Abstract
Background
The aim of this study was to provide an integrated analysis of safety and efficacy data for brimonidine tartrate ophthalmic solution 0.025 per cent (low‐dose; Bausch & Lomb Incorporated), a topical vasoconstrictor for relief of ocular redness.
Methods
Integrated efficacy data from two randomised, double‐masked, vehicle‐controlled studies in subjects with ocular redness as well as safety data from the two efficacy studies, a vehicle‐controlled safety study, and a pharmacokinetic study were analysed. Efficacy outcomes analysed included investigator‐assessed ocular redness (scale, 0–4) before treatment instillation and at five to 240-minutes post‐instillation on Day 1, at five-minutes post‐instillation on Days 15 and 29, and one-week after treatment discontinuation (Day 36), and redness self‐assessed by subjects recorded daily in diaries. Safety assessments included adverse events, ophthalmic examinations, and rebound redness upon treatment discontinuation. Drop comfort (scale, 0–10) was a tolerability measure.
Results
The efficacy population included 117 subjects (brimonidine, n = 78; vehicle, n = 39). The safety population included 635 subjects (brimonidine, n = 426; vehicle, n = 209). Investigator‐assessed ocular redness was significantly lower with brimonidine versus vehicle at all post‐instillation time points on Day 1 (mean change from pre‐instillation of −1.4-units for brimonidine and −0.2-units for vehicle; p < 0.0001). Subject‐assessed ocular redness was also significantly lower with brimonidine versus vehicle (mean treatment difference in average daily ratings of −0.9; p < 0.0001). There was no evidence of tachyphylaxis through Day 29 and rebound redness was rare. Incidence of ocular adverse events was low, the most common being reduced visual acuity (brimonidine, 4.0-per cent; vehicle, 4.3-per cent) and conjunctival hyperaemia (2.6 and 2.9-per cent, respectively). Both brimonidine and vehicle were rated as very comfortable (mean post‐instillation scores, 0.4–0.5).
Conclusion
In this integrated analysis, low‐dose brimonidine significantly reduced ocular redness with no tachyphylaxis, and minimal rebound redness, and was generally safe and well tolerated.
Ocular redness, which commonly results from inflammation of the conjunctiva and associated dilation of the conjunctival vessels, has a number of potential aetiologic factors including allergy, infection, dry eye, exposure to environmental irritants, and contact lens wear.Citation2010 Treatment should, whenever possible, be specific to the underlying cause (for example, antihistamines and mast cell stabilisers for allergic conjunctivitis and topical antibiotics for bacterial conjunctivitis).Citation2010 For ocular redness with no apparent underlying pathology, over‐the‐counter ocular vasoconstrictors (decongestants) may provide relief.Citation2010 These agents produce vasoconstriction via agonist activity at α‐adrenergic receptors, but vary in their receptor binding profiles.Citation2018 Phenylephrine and tetrahydrozoline exhibit relatively selective affinity for α1‐adrenergic receptors (selective α1‐receptor agonists) whereas naphazoline and oxymetazoline bind to both α1‐ and α2‐adrenergic receptors (mixed α1/α2‐receptor agonists).
Although ocular decongestants are effective for reducing ocular redness, continued use has been associated with tolerance or loss of effectiveness (that is, tachyphylaxis) and rebound redness upon treatment discontinuation.Citation1994 Tachyphylaxis appears related to a reduction of the α1‐receptor response, perhaps due to the sequestration of α1 receptors (an acute desensitisation mechanism) and subsequent downregulation of surface α1 receptors with chronic exposure to agonists.Citation1987 Due to the preferential expression of α1 receptors in arterial vessels, rebound redness after chronic use is thought to result from tissue ischaemia and the subsequent release of vasodilators, as well as loss of vascular tone due to α1‐receptor downregulation.Citation1997
Brimonidine is a highly selective α2‐receptor agonist with relatively low binding affinity for α1 receptors (ratio of α2: α1 binding affinity of ~1,000:1).Citation1998 Because α2 receptors are expressed predominantly in veins, it is possible that α2‐receptor agonists for ocular use may have a lower potential for tachyphylaxis and rebound redness.Citation2007 Brimonidine is approved by the US Food and Drug Administration in an ophthalmic solution (0.15 and 0.2-per cent) for lowering intraocular pressure in patients with open‐angle glaucoma or ocular hypertension,Citation1998 and in a topical gel (0.33-per cent) for the treatment of non‐transient facial erythema of rosacea in adults.Citation2013 Additionally, brimonidine instilled in the eyes at low doses (0.025–0.2 per cent) has been shown to control bleeding during ocular surgery,Citation2002 prevent bleeding from intravitreal injections,Citation2011 and induce conjunctival blanching before surgery,Citation2009 demonstrating the ocular vasoconstrictive effects of brimonidine. In December 2017, low‐dose brimonidine tartrate (0.025 per cent; Lumify eye drops, Bausch & Lomb Incorporated, Rochester, New York, USA) received approval in the USA to relieve redness of the eye due to minor irritations.Citation2017
The purpose of the analysis reported here is to provide a comprehensive summary of the available data on the efficacy and safety/tolerability profile of low‐dose brimonidine tartrate ophthalmic solution for the reduction of ocular redness. Efficacy studies of this low‐dose brimonidine formulation included adults with ocular redness of an undetermined nature, to represent the real‐world population of ocular decongestant users. Healthy paediatric subjects were included in a large safety study.
