Abstract
The influence of the addition of peat on arbuscular mycorrhizal formation and host plant growth was investigated using a pot experiment. Peat was mixed with Masa soil at different levels (0, 25, 50, 100, 150, 200 g kg−1) into which an arbuscular mycorrhizal fungus (AMF) Gigaspora margarita Becker & Hall was inoculated, and seedlings of Miscanthus sinensis Anderess were planted. There was a significant increase in plant growth with increasing levels of peat. Root colonization and the number of proliferating spores increased with increasing levels of peat. By decreasing the bulk density, increasing the maximum water-holding capacity and the content of total nitrogen, peat addition considerably improved the physical and chemical properties of the soil, which might result in the promotion of plant growth and AMF activity.
INTRODUCTION
It has generally been recognized that the effect of arbuscular mycorrhizal (AM) symbiosis associated with host plants is beneficial because growth and development of the plants are stimulated, drought tolerance increases and the association has a high potential for agriculture and land reclamation (CitationLiu and Li 2000). As a result of these characteristics, arbuscular mycorrhizal fungi (AMF) have been expected to promote plant growth in degraded and denuded lands (CitationYokoyama et al. 2005). Therefore, revegetation practices in degraded and denuded ecosystems often include the introduction of AMF with the plant seeds (CitationSaito 2000).
Soil factors influence AM formation. Organic matter enhances soil fertility and also stimulates the development of AMF in the soil (CitationHepper et al. 1983; CitationJoner and Jakobsen 1995).
Peat is often the major component of many organic amendments and its specific characteristics exert a significant influence on AMF (CitationLinderman and Davis 2003). CitationLinderman and Davis (2003) indicated that interactions between the fungal isolates, peat types and levels of peat application to the medium could be specific. However, in studies examining the effect of peat on AM formation, Canadian peat was the substrate most frequently used. The effect of peat from China on AM formation is poorly documented. Peat properties vary considerably with location. Different types of peat vary in their degree of decomposition. Plant species, climatic conditions and water quality affect the distinct characteristics of peat (Wang et al., 2001). Chinese peat was mostly composed of herbage and arbor and moss accounted for only 1%, whereas Canadian peat was mostly composed of moss (CitationWang et al. 2001). Therefore, there were large differences in the physical and chemical properties between Chinese and Canadian peat (CitationWang et al. 2001). In general, the pH of Chinese peat ranged from 5 to 7, whereas the pH of Canadian peat was lower (3.5–4). The maximum water-holding capacity of Canadian peat was much higher than that of Chinese peat. The organic matter content of Chinese peat was in the range of 30–50%, whereas Canadian peat was higher at 91–99%. The total N content of Chinese peat ranged from 1.4 to 2.4%, whereas Canadian peat ranged from 0.4 to 0.6% (CitationWang et al. 2001).
China has abundant peat resources, amounting to approximately 46 hundred million tons (CitationWang et al. 2001). Studies on the use of peat in China have been carried out for agriculture, horticulture and reforestation programs over a long period of time. Improvement in soil physical and chemical properties such as aeration, water retention and nutrient status with the addition of Chinese peat suggested that peat could be a factor that promotes the growth of AM fungi in soil, the infection of roots and the development of AM symbiosis. It is anticipated that a combination of peat and AM may considerably enhance plant growth and have beneficial effects on the stability of the ecosystems in degraded soil and in rehabilitation programs of degraded soils.
In the present study, attempts were made to investigate the effect of the addition of peat on root colonization of AMF in soil and plant growth. To our knowledge, no direct study examining the effect of peat from China on AM formation has been conducted.
MATERIALS AND METHODS
Soil amendments
Peat originating from China was purchased in Japan (Nibannsu Co., Japan). Masa soil, a decomposed granite soil, was sampled from the lower layers of mountain soil and is commercially available in Japan (Kakeuma Sangyo Co., Fukuoka, Japan). Masa soil was passed through a 2 mm mesh sieve and autoclaved twice at 121°C for 30 min before use. The peat was autoclaved twice at 80°C for 30 min.
Soil amendment consisted of 6 treatments, that is, the addition of 0, 25, 50, 100, 150 and 200 g kg−1 peat (0, 2.5, 5, 10, 15, 20% hereafter). The experiment was repeated 3 times with 3 replications per treatment.
