Abstract
The effects of peat extract solutions on spore germination and hyphal growth of the arbuscular mycorrhizal fungus Gigaspora margarita Becker & Hall were investigated. Spores were incubated in extract solutions of peat from China and peat moss from Canada, which were prepared at a weight ratio of 1:10, 1:20, 1:30, 1:50 and 1:100 for each substrate according to the weight ratio of water. Compared with peat and peat moss solutions, solutions of KH2PO4 and (NH4)2SO4 at various concentrations were used as media to investigate the effect of phosphorus (P) and nitrogen (N). Spores in the peat solutions showed the highest values for germination percentage, hyphal length and number of auxiliary cells, and these values were significantly higher than the values recorded for the other treatments. In contrast, in the peat moss extract solutions, which had a lower content of N and higher content of P than the peat solutions, the germination and hyphal growth of spores decreased. Spore growth was promoted with increased concentrations of the N solutions. However, P solutions exerted an inhibitory effect on spore germination and hyphal growth. Peat solutions showed a high N content and low P content, which might be related to the promotion of spore growth.
INTRODUCTION
Arbuscular mycorrhizal (AM) formation is influenced by a large number of environmental factors. Organic matter enhanced soil fertility and also stimulated the development of arbuscular mycorrhizal fungi (AMF) in soil (CitationHepper and Jakoben 1983; CitationHepper and Warner 1983; CitationJoner and Jakobsen 1995). Peat is often the major component of potting mixes, and its specific characteristics may significantly affect AM fungi (CitationLinderman and Davis 2003). Peat addition has been reported to both stimulate (CitationWang et al. 1993) and suppress (CitationBiermann et al. 1983; CitationGraham et al. 1984) root colonization. In further studies, it was suggested that the spread and efficiency of AMF as well as host plant growth could be affected by the nature of the peat used (CitationLinderman and Davis 2003;Citation Ponton et al. 1990). However, in these studies, Canadian peat was the substrate most frequently used. The effect of peat from China on AM formation is poorly documented. Peat properties vary considerably depending on location. Different types of peat vary in their degree of decomposition. Plant species, climatic conditions and hydrological factors affect the distinct characteristics of peat (Wang et al. 2001). Chinese peat was mostly composed of herbage and arbor and moss accounted for only 1%, whereas Canadian peat was mostly composed of moss (CitationWang et al. 2001). Therefore, there were large differences in the physical and chemical properties between Chinese and Canadian peat (CitationWang et al. 2001). In general, the pH of Chinese peat ranged from 5 to 7, whereas the pH of Canadian peat was lower (3.5–4.0). The maximum water-holding capacity of Canadian peat was much higher than that of Chinese peat. The organic matter content of Chinese peat was in the range of 30–50%, whereas the content of Canadian peat was higher (91–99%). The total nitrogen (N) content of Chinese peat ranged from 1.4 to 2.4%, whereas the N content of Canadian peat ranged from 0.4 to 0.6% (CitationWang et al. 2001).
China has abundant peat resources, amounting to approximately 46 hundred million tons (CitationWang et al. 2001). Studies on the use of peat in China have been carried out for agriculture, horticulture and reforestation programs over a long period of time. Improvement of the physical and chemical properties of Chinese peat, such as aeration, water retention and nutrient status suggested that peat could be a factor that promotes the growth of AM fungi in soil, the infection of roots and the development of AM symbiosis (N. Ma et al., unpubl. data, 2005). Thus, we attempted to investigate the effect of peat on AM formation to apply low-cost, high-quality amendments in reforestation programs and maximize peat value.
The AM infection of roots can be initiated by germ tubes arising from spores, by hyphae growing from other propagules or by external hyphae connected to active AM. Infection resulting from the first two processes (germination of spores or other propagules) is referred to as primary infection (CitationAllen 1992). The impact of these fungi on their hosts will depend on rapid spore germination and their ability to colonize (CitationTommerup 1983b). Therefore, the possible influence of peat on mycorrhizal development may, in part, be explained by the direct effect of peat on the germination of spores of AM fungi.
A number of studies have reported that spore germination and hyphal growth from resting spores of a variety of AM fungi were differently affected by factors including temperature (CitationTommerup 1983a), pH (CitationGreen et al. 1976), organic substrates (CitationHepper and Jakobsen 1983) and mineral nutrient concentrations (CitationDaniels and Trappe 1980). Thus, physicochemical improvement by peat could be expected to influence spore germination and hyphal growth.