Methods
Study design
This is an integrated analysis of data from four clinical studies on the efficacy and/or safety of brimonidine tartrate ophthalmic solution 0.025 per cent (Table ). Both the efficacy and safety analyses included two single‐centre randomised, double‐masked, vehicle‐controlled safety and efficacy studies of brimonidine tartrate ophthalmic solution (0.025 per cent) in subjects with ocular redness (Studies 1 and 2).Citation2018 Details of the study methods have been previously reported.Citation2018 In addition to these two studies, the safety analysis included a multi‐centre, double‐masked, randomised, vehicle‐controlled safety study (Study 3) and a single‐centre, open‐label, pharmacokinetic study (Study 4). Where used, the vehicle formulation was identical to the brimonidine formulation but without brimonidine tartrate.
Table 1. Summary of studies of brimonidine tartrate ophthalmic solution 0.025 per cent included in this integrated analysis
All studies were approved by an institutional review board (Alpha IRB, San Clemente, California, USA) and conducted in compliance with the ethics principles of the Declaration of Helsinki and the International Conference on Harmonisation Good Clinical Practice guidelines. All subjects (or a parent/guardian for subjects under the age of 18) provided written informed consent before study procedures were initiated.
Subjects
Both efficacy studies enrolled subjects (aged ≥ 40-years in Study 1; aged ≥ 18-years in Study 2) with ocular redness but otherwise stable ocular health.Citation2018 Eligible subjects in these two efficacy studies had a baseline redness score of > 1 in both eyes as assessed by investigators using the Ora Calibra Ocular Hyperemia Scale (Ora, Inc., Andover, Massachusetts, USA), which utilises a five‐point scale (0 = none, 1 = mild, 2 = moderate, 3 = severe, 4 = extremely severe); this scale is based on photographic standards and has been used in previous studies.Citation2013 Additional inclusion/exclusion details were previously described.Citation2018
Study 3 included subjects aged ≥ 5-years with ocular health within normal limits (including a best‐corrected visual acuity [BCVA] of 0.3 logarithm of the minimum angle of resolution or better in each eye); ocular redness was not a requirement. In addition to the exclusion criteria utilised in Study 2,Citation2018 subjects were also excluded if they had prior/anticipated concurrent use of monoamine oxidase inhibitors and/or antidepressants that affect noradrenergic transmission.
Study 4 included adults (≥ 18 and ≤ 55-years old) with normal ocular health (BCVA of 0.6 logarithm of minimum angle of resolution or better in each eye) and blood/urine analyses within normal limits. Exclusion criteria included known contraindications/sensitivity to study medications; any active systemic/ocular disorder other than refractive disorder; prior/anticipated concurrent use of alcohol/caffeine/xanthine consumption (≤ 48-hours of beginning study treatment), contact lenses (≤ 5-days of beginning study treatment), prescription or non‐prescription drugs (≤ 14-days of beginning study treatment), investigational drugs/devices (≤ 30-days of beginning study treatment), or long‐acting depot injectable/implant drugs; any abnormality of the lids, ocular surface, or lacrimal duct system that in the investigator's opinion could affect ophthalmic drop absorption; history (≤ 12-months of beginning study treatment) of chronic alcohol/illicit drug consumption or tobacco/nicotine‐containing product use; abnormal blood pressure; intraocular pressure < 5-mmHg or > 22-mmHg or a diagnosis of glaucoma.
Treatments and assessments
In the efficacy studies (Studies 1 and 2), treatment was instilled bilaterally four times daily for four-weeks (Days 1 to 29), with assessments of ocular redness (using the Ora Calibra Ocular Hyperemia Scale) beginning on Day 1 and concluding one-week after treatment discontinuation. Investigator assessments (allowing half‐unit increments) were conducted during treatment visits on Day 1, Day 15 ± 2, Day 29 ± 2, and one-week after treatment discontinuation on Day 36 ± 1, while subject redness assessments (whole‐unit increments) were completed during treatment (Days 1 to 29 ± 2) and for one-week after treatment discontinuation.
