Abstract
Objective
The aim of the present study was to evaluate psychophysiological responses to expressions of surprise, sadness, and disgust and the possible effect of the expresser's sex.
Method
The startle reflex, the skin conductance response (SCR), and heart rate (HR) were recorded in 50 participants while they viewed male and female expressions of surprise, sadness, and disgust.
Results
The results showed enhanced startle reflex potentiation and greater HR deceleration in response to disgust expressions and a larger SCR and greater HR deceleration in response to male expressions of disgust.
Conclusions
These results suggest higher activation of the defensive motivational system and an orienting response to expressions of disgust, especially those of male faces.
Funding information Universidad de San Buenaventura, Grant/Award Number: CHS 012‐010
WHAT IS ALREADY KNOWN ABOUT THIS TOPIC?
Expressions of anger and happiness enhance the startle reflex.
Male expressions of anger enhance the startle reflex compared with female expressions of anger.
Inconclusive results have been found with respect to skin conductance response and heart rate response.
WHAT THIS TOPIC ADDS?
Disgust expressions increased startle reflex potentiation and heart rate deceleration.
Male disgust expressions generated a greater skin conductance response and greater heart rate deceleration.
Disgust expressions activated the defensive motivational system and an orienting response, especially male disgust expressions.
INTRODUCTION
The emotional priming hypothesis of Lang (Citation1995) suggests that the appetitive system activates approach behaviour when consummation, procreation, or nurturance is the goal, and the defensive system activates defence mechanisms to protect the organism from threat. The activation of these systems occurs when the demands of the context require it (i.e., when there is behavioural relevance that generates the predisposition to a specific behaviour), which is observed in the modulation of defensive reactions (e.g., startle reflex) and autonomic responses (e.g., skin conductance response [SCR] and heart rate [HR]). The modulation of physiological reactions to stimuli with different affective content has been shown to be a good index of the affective valence that is experienced by the individual, the level of arousal, or the attentional response to a stimulus (Bradley & Lang, Citation1999a; Bradley & Lang, Citation1999b; Gantiva & Camacho, Citation2016; Gantiva, Delgado, & Romo‐González, Citation2015; Gantiva, Guerra, & Vila, Citation2011; Miccoli et al., Citation2014; Muñoz et al., Citation2013).
Emotional facial expressions are stimuli with high evolutionary relevance that allow individuals to communicate internal emotional states, making such expressions fundamental to social cognition (Alpers, Adolph, & Pauli, Citation2011; Morris et al., Citation1996). Emotional facial expressions are able to evoke emotional responses in daily social interactions (Dimberg, Citation1982) and generate an emotional contagion in empathic individuals (Dimberg, Andréasson, & Thunberg, Citation2011). In addition to pictures that convey emotions, facial expressions can capture attention (Mogg & Bradley, Citation2002), but the latter are holistically processed, as opposed to emotional pictures that require the integration of various elements (Farah, Wilson, Drain, & Tanaka, Citation1998).
The results of physiological responses to emotional facial expressions (with the exception of facial electromyography to evaluate rapid facial reactions; Dimberg et al., Citation2011) have been inconclusive and limited mainly to expressions of fear, anger, and happiness. For example, Balaban (Citation1995) and Springer, Rosas, McGetrick, and Bowers (Citation2007) found that expressions of anger enhance the startle reflex compared with expressions of happiness and fear. Although angry and fearful faces convey messages of “threat,” their priming effects on startle circuitry differ. Thus, angry expressions, representing a viewer‐directed threat with an unambiguous source (i.e., the expresser), may more effectively induce a motivational propensity to withdraw or escape. Conversely, the source of the threat is comparatively less clear for fearful faces. These results are more pronounced in women than in men (Anokhin & Golosheykin, Citation2010).
Other studies have reported an increase in the startle reflex in response to expressions of happiness (Alpers et al., Citation2011; Duval, Lovelace, Aarant, & Filion, Citation2013; Hess, Sabourin, & Kleck, Citation2007; Wangelin, Bradley, Kastner, & Lang, Citation2012). These results suggest that the startle reflex in response to facial expressions is modulated by arousal and not by valence. In the case of happy expressions, an increase in the startle reflex is associated with an increase in alertness because the facial expression itself, without a specific context, cannot activate the appetitive motivational system (Alpers et al., Citation2011).
Few studies have evaluated autonomic responses to emotional expressions, and the results have been inconclusive. For example, Vrana and Gross (Citation2004) found an increase in HR in response to happy expressions, but these results were not corroborated by Alpers et al. (Citation2011). Vrana and Gross (Citation2004) and Alpers et al. (Citation2011) reported a higher SCR to happy expressions, but Springer et al. (Citation2007) found no differences in the SCR to angry, fearful, happy, and neutral expressions.