Multiplication and maintenance
The endophytes used were obtained as spores of Gigaspora margarita MAFF520054 from the National Institute of Agrobiological Sciences, Tsukuba, Japan. Spores were multiplied in pot cultures on white clover for 3 months (CitationSaito 2001). The collected soil samples with spores were stored at 4°C until required. Newly developed spores were extracted by wet sieving and decanting 5 days before inoculation (CitationGerdemann and Nicolson 1963). Spores were picked up individually with pipettes, washed in sterile deionized water and stored.
Plant
The seeds of Miscanthus sinensis Anderess were purchased in Japan (Kaneko Co., Hiroshima, Japan). The seeds were sterilized in a 10% NaClO solution for approximately 40 min, rinsed thoroughly with sterile deionized water and germinated on moist filter paper in sterilized petri dishes at 25°C. The seeds germinated after approximately 2 weeks in a growth chamber. Three seedlings were either inoculated with 10 spores (inoculated plants) or were not inoculated (non-inoculated plants) and were placed into plastic pots with a capacity of 87.45 cm. The pots were placed in transparent Sunbags (44.0 cm × 20.5 cm) (Sigma, St Louis, MO, USA) in a growth chamber. Light was provided under a photoperiod of 12 h and the temperature was 25 ± 2°C (day and night). Sterile deionized water was supplied every 10 days.
Determination of plant growth and root colonization in each treatment was carried out at 40, 60, 90 and 120 days after incubation. Plants were harvested by cutting the shoots at the soil surface. Shoot fresh weight was recorded immediately. The roots were cleaned and stained with trypan blue, and the percentage of root colonization was assessed using the grid intersect method (CitationGiovannetti and Mosse 1980). The number of spores was counted using the wet sieving method (CitationGerdemann and Nicolson 1963).
Soils amended with peat were analyzed at the onset of the study for nutrient content. Maximum water-holding capacity was measured using the Hilgard method. Bulk density was measured using the core method (CitationBlack et al. 1965). The pH was measured using the pH Electrode Meter Method (CitationDojyo hyojun bunseki sokutei hou iinkai 1986). The concentration of available phosphorus (P) was determined using the Truog method, total nitrogen (N) content was determined using the Kjeldahl method, and total organic carbon content was determined using the Tyurin method (CitationDojyo hyojun bunseki sokutei hou iinkai 1986). The physical and chemical properties of the soil media are listed in .
Statistical analysis
The treatments were arranged as follows, inoculated treatment, non-inoculated treatment and rate of peat amendment, and each treatment was replicated 3 times. Differences between the treatments were determined using an anova and a Tukey test (P < 0.05). Statistical comparisons were considered significant at P < 0.05.
RESULTS AND DISCUSSION
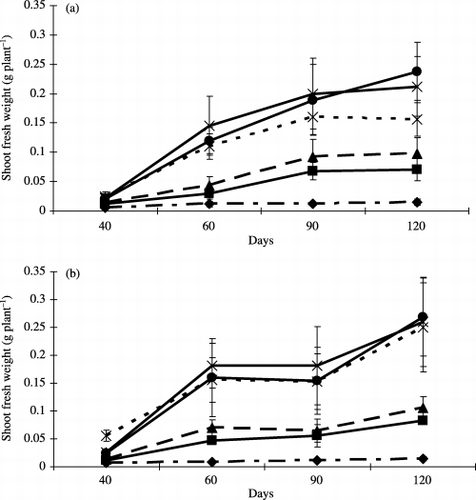
There was a significant increase in the shoot fresh weight of the non-inoculated plants when the level of added peat increased (). The shoot fresh weight of non-inoculated plants with 15% and 20% peat addition was higher than that with 10%, but there were no significant differences between the values at 60 and 90 days. The addition of peat exerted a significant promotive effect on plant growth to a certain extent.
With increasing levels of peat addition, the shoot fresh weight of the inoculated plants gradually increased
Table 1 Physical and chemical properties of the soil medium
Figure 1 Shoot fresh weight of (a) non-inoculated plants and (b) inoculated plants at each harvest time. Bars represent standard deviations of the means of three replicates. Peat addition rate: 0% (–⧫–), 2.5% (–▪–), 5% (–▴–), 10% (--×--), 15% (–×–) and 20% (–•–) (w/w).
Similarly, there was a significant increase in the total root length of the mycorrhizal plants when peat amendment increased from 2.5% to 20% (). The total root length of the plants in all treatments increased as the culture time increased.