In the present study we analyzed the effect of peat amendments on AM formation through direct contribution to spore growth during the early stages of fungal development. We estimated the effect of Chinese peat on spore germination and hyphal growth and investigated the key factors compared with Canadian peat. To our knowledge, no direct study on the effect of peat from China on spore growth has been conducted.
MATERIALS AND METHODS
Multiplication and maintenance
Spores of the AM fungus Gigaspora margarita MAFF520054 were obtained from the gene bank of the National Institute of Agrobiological Sciences, Tsukuba, Japan. Spores were multiplied in pot cultures on white clover for 3 months (CitationSaito 2001). Newly developed spores were extracted by wet sieving and decanting (CitationGerdemann and Nicolson 1963). Spores were picked up individually with pipettes, washed in sterile deionized water and stored at 4°C until required.
Types of media
Two types of peat, Chinese peat and Canadian peat, which were commercially available in Japan were used in the present study. One was a peat sample purchased from Nibannsu Company, Japan (Origin: China). The second was a peat moss sample purchased in Japan (Origin: Canada). Chinese peat was referred to as peat and Canadian peat was referred to as peat moss in the present experiment. Peat and peat moss were autoclaved twice at 80°C for 40 min. The substrates were mixed with deionized water according to the weight ratio and shaken for 1 h at room temperature. The filtered solution was sterilized by passage through a 0.2 µm Millipore membrane (Advantec, Tokyo, Japan). Aliquots (2 mL) of the solutions were dispensed into each well of a 24-multi-well plate, with each well containing one spore of G. margarita.
The extract solutions of peat were compared with those of peat moss. The ten extract solutions were prepared at a weight ratio of 1:10, 1:20, 1:30, 1:50 and 1:100 for peat and peat moss. The extract solutions were referred to as peat solutions and peat moss solutions. Sterile deionized water was prepared as a control. To investigate the key factor of the effect, solutions of (NH4)2SO4 at various concentrations with the same concentration of N as that in the peat solutions (from 1:10 to 1:100) and one additional solution with a concentration of 0.5-fold that of the 1:100 solution were used. The (NH4)2SO4 solutions were referred to as N solutions 1:10, 1:20, 1:30, 1:50, 1:100 and 1:200. Solutions of KH2PO4 at various concentrations with the same concentration of P as that in the peat moss solutions (1:10, 1:20 and 1:50) and one additional solution with a concentration twofold that of the 1:10 solution were used. The KH2PO4 solutions were referred to as P solutions 1:5, 1:10, 1:20 and 1:50.
The nutrient content of the extract solutions was analyzed at the start of the study. The pH was measured using the pH Electrode Meter Method, total N content was determined using the Kjeldahl method, total organic C content was determined using the Tyurin method and the concentration of available P was determined using the Truog method (CitationDojyo hyojun bunseki sokutei hou iinkai 1986). The indophenol blue method was used to determine the concentration of NH+ 4-N (CitationPage et al. 1982). The nutrient levels of the extract solutions are listed in .
Assessment of spore germination
Spores were surface sterilized for 15 min in a 2% chloramine T solution containing streptomycin (1.2 mg mL−1) and rinsed 5 times in sterile deionized water. Sterilized spores were transferred to the media using fine forceps. Each treatment consisted of 10 spores per multi-well plate, with 1 spore per well. Incubation was carried out at 25°C in darkness.
Germination was assessed by scoring the emergence of a germ tube at 7, 10 and 16 days after incubation.
Table 1 Extractable nutrient levels for the soil solutions
The growth status of individual spores was evaluated according to the stage of spore growth. shows the development stages of spore growth. There were four spore growth stages: (1) initial stage (A type): new spore without hypha, (2) second stage (B type): spore with one hypha, (3) developing stage (C type): extension of hypha, (4) mature stage (D type): auxiliary cell attached to the hypha. The index of growth refers to the weight assessment (Index = NA × 0 + NB × 1 + NC × 2 + ND × 3). NA, NB, NC and ND refer to the number of spores at one of the four stages, respectively. The index showed differences in the spore growth conditions in different solutions.
Statistical analysis
Experiments were conducted with two replicated multi-well plates per treatment. The data were subjected to an anova. When a significant (P < 0.05) treatment effect was found the mean values were compared using a Tukey test (P < 0.05).