Inconsistencies in physiological responses to emotional facial expressions may be attributable to the fact that emotional facial expressions can be easily coded, but the behavioural relevance of some emotional expressions is ambiguous because behavioural relevance depends on contextual demands. For example, when observing only happy facial expressions without a specific context, the behavioural relevance is not obvious. However, other emotional facial expressions may have evolved as a threat signal by themselves (e.g., anger) so that the individual automatically responds to it (Alpers et al., Citation2011).
The expresser's sex is another important factor in emotional expressions. Research has shown that male expressions of anger enhance the startle reflex compared with female expressions of anger (Hess, Adams Jr., & Kleck, Citation2007; Hess, Sabourin, & Kleck, Citation2007; Paulus, Musial, & Renn, Citation2014). This occurs because facial expressions convey emotional and social information. The morphological cues of male faces are more dominant than female faces, which is associated with threat and the need to prepare for avoidance or escape behaviours. Conversely, female faces have cues of affiliation that are associated with approach behaviours (Hess, Adams Jr., & Kleck, Citation2007).
Studies on the emotions of surprise, sadness, and disgust have focused on the physiological responses that characterise them (for review, see Kreibig, Citation2010) and on the ability of facial expressions of these emotions to generate emotional contagion (Lundqvist & Dimberg, Citation1995). However, no study to date has evaluated the startle reflex, SCR, and HR as indices of motivated behaviour and orientation/attention responses to expressions of surprise, sadness, and disgust. Evaluating such indices would allow obtaining information about the behavioural relevance of these emotions in daily social interactions.
Disgust is an emotion that serves as a disease‐avoidance mechanism. Disgust activates the defensive motivational system and avoidance behaviours to protect the organism against infections or diseases (Oaten, Stevenson, & Case, Citation2009). Expressions of sadness and surprise can be easily recognised, but they do not necessarily induce behavioural responses because they do not report the proximity of a threat; therefore, these expressions alone do not necessarily activate any of the primary motivational systems. Previous studies have shown that some emotional expressions, such as happiness, require a context to activate the appetitive motivational system. Conversely, expressions of anger do not need a context to activate the defensive motivational system (Alpers et al., Citation2011).
Additionally, the evolutionary function of emotions such as anger and fear is clearly established (i.e., fight/flight response; Springer et al., Citation2007), but there is less certainty about the evolutionary function of emotions such as sadness and surprise.
Based on these considerations, the objective of the present study was to examine psychophysiological responses to expressions of surprise, sadness, and disgust and examine the possible effect of the expresser's sex. We hypothesized that expressions of disgust are more behaviourally relevant than expressions of sadness and surprise. Therefore, we expected to observe a larger startle reflex and a greater orientation/attention response (i.e., higher SCR and greater HR deceleration) to expressions of disgust compared with expressions of sadness and surprise.
Additionally, the functional equivalence hypothesis (Hess, Adams Jr., & Kleck, Citation2007; Hess, Sabourin, & Kleck, Citation2007) proposes that morphological characteristics that indicate dominance (e.g., square jaw, heavy eyebrows, and high forehead) increase the emotional impact of facial expressions that indicate danger (i.e., anger and disgust). We expected to see higher psychophysiological responses (i.e., potentiation of the startle reflex, a higher SCR, and greater HR deceleration) to male facial expressions of disgust because male faces have morphological characteristics that indicate a greater likelihood of threat than female faces.
METHODS
Participants
Fifty participants (25 females), ranging from 22 to 42-years of age (M = 31.06-years, SD = 6.95), participated in the study. Social anxiety has been found to influence psychophysiological responses to facial expressions (Wangelin et al., Citation2012). Therefore, the Spanish version of the SPAI (Olivares, Garcia‐Lopez, Hidalgo, Turner, & Beidel, Citation1999; Turner, Beidel, Dancu, & Stanley, Citation1989) was applied to exclude possible effects of social anxiety. Scores were lower than normative data (M = 33.26, SD = 11.04). No participant reported current physical or psychological problems, and no participant was under pharmacological treatment. All of the participants had normal or corrected‐to‐normal vision and audition. All of the participants provided written informed consent. The study was approved by the Institution Review Board.