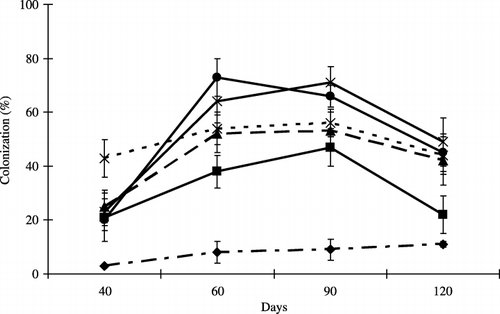
The time-course of culture from onset to 90 days revealed an increase in the root colonization of plants in
Figure 2 Total root length of inoculated plants. Bars represent standard deviations of the means of three replicates. Differences among the levels of peat addition were determined using an anova (P = 0.58, 0.04, 0.036 and 0.16 at 40, 60, 90 and 120 days, respectively). Peat addition rate: 0% (–⧫–), 2.5% (–▪–), 5% (–▴–), 10% (--×--), 15% (–×–) and 20% (–•–) (w/w).
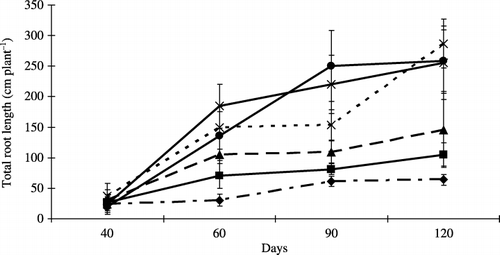
Figure 3 Root colonization at each harvest time. Bars represent standard deviations of the means of three replicates. Differences among the levels of peat addition were determined using an anova (P = 0.03, 0.001, 0.001 and 0.001 at 40, 60, 90 and 120 days, respectively). Peat addition rate: 0% (–⧫–), 2.5% (–▪–), 5% (–▴–), 10% (--×--), 15% (–×–) and 20% (–•–) (w/w).
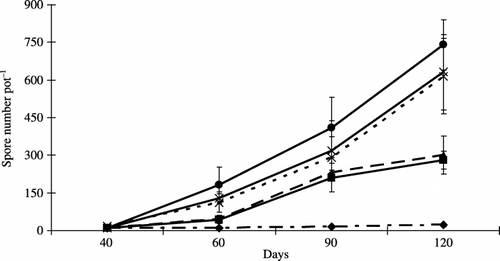
The number of proliferating spores in the treatments consisting of 0–20% peat addition increased at each harvest time (). The number of spores in the plants with 20% peat addition was the highest at each harvest time. The lowest number was recorded in the Masa soil at each harvest time, and the spore number in all treatments increased until the end of the culture.
In the present study, these observations confirmed the overall beneficial effect of peat on plant growth. In addition, the stimulatory effect of AMF on plant growth by the addition of peat was also observed. There was a significant increase in the root colonization percentage and spore number with increasing levels of peat. It was suggested that the incorporation of peat exerted a beneficial effect on AMF activity and AM formation.
The promotion of AM formation by the addition of peat presumably resulted from improvement of soil physical properties and soil nutrient conditions, such as P, N and organic matter contents ().
First, in the case of the soil physical properties, the addition of peat sharply reduced the bulk density of soil. As a result, total root length increased (), and the percentage of colonized root length increased (). This reduction in soil bulk density also led to an increase in soil porosity. For mycorrhizal formation, porous materials could be effective for AMF (CitationEzawa et al. 2002). In contrast, the optimum substrate for mycorrhizal infection should provide an adequate and balanced water supply, and an abundant oxygen supply for capillary root growth (CitationWang et al. 1993). The properties of peat were compatible with these two conditions. It is possible that because peat increased the percentage of soil pores, the elongation of roots and hyphae of AMF were enhanced. Moreover, the incorporation of peat made the soil more aerobic, which produced optimum conditions for spore growth and hyphal elongation.
Figure 4 Spore number per pot at each harvest time. Bars represent standard deviations of the means of three replicates. The differences among the levels of peat addition were determined using an anova (P = 0.01, 0.001, 0.04 and 0.001 at 40, 60, 90 and 120 days, respectively). Peat addition rate: 0% (–⧫–), 2.5% (–▪–), 5% (–▴–), 10% (--×--), 15% (–×–) and 20% (–•–) (w/w).
The availability of nutrients in soil is one of the major factors affecting the colonization of AMF, in particular the P content (CitationSmith and Read 1997). It is generally recognized that P fertilizer often adversely affects root colonization of many host species by AMF (CitationAbbott et al. 1984; CitationMiranda et al. 1994). These results indicate that, in general, AMF prefer soils with low P levels. In our experiment, as the levels of peat addition increased, the P content of soil slightly increased in the soil media (). Moreover, the concentration of P in the present experiment was much lower than that recorded in previous studies (CitationMiranda et al. 1994; CitationSylvia 1990). Thus, it is reasonable to assume that the colonization of these fungi was not depressed because the level of P in peat was low.