The primary efficacy outcome in this integrated analysis was ocular redness evaluated by the investigator before medication instillation and at five, 15, 30, 60, 90, 120, 180, and 240-minutes post‐instillation on Day 1. Secondary efficacy outcomes included investigator assessment of ocular redness at five-minutes post‐instillation at Day 15 and Day 29, investigator assessment of total clearance of ocular redness at each post‐instillation time point at each visit, and ocular redness as evaluated by the subject (captured in subject diaries) throughout the treatment period (Days 1 to 29). The potential for tachyphylaxis was evaluated using the change from pre‐instillation to five-minutes post‐instillation in investigator‐assessed ocular redness score on Day 15 and Day 29.
In the safety study (Study 3), treatment was instilled bilaterally four times daily for four-weeks. In the pharmacokinetic study (Study 4), treatment was instilled bilaterally once on the first day and then four times daily for the subsequent five-days, with plasma collections post‐instillation at 0.25, 0.5, 1, 1.5, 2, 3, 4, 6, 8, 12, 18, and 24-hours; brimonidine plasma concentrations were determined by liquid chromatography–tandem mass spectrometry. Safety assessments (Studies 1 through 4) included treatment‐emergent adverse events, BCVA, slitlamp biomicroscopy, dilated fundoscopy, intraocular pressure, physical examinations, and vital signs.
Rebound redness upon treatment discontinuation was assessed as a safety parameter in Studies 1 and 2 only, and was defined as any increase (Study 1) or an increase of ≥ 1 unit (Study 2) from the baseline redness score (Day 1 pre‐instillation), as evaluated by the investigator (Day 36) or as reported by the subject (overall daily average for Days 30 to 36). In Studies 1, 2, and 3, drop comfort was assessed using a 0–10 unit scale (0 = very comfortable; 10 = very uncomfortable) upon instillation and at 30-seconds and one-minute post‐instillation, and alertness (six‐point scale; 0 = fully alert, 5 = coma) was evaluated by investigators at on‐treatment visits; alertness was also assessed daily by subjects in diaries (Study 3 only).
Statistical analysis
The efficacy population (Studies 1 and 2) included all randomised subjects who received at least one dose of study medication and completed one post‐instillation ocular redness evaluation on Day 1. The safety population (Studies 1 through 4) included all subjects who received at least one dose of study medication. For the primary efficacy assessment (investigator‐assessed ocular redness on Day 1), brimonidine was compared with vehicle using a mixed‐effect repeated measure model that contained treatment, time point, treatment by time point interaction, and baseline (Day 1 pre‐instillation) score. Least‐squares means, standard errors, mean differences with 95-per cent confidence intervals, and p‐values were calculated. Investigator assessments on Day 15 and Day 29 were analysed using similar mixed‐effect repeated measure modelling. In addition, change in investigator‐assessed ocular redness (from pre‐ to post‐instillation time points) at each visit was analysed using two‐sample t‐tests. A responder analysis compared the percentage of subjects with total clearing (redness scores of 0 based on investigator assessment) in the two treatment groups using Fisher's exact test. For subject ratings of ocular redness (as recorded in diaries), mean scores from both eyes at each time point were used to calculate daily averages for each subject. The last observation carried forward method was used to impute missing data if no score was provided for an entire day. Diary data for post‐instillation time points were analysed using a mixed‐effect repeated measure model that contained treatment, time point, and the treatment by time point interaction.
Safety data were pooled across the four studies (Table ) and summarised using descriptive statistics. The Medical Dictionary for Regulatory Activities (version 16.1) was used to classify adverse events. Drop comfort was compared for low‐dose brimonidine versus vehicle using a two‐sample t‐test. Efficacy data were pooled across the two efficacy studies, with the exception of rebound redness, for which the definition varied between studies. Pooling is an appropriate analytic approach when access to patient‐level data is available and sources of potential heterogeneity are controlled. As the two efficacy studies included in this analysis were of similar design (randomised, double‐masked, vehicle‐controlled), with a similar subject population (adults with ocular redness of > 1 on a 0‐ to 4‐point scale) treated with the same product (brimonidine tartrate ophthalmic solution 0.025 per cent) for a similar length of time (four-weeks) and assessed using the same outcome measure (the Ora Calibra Ocular Hyperemia Scale), the potential for heterogeneity was limited, and pooling was selected as the preferred approach.
