Abstract
Calcium-ligand blots using 45Ca2+ revealed that there were at least four calcium-binding proteins (17, 18.5, 48 and 52 kDa) in the phloem sap of rice plants. Furthermore, an anti-spinach calmodulin antibody cross-reacted strongly with an 18.5 kDa protein from the phloem sap. When electrophoresed on native gels, the anti-calmodulin cross-reacting protein migrated more slowly in the presence of calcium than in its absence. Based on these results, we concluded that the cross-reacting protein was a calmodulin. An 125I-CaM overlay assay revealed that two calmodulin-binding proteins (20 kDa and 40 kDa) were present in the rice phloem sap. Based on these data, it is possible that a calmodulin signal cascade is present in the sieve tubes of rice plants.
INTRODUCTION
Sieve tubes play important roles in the long-distance transport of photoassimilates and micronutrients in plants. However, it is considered that plants utilize the network of these tubes for the transport of nutrients as well as substances involved in signaling and plant defense. These physiological functions are mediated by proteins in the sieve tubes (CitationRaven 1991). To date, several kinds of enzymatic activities have been detected in phloem sap and have, in general, been classified into the following eight categories: sugar metabolism, loading of phloem solutes, signal transduction, redox regulation, protein metabolism, macromolecular trafficking, defense and cell-structure-related proteins (CitationHayashi et al. 2000). Among these phloem proteins, those involved in signal transduction have attracted the interest of many researchers. For example, several protein kinases in rice (CitationNakamura et al. 1995) and pumpkin (CitationYoo et al. 2002) have been found in phloem sap.
For certain physiological signals that are transduced via sieve tubes, calcium ions are considered to play an important role. Indeed, protein kinase activities in phloem sap were calcium-dependent (rice CitationNakamura et al. 1995; pumpkin CitationYoo et al. 2002). These findings suggested that calcium ions are used not only as a nutrient but also as a second messenger in the sieve tubes. Total calcium ion concentrations, which have been measured in phloem sap from rice (CitationFukumorita et al. 1983), castor bean (CitationHall and Baker 1972) and tobacco (CitationHocking 1980), range from 4.0 to 4.6 mmol L−1. However, despite the high total calcium concentration, free calcium in sieve tubes occurs at micromolar levels (CitationBrauer et al. 1998). The environmental stimuli that cause rapid changes in the free calcium concentration in plants include red light, blue light, UV irradiation, low and high temperature, touch, hyperosmotic stress and oxidative stress (CitationSanders et al. 2002). Cytosolic calcium concentration must be tightly regulated to allow Ca2+-mediated transduction of physiological signals (CitationZielinski 1998), and plants utilize various calcium modulators to control intercellular calcium concentration. We assumed that calcium modulators would also be used to control the free calcium concentration in sieve tubes. However, little is known about the mechanisms or downstream effects of Ca2+ signaling in the phloem.
Many types of calcium-binding proteins have been detected in the plant kingdom and they control a large number of calcium-dependent processes in plants (CitationHepler and Wayne 1985). Calmodulin (CaM) is the most widely distributed calcium-modulator protein (CitationZielinski 1998). Although CaM itself does not display any enzymatic activity, it binds to Ca2+ and alters the activity of calmodulin-binding proteins (NAD kinase CitationAnderson and Cormier 1978; glutamate decarboxylase CitationLing et al. 1994; Ca2+-ATPases CitationAskerlund 1997; heat shock transcription factor CitationLi et al. 2004) in response to Ca2+ signals. Because of its conservation, CaM is assumed to be an essential Ca2+-dependent modulator of physiological processes in plant systems. Therefore, it might be expected that CaM is involved in the transduction of a variety of abiotic and biotic signals to sink organs and in the maintenance of calcium homeostasis in the sieve tubes. If calcium is to function in the sieve tubes, these cells must have a calcium-modulator and a calcium-buffering system.
In the present study, we used pure phloem sap collected from rice plants to demonstrate that CaM and calmodulin-related signal transduction systems are present in the sieve tubes of rice plants. The implications of the presence of CaM and a calmodulin-related signal pathway in these sieve tubes were examined.