Although it has been suggested that N suppresses root colonization by AMF (CitationBuwalda 1982; CitationJohnson et al. 1984), N addition has also been reported to stimulate root colonization by the application of nitrate or ammonium (CitationBrown et al. 1981), and by the application of increasing amounts of nitrate at three phosphate levels (CitationHepper 1983). In the present experiment, as the levels of peat addition increased, the total N content of soil markedly increased (). This increased N level might be one of the key factors of the stimulatory effect on root colonization.
Moreover, as the levels of peat addition increased, the value of the N/P ratio also increased in the soil media (). CitationHepper (1983) reported that the application of fertilizer with a high N/P ratio promoted the infection of lettuce with Glomus mosseae. The author suggested the importance of considering the ratio of applied N to P when studying the effect of either of these nutrients on AM infection. Therefore, the increase in the N/P ratio with the addition of peat might also be related to the beneficial effect on root colonization.
In addition, the amount of organic matter in the media might be another important factor. A number of experiments have shown that organic matter stimulates hyphal growth (CitationJoner and Jakobsen 1995). It is possible that peat addition increased the amount of organic matter in the soil, which led to an increase in AM colonization levels.
The addition of peat has been reported to both stimulate (CitationWang et al. 1993) and suppress (CitationBiermann et al. 1983; CitationGramam et al. 1984) root colonization. Studies have suggested that the spread and efficiency of AMF as well as host plant growth could be affected by the nature of the peat used (CitationLinderman and Davis 2003; CitationPonton et al. 1990). A peat-based substrate low in P and with good aeration improved AMF spread and efficiency (CitationWang et al. 1993). In the present study, the properties of peat from China were compatible with the above conditions. In contrast, in all previous studies analyzing the effect of peat that we examined, fertilizer solution had been applied. However, nutrient solutions were not used in our experiment and the stimulatory effect on root colonization and plant growth resulted from the direct role of peat.
In conclusion, the present study indicated that the application of peat to soil might promote plant growth and stimulate mycorrhizal formation. This effect of peat was ascribed to its physicochemical properties, which are favorable to AMF activity. To further identify the key factor of the promotive effect of peat, a comparison of peat and peat moss is currently being undertaken. Moreover, the combined use of a suitable peat and AMF could become a useful revegetation technique for successful reforestation programs of degraded soil in the future.
ACKNOWLEDGMENTS
We thank Dr Takahiro Tateishi, Iwate University, for valuable comments on the study. We also thank Mr Kimio Nakamura and Takumi Mitsuno for their assistance with the work and for helpful suggestions for the study.
REFERENCES
- Abbott , L , Robson , AD and Deboer , G . 1984 . The effect of phosphorus on the formation of hyphae in soil by the vesicular-arbuscular mycorrhizal fungus, Glomus fasciculatum . New Phytol , 97 : 437 – 446 .
- Biermann , B and Linderman , RG . 1983 . Effect of container plant growth medium and fertilizer phosphorus on establishment and host growth response to vesicular-arbuscular mycorrhizae . JAmerSocHortSci , 108 : 962 – 972 .
- Black , CA , Evans , DD , Ensminger , LE , White , JL and Clark , FE . 1965 . “ Particle density ” . In Methods of Soil Analysis Part 1 – Physical and Mineralogical Properties, Including Statistics of Measurement and Sampling , Edited by: Black , C.A. , Evans , D.D. , Ensminger , L.E. , White , J.L. and Clark , F.E. 375 – 377 . WI : American Society of Agronomy .
- Brown , RW , Schultz , RC and Kormanik , PP . 1981 . Response of vesicular-arbuscular endomycorrhizal sweetgum seedlings to three nitrogen fertilizers . For. Sci. , 27 : 413 – 420 .
- Buwalda , JG and Goh , KM . 1982 . Host-fungus competition for carbon as a cause of growth depressions in vesicular-arbuscular mycorrhizal ryegrass . Soil BiolBiochem , 14 : 103 – 106 .
- Dojyo hyojun bunseki sokutei hou iinkai . 1986 . Dojyo hyojun bunseki sokutei hou , Tokyo : Hakuyusya Incorporation . (in Japanese)
- Ezawa , T , Yamamoto , K and Yoshida , S . 2002 . Enhancement of the effectiveness of indigenous arbuscular mycorrhizal fungi by inorganic soil amendments . Soil SciPlant Nutr , 48 : 897 – 900 .