Stimuli and procedure
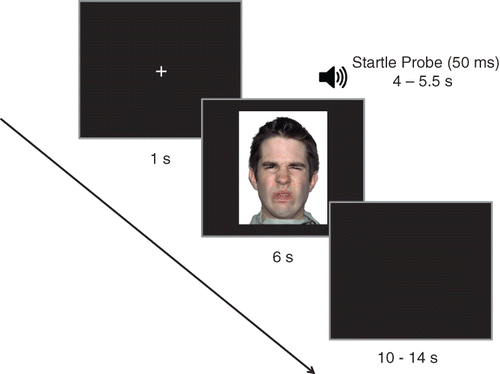
A total of 30 emotional face images (10 surprise faces, 10 sad faces, and 10 disgust faces) were selected from the NimStim Stimulus SetFootnote1 (Tottenham et al., Citation2009). Each expression was expressed by five men and five women. All of the pictures were presented twice in four counterbalanced orders that consisted of 60 slides each, with the constraint of not presenting the same emotional face category (i.e., surprise, sadness, and disgust) consecutively.
The pictures were presented for 6 s on a 19‐in. flat‐screen monitor that was located approximately 60-cm from the subject. To avoid the participants' predicting picture onset, the intertrial interval (ITI) varied randomly between 10 and 14-s. The startle probes consisted of 50-ms, 105-dB white‐noise bursts with an instantaneous rise time that were presented through headphones with binaural sound 4–5.5 s after picture onset (Blumenthal et al., Citation2005) (Figure 1). The startle probes were presented in a total of 36 trials, 12 trials per category (i.e., surprise, sadness, and disgust). For each category, the probe was presented in six male faces and six female faces. Six additional probes were presented during the ITIs. The pictures and startle probes were programmed using E‐Prime 2.0 software (Psychology Software Tools, Pittsburg, PA, USA).
Physiological measures
PowerLab 26T equipment (ADInstruments, Sydney, Australia) was used to record electromyographic activity, electrocardiographic activity, and skin conductance. The eye‐blink component of the startle response was measured with 4 mm Ag/AgCl electrodes over the left orbicularis oculi muscle (Blumenthal et al., Citation2005). Electromyographic activity was recorded using a 10–500-Hz frequency band filter that was rectified and integrated with a time constant of 20-ms. HR was derived from the electrocardiogram using three large Sensormedic electrodes that were filled with electrolyte paste at lead II. R‐waves were detected from the electrocardiogram data using peak detector software (LabChart 7.3, ADInstruments, Sydney, Australia). R‐R intervals were then converted to HR (in beats per minute [bpm]). Skin conductance was recorded from two dry, bright‐plated bipolar electrodes that were placed on the intermediate phalanges of digits II and IV of the nondominant hand. The signal was calibrated before each session to detect activity in the range of 0–40 μS (microSiemens) and recorded using an ADInstruments Model ML116 GSRAmp. All of the signals were acquired at a sampling rate of 1,000-Hz.
Data reduction
The peak magnitude of the startle reflex was determined in the 20–150-ms time frame following the stimulus onset relative to a 25-ms prestimulus baseline (Blumenthal et al., Citation2005). A total of 1.8% of the trials from all of the participants were excluded because of unstable baseline activity (>2 SDs above the mean baseline within subject). Average blink magnitude was calculated for probes presented during the ITI, and blinks were then converted to T‐scores using the mean and SD of the ITI distribution (Wangelin et al., Citation2012).
Reactions in skin conductance and HR were determined by subtracting activity in the 1 s before picture presentation from activity that occurred at each 0.5 s after picture onset. For skin conductance, the maximum change that occurred between 1 and 4 s after picture onset was scored, and a log transformation (log [SCR + 1]) was performed to normalise the data. To determine initial HR deceleration as the orienting response, the maximum deceleration from baseline in the first 3 s of picture viewing was calculated.
Self‐report measures
After physiological measures were recorded, the participants rated the 30 images of emotional faces in the valence and arousal dimensions using the Self‐Assessment Manikin (SAM; Bradley & Lang, Citation1994). Each dimension was represented by five humanoid figures that indicated different levels of intensity, ranging from one (the lower end) to nine (the upper end), with markings between the figures that indicated intermediate scores.
Statistical analysis
Physiological and self‐report measures were analysed using a 3 × 2 repeated‐measures analysis of variance (RM‐ANOVA), with Emotion (surprise, sadness, and disgust expressions) and Expresser Sex (women vs. men) as the repeated measures. When the assumption of sphericity was not met, the Greenhouse–Geisser correction was applied to the degrees of freedom in all cases. Post hoc analyses of the mean values were performed using paired multiple comparisons, adjusted with the Bonferroni correction. A significance level of .05 was used in all of the analyses. Effect sizes are reported as the partial eta squared (η p2; small ≥ 0.01, medium ≥ 0.06, large ≥ 0.14; Cohen, Citation1988). All of the statistical analyses were performed using Statistical Package for Social Sciences (SPSS) 20.0 software.