MATERIALS AND METHODS
Plant material and collection of phloem sap
Rice plants (Oryza sativa L., cv. Kantou) were grown hydroponically using a modified Kimura B nutrient medium (CitationHayashi and Chino 1986) in a greenhouse under natural light. Hydroponic solutions were renewed every 3 days. Plants at the 7th or 8th leaf stage were used for the collection of phloem sap. Phloem sap was collected from rice plants using a laser insect technique as previously described (CitationKawabe et al. 1980). Collected phloem sap was stored at −20°C until subsequent analysis.
Protein extraction and determination
Proteins of rice plants were extracted according to previously described methods (CitationNakamura et al. 1993). Leaves, seeds and root tissues from rice plants were dry-powdered with liquid nitrogen and homogenized in a mortar with a pestle in an ice-cold Tris buffer containing 25 mmol L−1 Tris-HCl (pH 6.8), 5% (w/v) β-mercaptoethanol, 5 mmol L−1 (p-amidinophenyl)methanesulfonyl fluoride hydrochloride and 25 µg mL−1 leupeptin. The extract was filtered through two layers of gauze and centrifuged at 16,000 g for 30 min at 4°C. The supernatant fraction was collected and stored at −20°C until analysis.
Total protein content in the phloem sap and plant extracts was determined according to the method of CitationBradford (1976). Protein Assay Reagent (Bio-Rad Laboratories, Hercules, CA, USA) was used in these determinations, with bovine γ-globulin as a standard.
Calcium-ligand blotting
Calcium-binding proteins were detected as previously described (CitationMaruyama et al. 1984). Proteins in the phloem sap of rice plants were electrophoresed in a 15% polyacrylamide gel containing 1 mmol L−1 ethylene glycol-bis(2-aminoethylether)-N,N,N′,N′-tetraacetic acid (EGTA) and transferred to a polyvinylidene difluoride (PVDF) membrane using Tris-glycine buffers (CitationTowbin et al. 1979). The membrane was washed in a washing buffer containing 10.0 mmol L−1 imidazole-HCl (pH 6.8), 60.0 mmol L−1 KCl and 5.0 mmol L−1 MgCl2 for 1 h with three changes. After washing, 45Ca2+ was added to the buffer (the final 45Ca2+ concentration was 2.5 µmol L−1) to detect calcium-binding proteins on the membrane. Subsequently, the membrane was washed in distilled water for 5 min and air-dried. Calcium-binding proteins on the dried membrane were detected using a bio-imaging analyzer system (BAS 2000; Fuji Film Co. Ltd, Tokyo, Japan).
Electrophoretic analysis of proteins that cross-react with anti-calmodulin serum
Proteins were separated using sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE) or two dimensional (2D)-PAGE. For 2D-PAGE analysis, the proteins were separated according to the method of CitationO’Farrell (1975). Gels consisting of 15% polyacrylamide gels were used for SDS-PAGE and 2D-PAGE. For the calcium mobility shift assay, proteins in the phloem sap were separated using native-PAGE, according to the method of CitationDavis (1964). After electrophoresis, proteins on the gels were transferred to a PVDF membrane using the method outlined above. The membrane was washed with phosphate-buffered saline (PBS) containing 73.0 mmol L−1 Na2HPO4, 37.0 mmol L−1 KH2PO4 and 40.0 mmol L−1 NaCl for 10 min. Subsequently, the membrane was blocked with a blocking solution (PBS with 0.05%[w/v] Tween 20 and 3.0%[w/v] gelatin) for 2 h. The blocked membrane was washed with PBS containing 0.05% (w/v) Tween 20 (TPBS) for 10 min with two changes and incubated overnight with antibody against spinach calmodulin (1:3,000 dilution) in a reaction buffer (TPBS with 0.5%[w/v] gelatin). After incubation, the membrane was washed with PBS for 40 min with three changes. The washed membrane was then incubated with a secondary antibody (peroxidase-conjugated) (Bio-Rad Laboratories) at 1:3,000 dilution. Immune complexes were detected using diaminobenzidine and hydrogen peroxide.