Results
Subject demographics and disposition
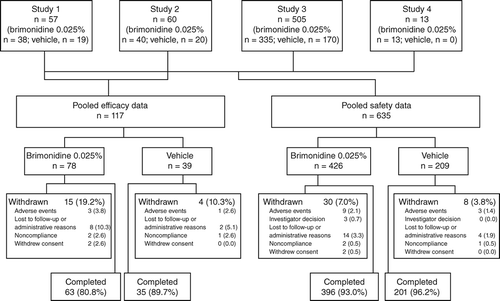
The efficacy population included 117 subjects (brimonidine, n = 78; vehicle, n = 39). The safety population included 635 subjects (brimonidine, n = 426; vehicle, n = 209). Baseline demographic characteristics were similar between treatment groups (Table ). Average age was 51.6-years in the efficacy population and 42.4-years in the safety population. Geriatric subjects (≥ 65-years) comprised 18.8-per cent of the efficacy population and 10.9-per cent of the safety population; the safety population also included paediatric subjects (7.9-per cent) aged ≥ 5 to 17-years.
Table 2. Subject demographic characteristics
The study completion rate was > 80-per cent in the efficacy analysis and > 90-per cent in the safety analysis (Figure 1). The most common reason for discontinuation was loss to follow‐up or administrative reasons.
Efficacy outcomes
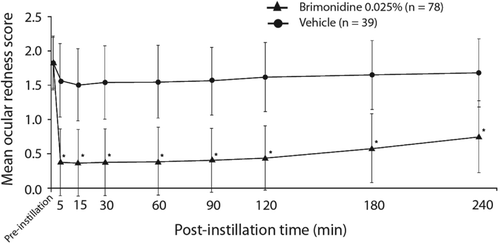
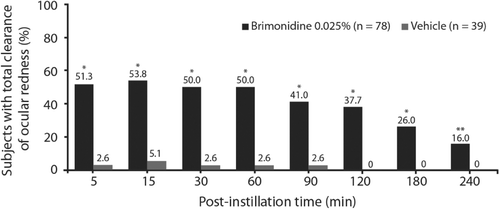
On Day 1, mean investigator‐assessed ocular redness scores were comparable between the treatment groups at baseline (1.8 in both groups) and significantly lower for low‐dose brimonidine compared with vehicle at all post‐instillation time points (all p < 0.0001) through four-hours (Figure 2). Similarly, the mean change in redness score from pre‐instillation to each post‐instillation time point was significantly greater for brimonidine relative to vehicle (p < 0.0001 for all). For the entire post‐instillation time period (five to 240-minutes), mean (standard error) redness score was 0.5 (0.05) in the low‐dose brimonidine group and 1.6 (0.07) in the vehicle group (p < 0.0001); mean change (standard error) from pre‐instillation redness score was −1.4 (0.05) for low‐dose brimonidine and −0.2 (0.07) for vehicle (p < 0.0001). Based on investigator evaluation (observed data only), total clearance of redness on Day 1 (Figure 3) was observed in a significantly greater number of subjects in the low‐dose brimonidine group as compared to the vehicle group at all time points post‐instillation (p ≤ 0.0077).
At‐home assessment of ocular redness, as recorded in subject diaries, showed significantly lower post‐instillation redness scores for subjects treated with low‐dose brimonidine compared with subjects in the vehicle group (Table ). Least‐squares mean post‐instillation redness scores were significantly lower for low‐dose brimonidine compared with vehicle between Days 1 and 15 and between Days 15 and 29 (both time points mean treatment difference of −0.9; p < 0.0001).
Table 3. Average daily ocular redness scores based on subject diary data (efficacy population)
For both investigator and at‐home ocular redness scores, subgroup analyses by age (adult, 18–64-years; geriatric ≥ 65-years), sex (male, female), race (White, Black), and iris colour (blue, brown, other) were generally consistent with results for the overall efficacy population.
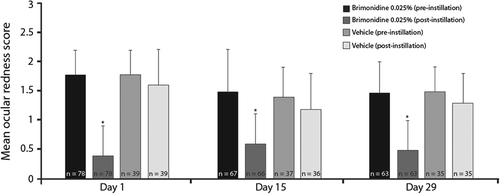
Evaluation of tachyphylaxis was based on investigator ratings of ocular redness after 15 and 29-days of treatment. In both treatment groups, mean pre‐dose redness scores appeared lower on Day 15 (brimonidine, 1.5; vehicle, 1.4) and Day 29 (1.5 in both groups) relative to Day 1 (1.8 in both groups). The mean decrease in redness scores from pre‐dose to five-minutes post‐dose was significantly greater for subjects treated with low‐dose brimonidine compared with vehicle on Day 15 and Day 29, as it was on Day 1 (all p < 0.0001), with ocular redness scores post‐instillation remaining consistent at all visits (Figure 4).