RESULTS
Startle reflex
The ANOVA of the startle reflex data revealed a significant main effect of Emotion (F2,98 = 3.93, p = .04, η p2 = .07). The startle reflex was greater for disgust faces (M = 73.62, SE = 12.38) than for surprise faces (M = 65.67, SE = 10.86; p = .01). No significant differences were found between disgust faces and sad faces (p = .38). There was no main effect of Expresser Sex (F < 1) and no significant Emotion × Expresser Sex interaction (F < 1).
Skin conductance response
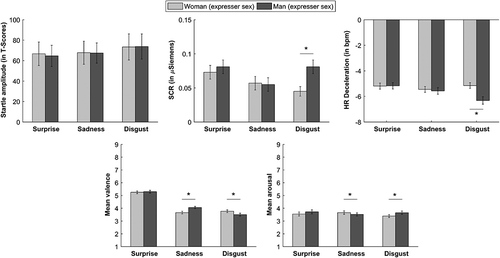
The ANOVA of the SCR data yielded no significant main effects of Emotion (F2,98 = 2.62, p = .07, η p2 = .02) or Expresser Sex (F1,49 = 3.24, p = .07, η p2 = .03). A significant Emotion × Expresser Sex interaction was found (F2,98 = 5.42, p = .005, η p2 = .05). Male disgust faces generated significantly larger SCRs compared with female disgust faces (p < .001; Figure 2).
Heart rate response
The ANOVA of the initial HR deceleration data revealed a significant main effect of Emotion (F2,98 = 4.75, p = .01, η p2 = .04). Disgust faces generated greater HR deceleration (M = −5.71, SE = .23) than surprise faces (M = −5.14, SE = .20; p = .02). A significant main effect of Expresser Sex was found (F1,49 = 6.16, p = .01, η p2 = .05). Male faces (M = −5.65, SE = .21) elicited higher HR deceleration than female faces (M = −5.24, SE = .19). The Emotion × Expresser Sex interaction was significant (F2,98 = 5.22, p = .009, η p2 = .05). Male disgust faces generated greater HR deceleration than female disgust faces (p < .001; Figure 2).
Subjective ratings
The ANOVA of the valence dimension revealed a significant main effect of Emotion (F2,98 = 142.82, p < .001, η p2 = .59). Disgust faces (M = 3.63, SE = .11) and sad faces (M = 3.86, SE = .09) were perceived as more aversive than surprise faces (M = 5.28, SE = .11; both p < .001), and disgust faces were perceived as more aversive than sad faces (p = .003). The Expresser Sex main effect was not significant (F1,49 = 2.43, p = .12, η p2 = .02). The Emotion × Expresser Sex interaction was significant (F2,98 = 18.95, p < .001, η p2 = .16). Female sad faces were perceived as more aversive than male sad faces, and male disgust faces were perceived as more aversive than female disgust faces (both p < .001; Figure 2).
The ANOVA of the arousal dimension yielded no significant main effects of Emotion (F < 1). A significant main effect of Expresser Sex was found (F1,49 = 4.04, p = .04, η p2 = .03). Female faces were perceived as less arousing (M = 3.53, SE = .13) than male faces (M = 3.63, SE = .13; p = .04). The Emotion × Expresser Sex interaction was significant (F2,98 = 7.17, p = .003, η p2 = .06). Female sad faces were perceived as more arousing than male sad faces (p = .03) and male disgust faces were perceived as more arousing than female disgust faces (p = .001) (Figure 2).
DISCUSSION
The objective of the present study was to examine psychophysiological responses to expressions of surprise, sadness, and disgust and examine the possible effects of the expresser's sex. We expected to observe a larger startle reflex and a greater orientation/attention response to expressions of disgust compared with expressions of sadness and surprise. We hypothesized that expressions of disgust are more behaviourally relevant than expressions of sadness and surprise. Our results only partially supported this hypothesis. The results showed that disgust faces generated a larger startle reflex and greater HR deceleration compared with surprise faces but not compared with sad faces. No significant differences in the SCR were found between expressions of disgust and expressions of sadness and surprise. With regard to the expresser's sex, male disgust faces generated a larger SCR and greater HR deceleration compared with female disgust faces.
The subjective measures yielded similar results. Disgust faces were perceived as more aversive than sad and surprise faces, and male disgust faces were more aversive than female disgust faces. Additionally, male faces were perceived as more arousing than female faces, and male disgust faces were perceived as more arousing than female disgust faces. Overall, these results suggest activation of the defensive motivational system and an orientation/attention response to expressions of disgust, especially those that are expressed by male faces, indicating the greater behavioural relevance of expressions of disgust. Moreover, the results support the functional equivalence hypothesis, in which the dominance characteristics of male faces increase the emotional response.
From a motivational perspective, threat and disgust pictures activate the defensive motivational system because they indicate the likelihood of harm to the individual (Bradley, Codispoti, Cuthbert, & Lang, Citation2001). These same results appear to be replicated with facial expressions of anger and disgust because these expressions convey the message of possible threat (e.g., harm and disease, respectively).