125I-CaM overlay assay
After separation of the rice phloem sap proteins by SDS-PAGE the proteins were transferred to a PVDF membrane using the method outlined above. Subsequently, cross-reaction of 125I-calmodulin with calmodulin-binding proteins was carried out according to the method of CitationLing and Assmann (1992). Calmodulin-binding proteins in the sap were detected using the method outlined above for calcium-ligand blotting.
Chemicals
The 45Ca2+ (specific activity, > 370 MBq [10 mCi]/mg) and 125I-calmodulin (specific activity, 1.85–5.55 MBq [50–150 µCi]/µg proteins) were purchased from NEN (Boston, MA, USA). Calmodulin from bovine brain and NAD kinase from chicken liver were purchased from Sigma (St Louis, MO, USA). All remaining chemicals used were of analytical grade. The anti-calmodulin antibody used in the present study was supplied by Dr S. Muto, Nagoya University, Nagoya, Japan.
RESULTS
Detection of calcium-binding proteins in the phloem sap of rice plants
The sugar profile of phloem sap is used as a marker of its purity. If the phloem sap, which contains only sucrose, is contaminated with the contents of broken plant cells, glucose or fructose will also be detected. It is generally recognized that neither glucose nor fructose are detected in rice phloem sap collected using an insect laser technique (CitationFukumorita and Chino 1982), indicating that this method yields very pure samples.
For the calcium-ligand blotting experiments, proteins from the sieve tubes of rice plants were transferred onto PVDF membranes from SDS-PAGE gels for 30 min (, lane 1) and for 60 min (, lane 2). Two different transfer times were used because, by staining for proteins remaining in the gels after electrotransfer, we observed that proteins with a molecular weight of > 40 kDa were not transferred after a short time (30 min) and that proteins with a relatively low molecular weight (< 20 kDa) moved all the way through the PVDF membranes during a long transfer (60 min). Calcium-binding proteins were then detected on the 45Ca2+-treated membranes (, lanes 1 and 2). Proteins with a molecular weight of 17 kDa and 18.5 kDa were detected in the sample that had been transferred for 30 min (, lane 1). In the sample that had been transferred for 60 min, a 48 kDa protein and a 52 kDa protein showed calcium-binding activity (, lane 2). These results demonstrated that at least four calcium-binding proteins were present in the sieve tubes of rice plants.
Detection of protein that cross-reacted with anti-calmodulin antibody
The molecular weight of plant calmodulins is approximately 17 kDa and they are highly acidic proteins (CitationZielinski 1998). Our calcium-ligand blot indicated that two calcium-binding proteins, with a molecular weight of 17 kDa and 18.5 kDa, could be detected in the phloem sap of rice plants when low molecular weight proteins were transferred to the PVDF membrane (). To
Figure 1 Detection of calcium-binding proteins in the phloem sap of rice plants. After electrophoresis and electroblotting, calcium-binding proteins were detected using 45Ca2+ as a probe. Phloem sap (80 µl, 12 µg protein) was electrophoresed and electroblotted to a polyvinylidene difluoride (PVDF) membrane for either 30 min (lane 1) or 60 min (lane 2). Bovine brain calmodulin (50 ng) was electrophoresed and electroblotted to the PVDF membrane as a positive control calcium-binding protein (lane 3).