Safety outcomes
In the safety population, the mean duration of exposure to the study medication was similar for low‐dose brimonidine (27.2 subject‐days) and vehicle (28.6 subject‐days). Total low‐dose brimonidine exposure equalled 11,597 subject‐days.
The incidence of ocular adverse events was similar in low‐dose brimonidine‐treated and vehicle‐treated subjects (Table ). The most common ocular adverse events were reduced visual acuity and conjunctival hyperaemia, all mild and deemed unrelated to treatment. Non‐ocular adverse events occurred in a similar percentage of subjects receiving low‐dose brimonidine (9.9-per cent) and vehicle (10.0-per cent). Non‐ocular adverse events occurring in ≥ 1-per cent of brimonidine‐ or vehicle‐treated subjects included headache (1.2 and 1.9-per cent, respectively), nasopharyngitis (0.7 and 1.9-per cent, respectively), and sinusitis (0.5 and 1.0-per cent, respectively); of these, only headache was considered to be treatment‐related by the investigator (0.7 and 0.5-per cent, respectively). There were no ocular adverse events and only one non‐ocular adverse event in the paediatric population (sinusitis in a vehicle‐treated subject).
Table 4. Incidence of ocular adverse events occurring in more than one subject in any treatment group (safety population)
Most adverse events (ocular and non‐ocular) were mild or moderate, and the majority were deemed not related to study treatment. Severe ocular adverse events were experienced by one vehicle‐treated subject (corneal abrasion and reduced visual acuity), and severe non‐ocular adverse events by two brimonidine‐treated subjects (one had gastroenteritis; one had sinusitis and methicillin‐resistant Staphylococcus aureus) and one vehicle‐treated subject (arrhythmia); the three severe/serious adverse events with brimonidine were not considered treatment‐related. The incidence of treatment‐related ocular adverse events was 5.2-per cent of subjects in the low‐dose brimonidine group and 4.8-per cent of subjects in the vehicle group, while the incidence of treatment‐related non‐ocular events was 2.1 and 1.4-per cent, respectively.
Study discontinuation due to adverse events occurred in 2.1 per cent of low‐dose brimonidine‐treated subjects (for contusion, corneal erosion, eye irritation, gastroenteritis, hypotension, instillation site burn, instillation site pain, nasal discomfort, sinusitis, and staphylococcal infection) and in 1.4-per cent of vehicle‐treated subjects (for bacterial pneumonia, headache, pyrexia, seasonal allergy, and sinusitis). Of the adverse events leading to study discontinuation, only hypotension and instillation site burn were considered to be treatment‐related.
Rebound redness after treatment discontinuation was minimal.Citation2018 The mean investigator‐evaluated ocular redness score on Day 36, one-week after brimonidine discontinuation, was 1.6 and similar to pre‐instillation scores on Days 1, 15, and 29. Likewise, the mean (standard deviation) ocular redness score recorded by brimonidine‐treated subjects in the week (Days 30–36) following treatment discontinuation (1.4 [0.9]) was similar to on‐treatment pre‐instillation scores (1.3 [0.9] for Days 1–15 and 1.3 [0.8] for Days 15–29). In Study 1, no rebound redness was identified based on either investigator assessment or subject diaries.Citation2018 In Study 2, rebound redness was identified in one subject (brimonidine) by investigator assessment and in seven subjects (four brimonidine‐treated and three vehicle‐treated) based on subject evaluation.Citation2018
Throughout the study, BCVA was generally similar among treatment groups, with mean changes (logarithm of the minimum angle of resolution) from baseline varying by less than ±0.04 at all visits. There were no meaningful changes from baseline based on slitlamp biomicroscopy or dilated fundoscopy results for either treatment group.
Mean intraocular pressure was 15-mmHg for each treatment group at baseline and remained similar (within 1-mmHg) across treatment groups through Day 29. Vital signs (heart rate, blood pressure, body weight) were similar among treatment groups, with no meaningful changes noted from baseline through Day 29. Physical examinations were considered normal at all visits, excepting a few subjects (five brimonidine, two vehicle) with non‐ocular findings deemed unrelated to treatment at Day 29. No safety concerns were noted in either the paediatric or geriatric populations.
Analysis of plasma samples in the pharmacokinetic study showed concentrations of brimonidine were below the lower limit of quantitation (0.025-ng/ml) at all time points with one exception; one subject had a detectable level (0.0253-ng/ml) at a single time point (one-hour following instillation of a single dose). No significant differences were observed between treatment groups for drop comfort. Mean (standard deviation) ratings of drop comfort in the low‐dose brimonidine and vehicle groups, respectively, were 0.5 (1.2) and 0.5 (1.0) upon instillation, 0.5 (1.1) and 0.4 (1.0) at 30-seconds post‐instillation, and 0.4 (1.0) and 0.4 (0.9) at one-minute post‐instillation.