Previous studies that used threat and disgust pictures (i.e., human attacks, animal attacks, mutilation, and pollution) reported greater HR deceleration and an increase in the startle reflex in response to these types of stimuli (Bradley et al., Citation2001; Yartz & Hawk, Citation2002), indicating a greater attentional response and preparation for a fight/flight response, respectively. Different studies have found potentiation of the startle reflex in response to expressions of anger (Alpers et al., Citation2011; Anokhin & Golosheykin, Citation2010; Hess, Adams Jr., & Kleck, Citation2007; Hess, Sabourin, & Kleck, Citation2007; Paulus et al., Citation2014; Springer et al., Citation2007) because they indicate a direct threat to the observer. The present study extends this finding to expressions of disgust.
Additionally, our results showed that expressions of disgust generated greater HR deceleration, indicating an increase in the attentional response (Bradley et al., Citation2001; Gantiva, Araujo, Aragão, & Hewitt, Citation2018). This is consistent with previous event‐related potential (ERP) studies that showed that expressions of disgust enhanced the amplitude of early posterior negativity, which is associated with the allocation of attentional and sensory resources (Ashley, Vuilleumier, & Swick, Citation2004).
With regard to the expresser's sex, we found that male expressions of disgust generated a larger SCR and greater HR deceleration, indicating a higher orientation/attention response to male expressions of disgust. According to the functional equivalence hypothesis (Hess, Adams Jr., & Kleck, Citation2007; Hess, Sabourin, & Kleck, Citation2007), an emotional message occurs as a result of the interaction between the facial expression and morphological characteristics of the person who is expressing the emotion. Thus, the morphological characteristics of domination, which are more common in men, intensify the emotional message that is conveyed by expressions that indicate damage (e.g., disgust expressions). This would explain the higher attentional response to male expressions of disgust. Similar results were found with expressions of anger, based on the startle reflex. Male expressions of anger enhanced the startle reflex compared with female expressions of anger (Hess, Adams Jr., & Kleck, Citation2007; Hess, Sabourin, & Kleck, Citation2007; Paulus et al., Citation2014), suggesting activation of the defensive motivational system (Bradley et al., Citation2001).
Altogether, these results suggest that morphological cues of dominance (e.g., square jaw, heavy eyebrows, and high forehead), which are more typical in men, influence expressions of disgust, generating a greater attentional response. In the case of expressions of anger, morphological cues of dominance generate more preparation for action.
These results may also be related to sex differences in the participants' sensitivity to disgust. Specifically, men reported experiencing less disgust compared with women (Haidt, McCauley, & Rozin, Citation1994). Therefore, a facial expression of disgust on a male face might signify to onlookers a far greater threat than an expression of disgust of a similar intensity on a woman's face.
Another possible explanation for the differences that were found in the SCR and HR deceleration in response to expressions of disgust in men and women may be related to social differences between men and women. Emotional face expressions are highly social in nature, indicating that the social meaning of a male expression may be different from the social meaning of a female expression. Previous studies found that the perception of situations with different social meaning (e.g., decision‐making related to unfair offers) increases the orientation response (i.e., HR deceleration; Osumi & Ohira, Citation2009). Thus, the enhancement of the SCR and HR deceleration in response to male disgust expressions suggests that male expressions have greater social relevance because they express a greater threat, perhaps because of differences in the social roles that are attributed to men and women, in which men only express emotions in situations of greater potential threat.
With regard to expressions of sadness, although they are evaluated as more aversive than expressions of surprise, no significant differences were found in the physiological responses that were generated by sadness expressions compared with the other expressions. Similar results have been found for expressions of fear (Paulus et al., Citation2014; Springer et al., Citation2007). These results confirm the importance of the functional impact of emotional stimuli, in which activation of the defensive motivational system involves both the negative assessment of the stimulus and the degree of direct threat that is indicated by the stimulus. For example, Bradley et al. (Citation2001) and Bernat, Patrick, Benning, and Tellegen (Citation2006) reported an increase in the startle reflex in response to pictures that indicated a direct threat but not in response to pictures of victimisation, in which another person suffered damage, although both types of stimuli were evaluated as aversive and were associated with similar levels of arousal.