RESULTS
Spore germination
Spores of G. margarita started to germinate within 4–6 days after incubation and the germ tube emerged through the subtending hypha. Spores continued to germinate during the 16-day period of incubation.
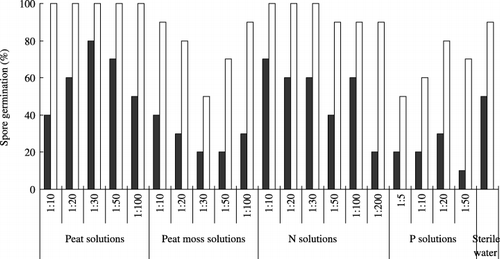
Germination of the spores increased over time in all solutions (). At 7 days, the germination percentage of the spores in all peat solutions was higher than 40%. However, the germination percentage of spores in all peat moss solutions was lower than 40%, which was below the level of spore germination in the control. Spores in the N solutions germinated at a rate of approximately 50%, whereas in the P solutions the spore germination percentage was less than 30%. This result indicated that spore growth was fastest in the peat solutions.
Although germination differed considerably between treatments in the early period, after 10 days almost all spores had germinated. At 10 days, spores treated with peat moss solutions had also germinated at a lower percentage than spores in the control. At the same time, all the spores in the peat solutions germinated.
Spore growth stages
There were significant differences in the indexes of spore growth stages among the treatments during the
Figure 1 Spore growth stages of Gigaspora margarita. Initial stage (A type): new spore without hypha; Second stage (B type): spore with one hypha; Developing stage (C type): extension of hypha; Mature stage (D type): auxiliary cell attached to the hypha.
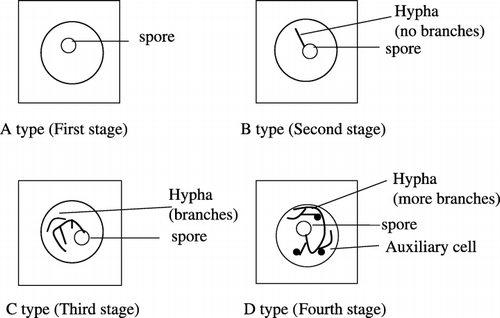
Figure 2 Percentage of spore germination of Gigaspora margarita after 7 (▪) and 10 days (□) of incubation in peat solutions, peat moss solutions, nitrogen (N) solutions, phosphorus (P) solutions and sterile deionized water.
Similarly, at 10 days the indexes of spore growth stages in the peat solutions and N solutions were higher than the control (). Although the spores grew better than those at 7 days, the indexes in the peat moss and P solutions were still lower than the control.
Hyphal extension
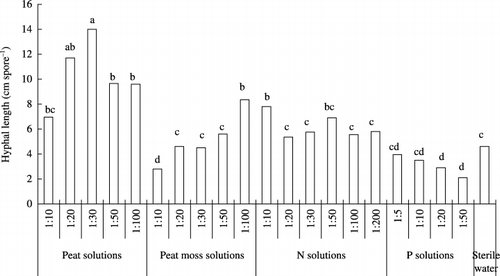
There were significant differences in hyphal length between the treatments at 10 days () and 16 days (). The time course of hyphal length demonstrated that hyphal extension in the peat solutions was the fastest of all the solutions.
Figure 3 Index of spore growth stage after 7 (▪) and 10 days (□) of incubation in peat solutions, peat moss solutions, nitrogen (N) solutions, phosphorus (P) solutions and sterile deionized water. Index was calculated according to the four growth stages (Index = NA × 0 + NB × 1 + NC × 2 + ND × 3). NA, NB, NC and ND denote the number of spores at the A, B, C and D spore growth stages, respectively.
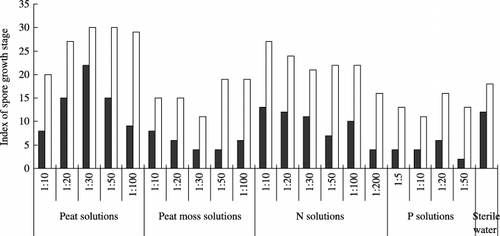
Figure 4 Hyphal length of Gigaspora margarita in peat solutions, peat moss solutions, nitrogen (N) solutions, phosphorus (P) solutions and sterile deionized water at 10 days. Means followed by the same letter are not significantly different (P < 0.05) according to a Tukey test.