Discussion
The results of this integrated analysis demonstrate the efficacy and safety of low‐dose brimonidine tartrate ophthalmic solution (0.025 per cent) in the treatment of ocular redness. At all post‐instillation evaluations, investigator‐assessed ocular redness was significantly lower in the low‐dose brimonidine group compared with the vehicle group. Similarly, subject‐assessed redness was significantly lower for low‐dose brimonidine compared with vehicle throughout the treatment period. The duration of action of redness reduction with low‐dose brimonidine was only evaluated in one of the two efficacy studies integrated hereinCitation2017 and demonstrated to be significant for up to eight-hours.
In the current integrated analysis, limited to the post‐instillation time points common to both studies, a robust reduction in ocular redness was confirmed over the four-hour post‐instillation period. Further, there was no evidence of tachyphylaxis over the one-month of treatment. The incidence of ocular and non‐ocular adverse events was low and similar between treatment groups, and there was minimal evidence of rebound redness upon treatment discontinuation. No safety concerns were identified on ophthalmic examination, and there were no substantial effects on intraocular pressure. Systemic exposure was negligible following topical administration, minimising concerns for systemic adverse effects, and both low‐dose brimonidine and its vehicle were considered very comfortable.
In cases where the cause of ocular redness is known, it would be appropriate to select treatments that specifically target the underlying aetiology (for example, allergic or infectious conjunctivitis).Citation2010 Topical vasoconstrictors may be considered for the management of ocular redness that has no obvious underlying pathology. However, the effectiveness of topical vasoconstrictors is often limited by the occurrence of tachyphylaxis and rebound redness.Citation1994 The mechanism of action of brimonidine (α2‐receptor agonist) differs from that of the available ocular vasoconstrictors, which are either selective α1‐receptor agonists or mixed α1/α2‐receptor agonists. This difference in adrenergic receptor binding may account for the absence of tachyphylaxis and minimal rebound redness observed in this analysis. In addition, there were no adverse event reports of mydriasis in the safety population of this analysis, in contrast to pupil dilation caused by α1‐receptor agonists.
Systemic and topical α2‐receptor agonists are known to have sedative properties and cardiovascular effects.Citation2015 Fatigue and drowsiness have been reported in studies of brimonidine tartrate ophthalmic solution 0.2-per cent,Citation1997 and somnolence is of particular concern in children.Citation2001 In this analysis of low‐dose brimonidine, fatigue was reported as an adverse event only once (adult subject) and there were no reports of somnolence; all paediatric subjects were deemed fully alert. Consistent with previous studies that showed minimal to no cardiovascular effects for higher‐dose brimonidine,Citation2000 no meaningful changes in mean heart rate or blood pressure were observed in this analysis. Miosis, previously reported with higher‐dose formulations of brimonidine,Citation2004 was also not reported in this analysis. Further, this analysis appears to confirm a likely dose‐dependent association for ocular allergic reactions, which have been reported in studies of 12-months in duration with brimonidine 0.2-per cent,Citation1998 and at a lower incidence with 0.15-per cent,Citation2002 but were not observed in this analysis of four safety studies of brimonidine tartrate ophthalmic solution 0.025 per cent over four-weeks of treatment. Long‐term studies with low‐dose brimonidine are warranted to confirm whether the low propensity of ocular allergy is demonstrated with continued use.
Limitations of these analyses include the relatively small sample sizes of paediatric and geriatric subjects. The efficacy of low‐dose brimonidine tartrate ophthalmic solution as an ocular redness reducer was demonstrated in this analysis; however, a larger database of treated subjects would provide a more complete safety profile, particularly for adverse events that infrequently occur.
In conclusion, this integrated analysis of safety and efficacy data found that low‐dose brimonidine tartrate ophthalmic solution (0.025 per cent) was effective in reducing ocular redness, generally safe, and well tolerated. The lack of tachyphylaxis and rare rebound redness observed after month‐long use suggests that use of low‐dose brimonidine does not appear to be limited by the side effects of other marketed vasoconstrictors. A longer period of clinical use in larger populations is needed to provide additional information regarding long‐term effectiveness.