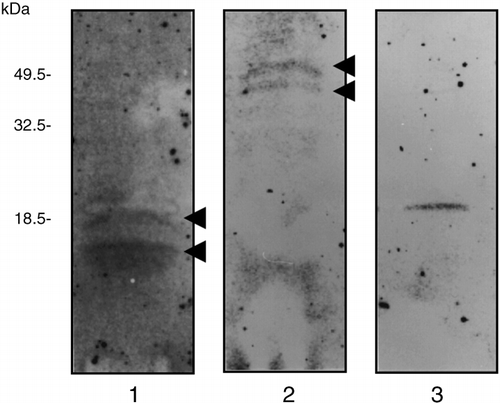
Figure 2 Immunoblot analysis of rice phloem sap proteins and bovine brain calmodulin. (A) After sodium dodecylsulfate–polyacrylamide gel electrophoresis (SDS-PAGE) separation of 0.6 µg phloem sap protein in the absence of calcium ions, proteins on the gel were detected by silver staining (silver staining lane). After electrotransfer of 0.6 µg phloem sap proteins, which were separated on the SDS-PAGE gel in the absence of calcium ions, to a polyvinylidene difluoride (PVDF) membrane, proteins recognized by the anti-calmodulin antibody were detected (immunological analysis lane). (B) After 2D-PAGE separation of the proteins in the phloem sap (4 µl, 0.6 µg protein) and bovine brain calmodulin (1 µg), proteins recognized by the anti-calmodulin antibody were detected on the PVDF membrane. IEF, isoelectric focusing.
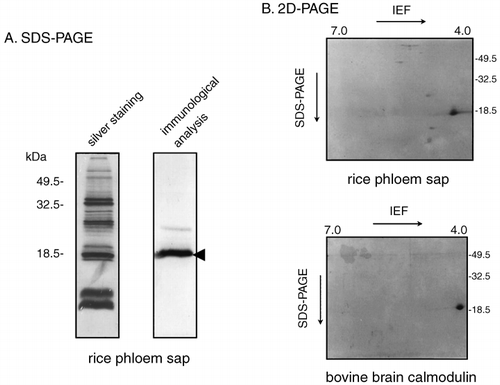
We repeated the immunoblot analysis on phloem sap proteins that had been separated using 2D-PAGE to determine the isoelectric point of this antibody-recognized protein. The anti-calmodulin antibodies detected a protein with a molecular weight of 18.5 kDa and an acidic isoelectric point of approximately 4.0 (). The mobility of this protein in the 2D-PAGE separation resembled that of bovine brain calmodulin (). Several more weakly cross-reacting spots were also detected on the PVDF membrane after 2D separation of the phloem sap proteins ().
Calcium mobility shift assay of protein recognized by anti-calmodulin antibody
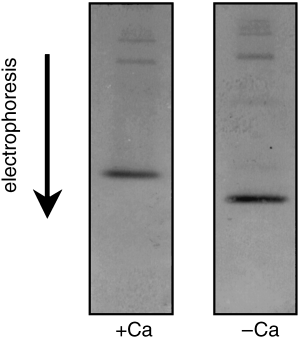
CitationVan Eldik et al. (1980) reported that the mobility of calmodulin in electrophoresis gels shifted in the presence of calcium ions. A calcium-dependent mobility shift assay was carried out on the phloem sap protein that was strongly recognized by the anti-calmodulin antibody. The mobility of this protein in native PAGE decreased in the presence of calcium ions ().
Comparison of anti-calmodulin cross-reacting proteins from various rice organs
Because calmodulin is present in most plant organs, we compared calmodulins in various rice organs in terms
Figure 3 Calcium mobility shift assay of rice phloem sap protein that reacts with anti-calmodulin antibody. Rice phloem sap (4 µl, 0.6 µg protein) was electrophoresed in the presence of calcium ions (1 mmol L−1 CaCl2 in the native-PAGE gel; left-hand panel) and immunoblotted with antiserum directed against spinach calmodulin (diluted to 1/3,000). The same experiment was repeated using a calcium-free native-PAGE gel (right-hand panel).