Overall, our results indicate higher activation of the defensive motivational system in response to expressions of disgust and a greater attentional response and arousal in response to male expressions of disgust. Our results may be explained by the motivational theory of emotion (Bradley & Lang, Citation2007; Lang, Citation1995), in which the emotional response depends on the proximity of the threat. Our results also corroborate the functional equivalence hypothesis (Hess, Adams Jr., & Kleck, Citation2007; Hess, Sabourin, & Kleck, Citation2007), in which emotional information depends on the interaction between facial expressions and the morphological characteristics of emotional expressions. According to the defence cascade model (Lang, Bradley, & Cuthbert, Citation1997), the results of the present study and the previous results of Springer et al. (Citation2007), Anokhin and Golosheykin (Citation2010), Alpers et al. (Citation2011), and Paulus et al. (Citation2014) indicate that expressions of anger and disgust (especially those of males) activate the defensive motivational system and prepare the observer for avoidance and escape behaviours (i.e., increased startle reflex, increased SCR, and HR deceleration). Expressions of fear and sadness did not potentiate defensive reflexes despite being assessed as negative because they did not indicate a direct or proximal threat to the individual.
The relevance of our findings should be evaluated by considering several limitations. First, no neutral expressions were used. However, the amplitude of the startle reflex was expressed as the mean startle response during the ITI (i.e., neutral stimulus). Future studies should analyse psychophysiological responses to the six basic emotions and neutral expressions (Ekman & Friesen, Citation1971) to identify differences between each of these emotions. Second, comparisons of our results with other studies should be done with caution because they were made with expressions of happiness, anger, and fear (Alpers et al., Citation2011; Anokhin & Golosheykin, Citation2010; Duval et al., Citation2013; Hess, Adams Jr., & Kleck, Citation2007; Hess, Sabourin, & Kleck, Citation2007; Paulus et al., Citation2014; Springer et al., Citation2007). Additionally, Alpers et al. (Citation2011) recorded SCRs and HR in response to only emotional expressions of women. Third, the psychophysiological responses that were analysed in the present study were limited. Future research should also record the activity of the corrugator and zygomatic muscles in an effort to corroborate the findings of Dimberg (Citation1982) with regard to facial mimicry. It would also be useful to integrate the psychophysiological responses that were observed in the present study with ERPs that are recorded electroencephalographically to investigate orientation, encoding processes, attention, and arousal (Batty & Taylor, Citation2003). Fourth, because of the small sample size, we were unable to analyse interactions between observer sex, emotion, and expresser sex. Such interactions have not been analysed in other studies of psychophysiological responses to different emotions (e.g., Alpers et al., Citation2011; Anokhin & Golosheykin, Citation2010; Hess, Adams Jr., & Kleck, Citation2007; Hess, Sabourin, & Kleck, Citation2007; Paulus et al., Citation2014) and thus should be addressed in the future.
CONCLUSION
Disgust facial expressions generated a larger startle reflex and greater HR deceleration. Male disgust faces generated a higher SCR and greater HR deceleration than female disgust faces. This study extends previous reports (Alpers et al., Citation2011), suggesting that disgust facial expressions have sufficient behavioural relevance to activate the defensive motivational system and generate attentional engagement because they indicate the probability of harm. Additionally, the results support the functional equivalence hypothesis (Hess, Adams Jr., & Kleck, Citation2007; Hess, Sabourin, & Kleck, Citation2007), which proposes that morphological traits of dominance increase the emotional message that is conveyed in facial expressions that indicate the probability of threat or damage.
ACKNOWLEDGEMENTS
Funding for this study was provided by the Universidad de San Buenaventura (Ref. CHS 012‐010).
Additional information
Funding
Notes
Funding information Universidad de San Buenaventura, Grant/Award Number: CHS 012‐010
1. NimStim: 01F_DI_O; 01F_SA_O; 01F_SP_O; 03F_DI_O; 03F_SA_O; 03F_SP_O; 05F_DI_O; 05F_SA_O; 05F_SP_O; 07F_DI_O; 07F_SA_O; 07F_SP_O; 09F_DI_O; 09F_SA_O; 09F_SP_O; 20M_DI_O; 20M_SA_O; 20M_SP_O; 23M_DI_O; 23M_SA_O; 23M_SP_O; 34M_DI_O; 34M_SA_O; 34M_SP_O; 37M_DI_O; 37M_SA_O; 37M_SP_O; 42M_DI_O; 42M_SA_O; 42M_SP_O.
REFERENCES
- Alpers, G. W., Adolph, D., & Pauli, P. (2011). Emotional scenes and facial expressions elicit different psychophysiological responses. International Journal of Psychophysiology, 80(3), 173–181. https://doi.org/10.1016/j.ijpsycho.2011.01.010
- Anokhin, A. P., & Golosheykin, S. (2010). Startle modulation by affective faces. Biological Psychology, 83, 37–40. https://doi.org/10.1016/j.biopsycho.2009.10.001
- Ashley, V., Vuilleumier, P., & Swick, D. (2004). Time course and specificity of event related potentials to emotional expressions. Neuroreport, 15, 211–216.