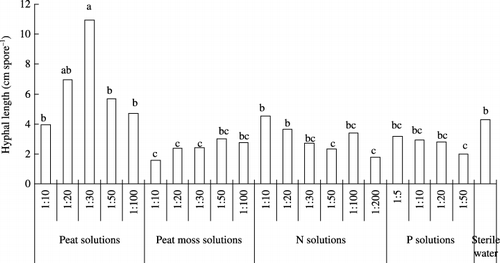
At 10 days, there were significant differences in hyphal length between the peat and peat moss solutions (). With decreasing concentration in the peat solutions, hyphal length increased and then decreased, with the highest value recorded at a concentration of 1:30. Hyphal length in the peat solutions was extremely high compared with the other treatments. In contrast, hyphal length in the peat moss solutions was lower than the control. The lower the concentration of the peat moss solutions, the greater the hyphal length. Hyphal length in the P solutions was lower than lengths recorded in the control. Hyphal length decreased as the concentration of the N solution decreased.
At 16 days, hyphal length was very different in the peat and peat moss solutions (). Hyphal length in all peat solutions was very high, 3.1 to 1.5-fold higher
than the control, whereas hyphal length in the peat moss solutions was similar to or lower than the control, except for the 1:100 solution. Moreover, in the peat moss solutions, hyphal length slightly increased when the concentration decreased from 1:30 to 1:100. Hyphal length in the control was much higher than that recorded in the P solutions, but was slightly lower than that in the N solutions.Auxiliary cells
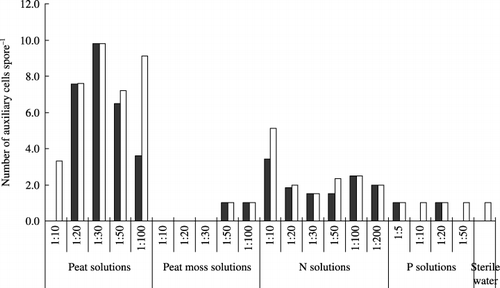
Auxiliary cells were observed in all peat solutions, N solutions, P solutions, peat moss solutions (1:50 and 1:100) and the control (). There was a positive significant correlation between hyphal length and the number of auxiliary cells across all treatments (r = 0.90 at 10 days and 0.88 at 16 days). Auxiliary cells were more frequently observed in the peat solutions. There were hardly any auxiliary cells in the peat moss solutions, except for the 1:50 and 1:100 solutions. The number of auxiliary cells in the N solutions was higher than the control, presumably because N significantly stimulated the growth of auxiliary cells. In contrast, the number of auxiliary cells in the P solutions was similar to that recorded in the control.
DISCUSSION
Spore germination of G. margarita was affected by the different extract solutions. Spores in the peat solutions showed the highest values for germination percentage, hyphal length and the number of auxiliary cells. These values were significantly higher than those recorded in the peat moss solutions and in the control. These results strongly suggested that the peat solutions exerted a promotive effect on spore germination and hyphal extension.
The effects of peat and peat moss on spore growth were totally different. The peat solutions stimulated spore germination and hyphal growth, whereas the peat moss solutions exerted a strong inhibitory effect at the early stage of spore growth. At the end of incubation, the spore germination percentage and hyphal length were lower than those recorded in the control. Thus, the results clearly suggested that peat moss exerted an inhibitory effect on spore growth. CitationLinderman and Davis (2003) demonstrated that because peats differed in their physical, chemical and biological properties, the outcome of mycorrhizal inoculation into media could not be predicted because of this variation. Our results also demonstrated that the effects of various peats on spore growth were different. Therefore, the present study suggested that the direct effect of various peats on the fungi might, in part, account for the difference in the impact on AM symbiosis development.
The spores that led to the most vigorous hyphal growth in peat solution 1:30 during the incubation period showed a higher sensitivity to the concentration of the peat solution. CitationDaniels and Graham (1976) suggested that some nutrients extracted from soil were necessary for
Figure 6 Number of auxiliary cells of Gigaspora margarita in peat solutions, peat moss solutions, nitrogen (N) solutions, phosphorus (P) solutions and sterile deionized water at 10 days (▪) and 16 days (□).
Spore germination may be affected by many factors, such as root exudates (CitationTawaraya et al. 1996), flavonoids (CitationBecard et al. 1992), nutrients extracted from soil (CitationDaniels and Graham 1976), amino acids (CitationHepper and Jakobsen 1983), vitamins (CitationDaniels and Graham 1976), plant sesquiterpenes (CitationAkiyama et al. 2005), soil P (CitationMiranda et al. 1994) and pH value (CitationGreen et al. 1976).