Detection of calmodulin-binding proteins in the sieve tubes of rice plants
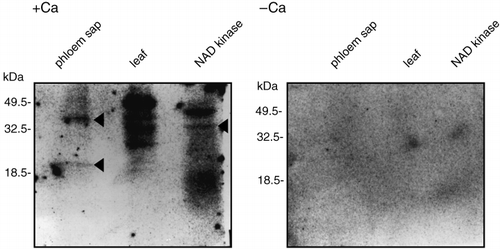
In calmodulin-mediated signaling cascades, signal transduction depends on the binding of calmodulin to a downstream signaling protein. Therefore, calmodulin-binding proteins were likely to be present in the phloem sap if a calmodulin-related signal cascade functioned in the sieve tubes of rice plants. To identify calmodulin-binding proteins in the phloem sap of rice plants, an 125I-CaM overlay assay was carried out. When the reaction buffer contained calcium ions, calmodulin bound to two proteins (20 kDa and 40 kDa) in the phloem sap of rice plants (arrowhead in ; phloem sap lane). However, these proteins did not bind to calmodulin when the reaction buffer did not contain calcium ions (). The results indicated that these phloem sap proteins could bind to calmodulin in a calcium-dependent manner. In the rice leaf extract used as a positive control, which was
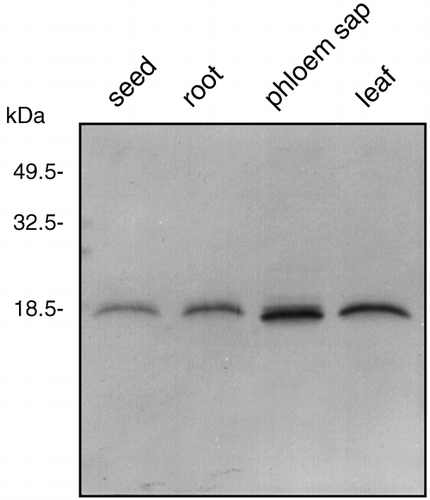
Figure 4 Immunoblot analysis of proteins from rice phloem sap, rice leaves, rice seeds and rice roots. Proteins from rice seeds (0.6 µg), rice roots (0.6 µg), rice phloem sap (0.6 µg) and rice leaves (0.6 µg) were subjected to sodium dodecylsulfate–polyacrylamide gel electrophoresis separation and immunoblotted with antiserum directed against spinach calmodulin, which was diluted to 1:3,000.
DISCUSSION
In the present study, we demonstrated that at least four calcium-binding proteins (17, 18.5, 48 and 50 kDa) were present in the phloem sap of rice plants (). In the plant kingdom, several types of calcium-binding proteins have been detected in various organs. Calcium-binding proteins with a low molecular weight were detected in the pollen of Brassica napus and Arabidopsis thaliana, and it was suggested that they were related to pollination (CitationRozwadowski et al. 1999). In certain floral tissues of A. thaliana, calreticulin was detected and might play a role in anther maturation or dehiscence (CitationNelson et al. 1997). In pea seedlings, an annexin-like protein was detected and immunolocalized in young developing vascular cells producing wall thickening and peripheral root-cap cells releasing slime (CitationClark et al. 1992). CitationMcEuen and Sabnis (1981)
Figure 5 125I-CaM overlay assay of proteins derived from rice phloem sap. Phloem sap (100 µl, 15 µg protein), proteins extracted from rice leaf (20 µg) and NAD kinase from chicken liver (12.5 µg) were electrophoresed and transferred to a polyvinylidene difluoride (PVDF) membrane. Reaction of proteins on the membrane with 125I-CaM was carried out in the presence (1 mmol L−1) or absence of calcium ions.
In a previous report, we demonstrated that approximately 58.0 kDa calcium-dependent protein kinase (CDPK) activity was present in the phloem sap from rice plants (CitationNakamura et al. 1995). Although previous studies have found that CDPK bound directly to calcium ions (CitationHarmon et al. 1987), in the present study we did not detect a calcium-binding protein with a molecular weight of 58 kDa. However, because we used 15% polyacrylamide gels for the SDS-PAGE analysis it was very difficult to clearly resolve proteins with a molecular weight over 50 kDa. Therefore, it is still possible that one of the calcium-binding proteins detected in this experiment was CDPK. Further studies should be carried out to resolve this issue.
We expected that either the 17 kDa or 18.5 kDa calcium-binding protein we detected could actually be calmodulin. Immunoblot analysis revealed that an 18.5 kDa protein cross-reacted strongly with an antibody against spinach calmodulin (). This analysis also revealed that proteins with a molecular weight of 23 kDa were recognized weakly by the anti-calmodulin antibody. Because we used a polyclonal antibody in the experiment, these proteins in the phloem sap might have a similar amino acid sequence to that recognized by the anti-calmodulin antibody. In the 2D-PAGE gels, the strongly cross-reacting protein showed the same molecular weight (18.5 kDa) and isoelectric point (acidic) as authentic calmodulin (). These analyses indicated that a protein with a molecular weight of 18.5 kDa in the rice phloem sap displayed the properties of calmodulin.