- Balaban, M. T. (1995). Affective influences on startle in five‐month‐old infants: Reactions to facial expressions of emotions. Child Development, 66, 28–36.
- Batty, M., & Taylor, M. J. (2003). Early processing of the six basic facial emotional expressions. Cognitive Brain Research, 17(3), 613–620. https://doi.org/10.1016/S0926-6410(03)00174-5
- Bernat, E., Patrick, C. J., Benning, S. D., & Tellegen, A. (2006). Effects of picture content and intensity on affective physiological response. Psychophysiology, 43, 93–103.
- Blumenthal, T. D., Cuthbert, B. N., Filion, D. L., Hackley, S., Lipp, O. V., & van Boxtel, A. (2005). Committee report: Guidelines for human startle eyeblink electromyographic studies. Psychophysiology, 42, 1–15.
- Bradley, M. M., Codispoti, M., Cuthbert, B., & Lang, P. J. (2001). Emotion and motivation I: Defensive and appetitive reactions in picture processing. Emotion, 1(3), 276–298. https://doi.org/10.1037//1528-3542.1.3.276
- Bradley, M. M., & Lang, P. J. (1994). Measuring emotion: The self‐assessment manikin and the semantic differential. Journal of Behavior Therapy and Experimental Psychiatry, 25, 49–59.
- Bradley, M. M., & Lang, P. J. (1999a). International affective digitized sounds (IADS): Stimuli, instruction manual and affective ratings (Tech. Rep. No. B‐2). Gainesville, FL: The Center for Research in Psychophysiology, University of Florida.
- Bradley, M. M., & Lang, P. J. (1999b). Affective Norms for English Words (ANEW): Instruction manual and affective ratings. Gainesville, FL: Center for Research in Psychophysiology, University of Florida.
- Bradley, M. M., & Lang, P. J. (2007). Emotion and motivation. In J. Cacioppo, L. Tassinary, & G. Berntson (Eds.), The handbook of psychophysiology. New York, NY: Cambridge University Press.
- Cohen, J. (1988). Statistical power analysis for the behavioral sciences (2nd ed.). Hillsdale, NJ: Erlbaum.
- Dimberg, U. (1982). Facial reactions to facial expressions. Psychophysiology, 19, 643–647.
- Dimberg, U., Andréasson, P., & Thunberg, M. (2011). Emotional empathy and facial reactions to facial expressions. Journal of Psychophysiology, 25(1), 26–31. https://doi.org/10.1027/0269-8803/a000029
- Duval, E. R., Lovelace, C. T., Aarant, J., & Filion, D. L. (2013). The time course of face processing: Startle eyeblink response modulation by face gender and expression. International Journal of Psychophysiology, 90(3), 354–357. https://doi.org/10.1016/j.ijpsycho.2013.08.006
- Ekman, P., & Friesen, W. V. (1971). Constants across cultures in the face and emotion. Journal of Personality and Social Psychology, 17(2), 124–129.
- Farah, M. J., Wilson, K. D., Drain, M., & Tanaka, J. N. (1998). What is “special” about face perception? Psychological Review, 105(3), 482–498.
- Gantiva, C., Araujo, A., Aragão, N., & Hewitt, N. (2018). Modulation of physiological responses as indices of attentional bias in dysphoria. International Journal of Mental Health and Addiction, 16(2), 328–338. https://doi.org/10.1007/s11469-017-9774-7
- Gantiva, C., & Camacho, K. (2016). Características de la respuesta emocional generada por las palabras: Un estudio experimental desde la emoción y la motivación. Psychologia, 10(2), 55–62.
- Gantiva, C., Delgado, R., & Romo‐gonzález, T. (2015). Emotional reactions to alcohol‐related words: Differences between low‐and high‐risk drinkers. Addictive Behaviors, 50, 60–63. https://doi.org/10.1016/j.addbeh.2015.06.004
- Gantiva, C., Guerra, P., & Vila, J. (2011). Validación colombiana del sistema internacional de imágenes afectivas: Evidencias del origen transcultural de la emoción. Acta Colombiana de Psicología, 14(2), 103–111.
- Haidt, J., Mccauley, C., & Rozin, P. (1994). Individual differences in sensitivity to disgust: A scale sampling seven domains of disgust elicitors. Personality and Individual Differences, 16(5), 701–713. https://doi.org/10.1016/0191‐8869(94)90212‐7
- Hess, U., Adams, R. B., Jr., & Kleck, R. E. (2007). When two do the same it might not mean the same: The perception of emotional expressions shown by men and women. In U. Hess & P. Philippot (Eds.), Group dynamics and emotional expression (pp. 33–52). New York, NY: Cambridge University Press.