ACKNOWLEDGEMENTS
Editorial and medical writing assistance was provided by Synchrony Medical Communications, LLC, West Chester, Pennsylvania, USA, and funded by Bausch & Lomb Incorporated, Rochester, New York. Studies were funded by Eye Therapies, LLC, Dana Point, California or Bausch & Lomb Incorporated, Rochester, New York. SLA, GLT, and EM were study investigators. SLA has a consulting agreement with Realm Therapeutics. GLT has received consultancy fees from Ora, Inc., reimbursement of meeting traveling expenses from Alcon Research Ltd., and research grants from Allergan. EM has received research grants from the following companies: Aciex, Acucela, Alcon Research Ltd., Allergan, AstraZeneca, Bausch + Lomb, Inotek Pharma, InSite Vision, Lexicon Pharma, Mimetogen, and Ocular Therapeutix. JLV is an employee of Bausch + Lomb, a division of Valeant Pharmaceuticals North America LLC.
Additional information
Funding
REFERENCES
- Cronau H, Kankanala RR, Mauger T. Diagnosis and management of red eye in primary care. Am Fam Physician 2010; 81: 137–144.
- Galor A, Jeng BH. Red eye for the internist: when to treat, when to refer. Cleve Clin J Med 2008; 75: 137–144.
- Dunlop AL, Wells JR. Approach to red eye for primary care practitioners. Prim Care 2015; 42: 267–284.
- Abelson MB, Yamamoto GK, Allansmith MR. Effects of ocular decongestants. Arch Ophthalmol 1980; 98: 856–858.
- Torkildsen GL, Sanfilippo CM, Decory HH et al. Evaluation of efficacy and safety of brimonidine tartrate ophthalmic solution, 0.025% for treatment of ocular redness. Curr Eye Res 2018; 43: 43–51.
- Adamczyk DT, Jaanus SD. Antiallergy drugs and decongestants. In: Bartlett JD, Jaanus SD, eds. Clinical Ocular Pharmacology, 5th ed. St Louis, MO: Butterworth‐Heinemann, 2008.
- Spector SL, Raizman MB. Conjunctivitis medicamentosa. J Allergy Clin Immunol 1994; 94: 134–136.
- Soparkar CN, Wilhelmus KR, Koch DD et al. Acute and chronic conjunctivitis due to over‐the‐counter ophthalmic decongestants. Arch Ophthalmol 1997; 115: 34–38.
- Vaidyanathan S, Williamson P, Clearie K et al. Fluticasone reverses oxymetazoline‐induced tachyphylaxis of response and rebound congestion. Am J Respir Crit Care Med 2010; 182: 19–24.
- Bielory L, Katelaris CH, Lightman S et al. Treating the ocular component of allergic rhinoconjunctivitis and related eye disorders. MedGenMed 2007; 9: 35.
- Fratelli M, De blasi A. Agonist‐induced alpha 1‐adrenergic receptor changes. Evidence for receptor sequestration. FEBS Lett 1987; 212: 149–153.
- Guimaraes S, Moura D. Vascular adrenoceptors: an update. Pharmacol Rev 2001; 53: 319–356.
- Adkins JC, Balfour JA. Brimonidine. A review of its pharmacological properties and clinical potential in the management of open‐angle glaucoma and ocular hypertension. Drugs Aging 1998; 12: 225–241.
- Fudemberg SJ, Batiste C, Katz LJ. Efficacy, safety, and current applications of brimonidine. Expert Opin Drug Saf 2008; 7: 795–799.
- Rahman MQ, Ramaesh K, Montgomery DM. Brimonidine for glaucoma. Expert Opin Drug Saf 2010; 9: 483–491.
- Corboz MR, Mutter JC, Rivelli MA et al. alpha2‐adrenoceptor agonists as nasal decongestants. Pulm Pharmacol Ther 2007; 20: 149–156.
- Corboz MR, Rivelli MA, Mingo GG et al. Mechanism of decongestant activity of alpha 2‐adrenoceptor agonists. Pulm Pharmacol Ther 2008; 21: 449–454.
- Fowler J Jr, Jackson M, Moore A et al. Efficacy and safety of once‐daily topical brimonidine tartrate gel 0.5% for the treatment of moderate to severe facial erythema of rosacea: results of two randomized, double‐blind, and vehicle‐controlled pivotal studies. J Drugs Dermatol 2013; 12: 650–656.
- Tong LX, Moore AY. Brimonidine tartrate for the treatment of facial flushing and erythema in rosacea. Expert Rev Clin Pharmacol 2014; 7: 567–577.
- Norden RA. Effect of prophylactic brimonidine on bleeding complications and flap adherence after laser in situ keratomileusis. J Refract Surg 2002; 18: 468–471.
- Desco MC, Navea A, Ferrer E et al. Effect of prophylactic brimonidine on bleeding complications after cataract surgery. Eur J Ophthalmol 2005; 15: 228–232.
- Hong S, Kim CY, Seong GJ et al. Effect of prophylactic brimonidine instillation on bleeding during strabismus surgery in adults. Am J Ophthalmol 2007; 144: 469–470.