- Gerdemann , JW and Nicolson , TH . 1963 . Spores of mycorrhizal Endogonespecies extracted from soil by wet sieving and decanting . TransBrMycolSoc , 46 : 235 – 244 .
- Giovannetti , M and Mosse , B . 1980 . An evaluation of techniques for measuring vesicular-arbuscular mycorrhizal infection in roots . New Phytol , 84 : 489 – 500 .
- Graham , JH and Timmer , LW . 1984 . Vesicular-arbuscular mycorrhizal development and growth response of rough lemon in soil and soilless media: effect of phosphorus source . JAmerSocHortSci , 109 : 118 – 121 .
- Hepper , CM . 1983 . The effect of nitrate and phosphate on the vesicular-arbuscular mycorrhizal infection of lettuce . New Phytol , 93 : 389 – 399 .
- Hepper , CM and Warner , A . 1983 . Role of organic matter in growth of a vesicular-arbuscular mycorrhizal fungus in soil . TransBrMycolSoc , 81 : 155 – 156 .
- Johnson , CR , Jarrell , WM and Menge , JA . 1984 . Influence of ammonium : nitrate ratio and solution pH on mycorrhizal infection, growth and nutrient composition of Chrysanthemum morifolium var. Circus . Plant Soil , 77 : 151 – 157 .
- Joner , EJ and Jakobsen , I . 1995 . Growth and extracellular phosphatase activity of arbuscular mycorrhizal hyphae as influenced by soil organic matter . Soil BiolBiochem , 27 : 1153 – 1159 .
- Linderman , RG and Davis , EA . 2003 . Soil amendment with different peat mosses affects mycorrhizae of onion . HortTechnology , 13 : 285 – 289 .
- Liu , RJ and Li , XL . 2000 . “ Function and mechanism of mycorrhiza ” . In Arbuscular Mycorrhizae and its Application , Edited by: Liu , R.J. and Li , X.L. 98 – 145 . Beijing : Science Press . (in Chinese)
- Miranda , JCC and Harris , PJ . 1994 . Effects of soil phosphorus on spore germination and hyphal growth of arbuscular mycorrhizal fungi . New Phytol , 128 : 103 – 108 .
- Ponton , F , Piche , Y , Parent , S and Caron , M . 1990 . The use of vesicular-arbuscular mycorrhizae in Boston fern production: I. Effects of peat-based mixes . Hortscience , 25 : 183 – 189 .
- Saito , M . 2000 . “ Use of VA mycorrhizal fungi ” . In Biseibutsu no Shizaika: Kenkyu no Saizensen (Microorganisms Resources: Its Characterization and Utilization) , Edited by: Suzui , T. , Okada , M. Kunimi , Y. 55 – 70 . Tokyo : Soft Science Incorporation . (in Japanese)
- Saito , M . 2001 . MAFF Microorganism Genetic Resources Manual No9Maintenance of Living Culture Collection of Arbuscular Mycorrhizal Fungi , Tsukuba : National Institute of Agrobiological Resources, Ministry of Agriculture, Forestry and Fisheries . (in Japanese)
- Smith , SE and Read , DJ . 1997 . “ Mineral nutrition, heavy metal accumulation and water relations of VA mycorrhizal plants ” . In Mycorrhizal Symbiosis , 2nd edn , Edited by: Smith , S.E. and Read , D.J. 126 – 160 . San Diego : Academic Press .
- Sylvia , DM and Neal , LH . 1990 . Nitrogen affects the phosphorus response of VA mycorrhiza . New Phytol , 115 : 303 – 310 .
- Wang , H , Parent , S , Gosselin , A and Desjardins , Y . 1993 . Vesicular-arbuscular mycorrhizal peat-based substrates enhance symbiosis establishment and growth of three micropropagated species . JAmerSocHortSci , 118 : 896 – 901 .
- Wang , ZHQ , Li , SHG , Kawakami , H and Nijima , Y . 2001 . “ The distribution, features and evaluation of the resources of peat composed of rotten mosses in China ” . In Practice and Mechanism of Making the Deserts Green With Peat Composed of Rotten Mosses , Edited by: Wang , Z.H.Q. , Li , S.H.G. , Kawakami , H. and Nijima , Y. 27 – 28 . Beijing : Science Press . (in Chinese)
- Yokoyama , K , Tateishi , T , Saito , M and Marumoto , T . 2005 . Application of a molecular method for the identification of a Gigaspora margaritaisolate released in a field . Soil SciPlant Nutr , 51 : 125 – 128 .