We used a mobility shift assay to further investigate the nature of the calmodulin-like protein in the rice phloem sap. CitationMarmé and Dieter (1983) previously reported that the mobility of calmodulin in native PAGE was decreased by calcium ions. A similar mobility shift was observed for the anti-calmodulin cross-reacting protein in the rice phloem sap (). In the upper parts of the immunoblots we could detect two additional weak bands, which migrated at 23 kDa. However, we could not detect a calcium-dependent mobility shift for these proteins. We concluded that the 18.5 kDa rice phloem sap protein actually is calmodulin based on its: (1) calcium-binding activity, (2) cross-reactivity with an anti-calmodulin antibody, (3) molecular weight and isoelectric point, (4) calcium-dependent mobility shift in native-PAGE separation.
Recently, calmodulin has been detected in the phloem exudate from Ricinus communis L. using quadrupole time of flight (Q-TOF) mass spectrometry (CitationBarnes et al. 2004). Because the phloem exudate from Ricinus was collected using an incision method, the researchers suggested that the calmodulin detected in the exudates was related to plant wounding and was expressed by the companion cells in response to mechanical stress (CitationBarnes et al. 2004). However, because our method for collecting phloem sap from rice is not stressful for the plants, our results demonstrate that calmodulin is present in unwounded sieve elements. We suggest that calmodulin in the sieve elements is involved in calcium sensing and signaling.
The molecular weight and amount of calmodulin in the phloem sap were compared with the amounts in roots, leaves and seeds of rice plants. When an equal amount of soluble protein (0.6 µg) from each sample was subjected to SDS-PAGE and immunoblot analysis, a calmodulin band with a similar molecular weight was detected in every sample (). Sieve tube proteins are synthesized in the companion cells and transported to sieve tubes via plasmodesmata (CitationIshiwatari et al. 1995). Microinjection experiments revealed that 3–20 kDa fluorescent dextrans can pass freely through plasmadesmata (CitationKempers and van Bel 1997). The molecular weight of calmodulin in the phloem sap is 18.5 kDa and was not modified when this protein was loaded into the sieve tubes. Therefore, calmodulin should be able to pass freely throughout the plant via plasmadesmata. Calmodulin concentration in the phloem sap from rice plants appeared to be slightly higher than the concentrations recorded in other tissues (). Sieve tubes might contain more calmodulin to maintain calcium homeostasis in the phloem sap because they lack calcium-storage organelles.
Calmodulin must bind to a calmodulin-binding protein in a calcium-dependent manner when it functions as a modulator in the cell (CitationCheung et al. 1978). At present, it is difficult to identify enzymatic activities that are controlled by calmodulin in the sieve tubes of rice plants. As an alternative approach, we attempted to detect calmodulin-binding proteins in phloem sap. We detected two calmodulin-binding proteins (20 kDa and 40 kDa) in the phloem sap of rice plants (arrowhead in ; phloem sap lane) and this binding was characterized by calcium dependency (). Therefore, the enzymatic activities of these proteins might be controlled by calmodulin. In plants, NAD kinase (CitationHarding et al. 1997) and glutamate decarboxylase (CitationLing et al. 1994) were detected as calmodulin-binding proteins and were related to signaling during pathogen response. Because many types of plant nutrients are transported via sieve tubes, these tubes are prone to attacks by insects and microorganisms. These calmodulin-binding proteins in the sieve tubes might act to protect these tubes from some attacks. However, large amounts of phloem sap would be needed to identify these enzymatic activities.