- Gupta A, Kekunnaya R, Sachdeva V et al. Strabismus surgery hemostasis. Ophthalmology 2012; 119: 649–650.
- Pasquali TA, Aufderheide A, Brinton JP et al. Dilute brimonidine to improve patient comfort and subconjunctival hemorrhage after LASIK. J Refract Surg 2013; 29: 469–475.
- Kim CS, Nam KY, Kim JY. Effect of prophylactic topical brimonidine (0.15%) administration on the development of subconjunctival hemorrhage after intravitreal injection. Retina 2011; 31: 389–392.
- Dahlmann‐noor AH, Cosgrave E, Lowe S et al. Brimonidine and apraclonidine as vasoconstrictors in adjustable strabismus surgery. J AAPOS 2009; 13: 123–126.
- Derick RJ, Robin AL, Walters TR et al. Brimonidine tartrate: a one‐month dose response study. Ophthalmology 1997; 104: 131–136.
- United States Department of Health and Human Services. Food and Drug Administration. Approval Package for: Lumify Ophthalmic Solution, 0.025%; 2017. [Cited 13 Aug 2018.] Available at: https://www.accessdata.fda.gov/drugsatfda_docs/nda/2017/208144Orig1s0000Approv.pdf.
- Mclaurin E, Cavet ME, Gomes PJ et al. Brimonidine ophthalmic solution 0.025% for reduction of ocular redness: a randomized clinical trial. Optom Vis Sci 2018; 95: 264–271.
- Gomes PJ, Ousler GW, Welch DL et al. Exacerbation of signs and symptoms of allergic conjunctivitis by a controlled adverse environment challenge in subjects with a history of dry eye and ocular allergy. Clin Ophthalmol 2013; 7: 157–165.
- Abelson MB, Gomes PJ, Vogelson CT et al. Clinical efficacy of olopatadine hydrochloride ophthalmic solution 0.2% compared with placebo in patients with allergic conjunctivitis or rhinoconjunctivitis: a randomized, double‐masked environmental study. Clin Ther 2004; 26: 1237–1248.
- Giovannitti JA Jr, Thoms SM, Crawford JJ. Alpha‐2 adrenergic receptor agonists: a review of current clinical applications. Anesth Prog 2015; 62: 31–39.
- Carollo DS, Nossaman BD, Ramadhyani U. Dexmedetomidine: a review of clinical applications. Curr Opin Anaesthesiol 2008; 21: 457–461.
- Walters TR. Development and use of brimonidine in treating acute and chronic elevations of intraocular pressure: a review of safety, efficacy, dose response, and dosing studies. Surv Ophthalmol 1996; 41 (Suppl 1): S19–S26.
- Lee DA, Gornbein J, Abrams C. The effectiveness and safety of brimonidine as mono‐, combination, or replacement therapy for patients with primary open‐angle glaucoma or ocular hypertension: a post hoc analysis of an open‐label community trial. Glaucoma Trial Study Group. J Ocul Pharmacol Ther 2000; 16: 3–18.
- Cantor LB. The evolving pharmacotherapeutic profile of brimonidine, an alpha 2‐adrenergic agonist, after four years of continuous use. Expert Opin Pharmacother 2000; 1: 815–834.
- Enyedi LB, Freedman SF. Safety and efficacy of brimonidine in children with glaucoma. J AAPOS 2001; 5: 281–284.
- Bowman RJ, Cope J, Nischal KK. Ocular and systemic side effects of brimonidine 0.2% eye drops (Alphagan) in children. Eye (Lond) 2004; 18: 24–26.
- Kesler A, Shemesh G, Rothkoff L et al. Effect of brimonidine tartrate 0.2% ophthalmic solution on pupil size. J Cataract Refract Surg 2004; 30: 1707–1710.
- Thordsen JE, Bower KS, Warren BB et al. Miotic effect of brimonidine tartrate 0.15% ophthalmic solution in normal eyes. J Cataract Refract Surg 2004; 30: 1702–1706.
- Leblanc RP. Twelve‐month results of an ongoing randomized trial comparing brimonidine tartrate 0.2% and timolol 0.5% given twice daily in patients with glaucoma or ocular hypertension. Brimonidine Study Group 2. Ophthalmology 1998; 105: 1960–1967.
- Schuman JS, Horwitz B, Choplin NT et al. A 1‐year study of brimonidine twice daily in glaucoma and ocular hypertension. A controlled, randomized, multicenter clinical trial. Chronic Brimonidine Study Group. Arch Ophthalmol 1997; 115: 847–852.
- Katz LJ. Twelve‐month evaluation of brimonidine‐purite versus brimonidine in patients with glaucoma or ocular hypertension. J Glaucoma 2002; 11: 119–126.