. M., Mathews, A., & Macleod, C. (1996). The emotional Stroop task and psychopathology. Psychological Bulletin, 120(1), 3–24.
- Williams, P., Heathcote, A., Nesbitt, K., & Eidels, A. (2016). Post‐error recklessness and the hot hand. Judgement and Decision Making, 11(2), 174–184.
- Wyble, B., Sharma, D., & Bowman, H. (2008). Strategic regulation of cognitive control by emotional salience: A neural network model. Cognition and Emotion, 22(6), 1,019–1,051.