The experiments reported in this study present evidence that a calmodulin-related signal pathway is located in the sieve tubes of rice plants. In previous studies, the presence of CDPK in sieve tubes was also demonstrated (CitationNakamura et al. 1995). Together, these findings indicate that sieve tubes play an important role in transporting environmental signals that are mediated by calcium ions. Further characterization of calmodulins and calmodulin-binding proteins might enable us to elucidate the mechanisms of signaling in sieve tubes.
ACKNOWLEDGMENT
We thank Dr Shoshi Muto (Nagoya University) for providing us with the antibody against spinach calmodulin.
Notes
Present address: 694 Futago, Aki, Higashikunisaki, Oita 873-0356, Japan.
REFERENCES
- Anderson , JM and Cormier , MJ . 1978 . Calcium-dependent regulation of NAD kinase in higher plant . BiochemBiophysResCommun , 84 : 595 – 602 .
- Askerlund , P . 1997 . Calmodulin-stimulated Ca2+-ATPases in the vacuolar and plasma membranes in cauliflower . Plant Physiol , 114 : 999 – 1007 .
- Barnes , A , Bale , J , Constantinidou , C , Ashton , P , Jones , A and Pritchard , J . 2004 . Determining protein identity from sieve element sap in Ricinus communisL. by quadrupole time of flight (Q-TOF) mass spectrometry . JExpBot , 55 : 1473 – 1481 .
- Bradford , MM . 1976 . A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding . AnalBiochem , 72 : 248 – 254 .
- Brauer , M , Zhong , W-J , Jelitto , T , Schobert , C , Sanders , D and Komor , E . 1998 . Free calcium ion concentration in the sieve-tube sap of Ricinus communisL. seedlings . Planta , 206 : 103 – 107 .
- Cheung , WT , Lynch , TJ and Wallace , RW . 1978 . An endogeneous Ca2+-dependent activator protein of brain adenylate cyclase and cyclic nucleotide phosphodiesterase . AdvCyclic Nucleotide Res , 9 : 233 – 251 .
- Clark , GB , Dauwalder , M and Roux , SJ . 1992 . Purification and immunolocalization of annexin-like protein in pea seedlings . Planta , 187 : 1 – 9 .
- Davis , BJ . 1964 . Disc electrophoresis II: Method and application to human serum proteins . AnnNYAcadSci , 121 : 404 – 427 .
- Fukumorita , T and Chino , M . 1982 . Sugar, amino acid and inorganic contents in rice phloem sap . Plant Cell Physiol , 23 : 273 – 283 .
- Fukumorita , T , Noziri , Y , Haraguchi , H and Chino , M . 1983 . Inorganic content in rice phloem sap . Soil SciPlant Nutr , 29 : 185 – 192 .
- Hall , SM and Baker , DA . 1972 . The chemical composition of Ricinusphloem exudate . Planta , 106 : 131 – 140 .
- Harding , SA , Oh , SH and Roberts , DM . 1997 . Transgenic tobacco expressing a foreign calmodulin gene shows an enhanced production of active oxygen species . EMBO J , 16 : 1137 – 1144 .
- Harmon , AC , Putnam-Evans , C and Cormier , M . 1987 . A calcium-dependent but calmodulin-independent protein kinase from soy bean . Plant Physiol , 83 : 830 – 837 .
- Hayashi , H and Chino , M . 1986 . Collection of pure phloem sap from wheat and its chemical composition . Plant Cell Physiol , 27 : 1387 – 1393 .
- Hayashi , H , Fukuda , A , Suzui , N and Fujimaki , S . 2000 . Proteins in the sieve element–companion cell complexes: their detection, localization and possible functions . AustJPlant Physiol , 27 : 489 – 496 .
- Hepler , PK and Wayne , RO . 1985 . Calcium and plant development . AnnuRevPlant Physiol , 36 : 397 – 439 .
- Hocking , PJ . 1980 . The composition of phloem exudate and xylem sap from tree tobacco (Nicotiana glaucaGroh) . AnnBot , 45 : 633 – 643 .
- Ishiwatari , Y , Honda , C Kawashima , I . 1995 . Thioredoxin h is one of the major proteins in rice phloem sap . Planta , 205 : 12 – 22 .