- Hess, U., Sabourin, G., & Kleck, R. E. (2007). Postauricular and eyeblink startle responses to facial expressions. Psychophysiology, 44, 431–435. https://doi.org/10.1111/j.14698986.2007.00516.x
- Kreibig, S. D. (2010). Autonomic nervous system activity in emotion: A review. Biological Psychology, 84(3), 394–421. https://doi.org/10.1016/j.biopsycho.2010.03.010
- Lang, P. J. (1995). The emotion probe: Studies of motivation and attention. American Psychologist, 50, 372–385.
- Lang, P. J., Bradley, M. M., & Cuthbert, B. N. (1997). Motivated attention: Affect, activation, and action. In P. J. Lang, R. F. Simons, & M. T. Balaban (Eds.), Attention and orienting: Sensory and motivational processes (pp. 97–135). Hillsdale, NJ: Erlbaum.
- Lundqvist, L.‐O., & Dimberg, U. (1995). Facial expressions are contagious. Journal of Psychophysiology, 9, 203–211.
- Miccoli, L., Delgado, R., Rodríguez‐ruiz, S., Guerra, P., García‐mármol, E., & Fernández‐santaella, M. C. (2014). Meet OLAF, a good friend of the IAPS! The open library of affective foods: A tool to investigate the emotional impact of food in adolescents. PLoS One, 9(12), e114515. https://doi.org/10.1371/journal.pone.0114515
- Mogg, K., & Bradley, B. P. (2002). Selective orienting of attention to masked threat faces in social anxiety. Behaviour Research and Therapy, 40(12), 1403–1414. https://doi.org/10.1016/S0005-7967(02)00017-7
- Morris, J. S., Frith, C. D., Perrett, D. I., Rowland, D., Young, A. W., Calder, A. J., & Dolan, R. J. (1996). A differential neural response in the human amygdala to fearful and happy facial expressions. Nature, 383(6603), 812–815.
- Muñoz, M. A., Viedma‐del‐jesús, M. I., Rossello, F., Sanchez‐nacher, N., Montoya, P., & Vila, J. (2013). The emotional impact of European tobacco‐warning images. Tobacco Control, 22(2), 123–129. https://doi.org/10.1136/tobaccocontrol-2011-050070
- Oaten, M., Stevenson, R. J., & Case, T. I. (2009). Disgust as a disease‐avoidance mechanism. Psychological Bulletin, 135(2), 303–321. https://doi.org/10.1037/a0014823
- Olivares, J., Garcia‐lopez, L. J., Hidalgo, M. D., Turner, S. M., & Beidel, D. C. (1999). The social phobia and anxiety inventory: Reliability and validity in a Spanish adolescent population. Journal of Psychopathology and Behavioral Assessment, 21, 67–78.
- Osumi, T., & Ohira, H. (2009). Cardiac responses predict decisions: An investigation of the relation between orienting response and decisions in the ultimatum game. International Journal of Psychophysiology, 74(1), 74–79. https://doi.org/10.1016/j.ijpsycho.2009.07.007
- Paulus, A., Musial, E., & Renn, K. (2014). Gender of the expresser moderates the effect of emotional faces on the startle reflex. Cognition and Emotion, 28(8), 1493–1501. https://doi.org/10.1080/02699931.2014.886557
- Springer, U. S., Rosas, A., Mcgetrick, J., & Bowers, D. (2007). Differences in startle reactivity during the perception of angry and fearful faces. Emotion, 7(3), 516–525. https://doi.org/10.1037/1528-3542.7.3.516
- Tottenham, N., Tanaka, J. W., Leon, A. C., Mccarry, T., Nurse, M., Hare, T. A., … Nelson, C. (2009). The NimStim set of facial expressions: Judgments from untrained research participants. Psychiatry Research, 168(3), 242–249. https://doi.org/10.1016/j.psychres.2008.05.006
- Turner, S. M., Beidel, D. C., Dancu, C. V., & Stanley, M. A. (1989). An empirically derived inventory to measure social fears and anxiety: The social phobia and anxiety inventory. Psychological Assessment, 1, 35–40.
- Vrana, S. R., & Gross, D. (2004). Reactions to facial expressions: Effects of social context and speech anxiety on responses to neutral, anger, and joy expressions. Biological Psychology, 66(1), 63–78.
- Wangelin, B. C., Bradley, M. M., Kastner, A., & Lang, P. J. (2012). Affective engagement for facial expressions and emotional scenes: The influence of social anxiety. Biological Psychology, 91(1), 103–110. https://doi.org/10.1016/j.biopsycho.2012.05.002
- Yartz, A. R., & Hawk, L. W. (2002). Addressing the specificity of affective startle modulation: Fear versus disgust. Biological Psychology, 59(1), 55–68.