- Kawabe , S , Fukumorita , T and Chino , M . 1980 . Collection of rice phloem sap from stylets of homopterous insects severed by YAG laser . Plant Cell Physiol , 21 : 1319 – 1327 .
- Kempers , R and van Bel , AJE . 1997 . Symplasmic connections between sieve element and companion cell in the stem phloem of Vicia fabaL. have a molecular exclusion limit of at least 10 kD . Planta , 201 : 195 – 201 .
- Li , B , Liu , H-T , Sun , D-Y and Zhou , RG . 2004 . Ca2+and calmodulin modulate DNA-binding activity of maize heat shock transcription factor in vitro . Plant Cell Physiol , 45 : 627 – 634 .
- Ling , V and Assmann , SM . 1992 . Cellular distribution of calmodulin and calmodulin-binding proteins in Vicia fabaL . Plant Physiol , 100 : 970 – 978 .
- Ling , V , Snedden , WA , Shelp , BJ and Assmann , SM . 1994 . Analysis of a soluble calmodulin binding protein from Favabean roots: identification of glutamate decarboxylase as a calmodulin-activated enzyme . Plant Cell , 6 : 1135 – 1143 .
- Marmé , D and Dieter , P . 1983 . “ Role of Ca2+and calmodulin in plants ” . In Calcium and Cell Function , Edited by: Cheng , WY . Vol. IV , 263 – 311 . London : Academic Press .
- Maruyama , K , Mikawa , T and Ebashi , S . 1984 . Detection of calcium binding proteins by 45Ca autoradiography on nitrocellulose after sodium dodecyl sulfate gel . JBiochem , 95 : 511 – 519 .
- McEuen , AR and Sabnis , DD . 1981 . Calcium-binding protein in sieve tube exudate . Planta , 151 : 531 – 534 .
- Nakamura , S , Hayashi , H , Mori , S and Chino , M . 1993 . Protein phosphorylation in the sieve tubes of rice plants . Plant Cell Physiol , 34 : 927 – 933 .
- Nakamura , S , Hayashi , H , Mori , S and Chino , M . 1995 . Detection and characterization of protein kinases in rice phloem sap . Plant Cell Physiol , 36 : 19 – 27 .
- Nelson , DE , Glaunsinger , B and Bohnert , HJ . 1997 . Abundant accumulation of the calcium-binding molecular chaperone calreticulin in specific floral tissues of Arabidopsis thaliana . Plant Physiol , 114 : 29 – 37 .
- O’Farrell , PH . 1975 . High resolution two-dimensional electrophoresis of proteins . JBiolChem , 250 : 4007 – 4021 .
- Raven , JA . 1991 . Long-term functioning of enucleate sieve elements: possible mechanisms of damage avoidance and damage repair . Plant Cell Environ , 14 : 139 – 146 .
- Rozwadowski , K , Zhao , R Jackman , L . 1999 . Characterization and immunolocalization of a cytosolic calcium-binding protein from Brassica napusand Arabidopsispollen . Plant Physiol , 120 : 787 – 797 .
- Sanders , D , Pelloux , J , Brownlee , C and Harper , JF . 2002 . Calcium at the Crossroad of Signaling . Plant Cell , 14 : S401 – S417 .
- Towbin , H , Staehelin , T and Gordon , J . 1979 . Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications . ProcNatl. AcadSciUSA , 76 : 4350 – 4354 .
- Van Eldik , LJ , Grossman , AR , Iverson , DB and Watterson , DM . 1980 . Isolation and characterization of calmodulin from spinach leaves and in vitro translation mixtures . ProcNatl AcadSciUSA , 77 : 1912 – 1916 .
- Yoo , B-C , Lee , J-Y and Lucas , WJ . 2002 . Analysis of the complexity of protein kinases within the phloem tube system . JBiolChem , 277 : 15 325 – 15 . 332
- Zielinski , RE . 1998 . Calmodulin and calmodulin-binding proteins in plants . AnnuRevPlant PhysiolPlant MolBiol , 49 : 697 – 725 .
- Present address: 694 Futago, Aki, Higashikunisaki, Oita 873-0356, Japan.