As a result of the complex composition of peat, it remains to be determined which specific ingredient of the organic matter in peat is responsible for the promotive effect. The main components of peat include inorganic substances such as mineral salts, metallic oxides and trace elements, and organic matter such as bitumen, carbohydrates, humic acids and lignin (Murayi 1998). The peat extract solutions probably contained mineral salts, hemicellulose, amino acids and vitamins that were related to spore growth. Because it was very difficult to identify the key organic matter factor in the peat extract solutions, in our experiment the role of the inorganic substances was investigated. We focused on the N and P contents, which were varied greatly between the peat and peat moss solutions.
First, N concentration in the media might be an important factor. Nitrogen addition has been reported to stimulate root colonization by the application of nitrate or ammonium (CitationBrown et al. 1981; CitationHepper 1983). In our experiment, the promotion of spore growth might also be related to the concentration of N in the peat solutions, which was twofold higher than that in the peat moss solutions (). With a decrease in the concentration in the N solutions, hyphal length decreased. A stimulation peak was observed at a concentration of 1:10 at 10 and 16 days (,) for hyphal length and the number of auxiliary cells (). It is possible that the maximum values were recorded in the 1:10 solution or in the solutions with a slightly higher concentration. The promotive effect on spore growth became gradually weaker after the N concentration exceeded the level of the 1:10 solution. This result suggested that N at a certain concentration exerted a stimulatory effect on spore growth.
A number of studies have shown that P may directly inhibit the growth of fungus in soil, which is one mechanism that might account for the inhibition or limitation of mycorrhizal development at high P levels (CitationNagahashi et al. 1996). It appears that high soil P levels may inhibit both spore germination and hyphal growth (CitationMiranda et al. 1994; CitationTawaraya et al. 1996). In our experiment, the content of P in the P solutions was the same as the concentration in the peat moss solutions. In the P solutions, spore growth was restricted with an increase in the dilution. Spore growth was inhibited at higher concentrations of P. In peat, the level of available P was lower than that recorded in peat moss (). Hence, it is possible that the low amount of P in peat exerted a negligible inhibitory impact on spore germination and hyphal growth, in contrast to peat moss, which showed a high content of soluble P.
The pH can also be considered to be a possible factor affecting spore germination and hyphal growth. For the peat and peat moss solutions, there was a positive correlation between hyphal length and the pH of the extract solutions (r = 0.75 at 10 days and 0.85 at 16 days).
Finally, in the peat solution, improved germination and growth may also be related to organic matter. Amino acids and vitamins, which can exert a beneficial effect on spore germination (CitationDaniels and Graham 1976), might be present in the peat extract solutions. Recently, it has been reported that plant sesquiterpenes induced extensive hyphal branching in the germinating spores of the AM fungus G. margarita (CitationAkiyama et al. 2005). It remains to be determined whether the peat and peat moss extract solutions contain hormones. If hormones were present in the peat solutions the direct effect on spore growth may not be confirmed because of the stronger stimulatory effect with dilution of the solutions.
In conclusion, our results indicated that Chinese peat stimulated spore germination and hyphal growth of the AM fungus G. margarita, in contrast to the effect of Canadian peat moss. This stimulatory effect might be associated with the complex effects of N, P, pH and organic matter. A high level of N and low level of P in the peat solutions exerted a beneficial effect on spore growth. This is consistent with the high N/P ratio that promoted root colonization (CitationHepper 1983). Therefore, it is suggested that levels of N and P should be one of the indicators used to select suitable peat. Further studies should be carried out to identify the key factors of the promotive effect. Various types of peat should be analyzed to identify the key factors in each peat type; however, single factors such as pH, total C content, inorganic N content and mineral salts should also be analyzed. The key factors of the effect on spore germination and hyphal growth may shed light on how to select suitable peat for reforestation programs.
As spore germination is the first step in mycorrhizal development, it is possible that the effect of peat on spore germination may be related to mycorrhizal development on host roots and the subsequent influence on host plant growth. Therefore, it is reasonable to assume that peat amendment may increase the beneficial effect of AM on plant growth. While studies on the effects of peat on AM establishment on host plants should be carried out, the beneficial effect of peat on spore germination suggests that peat could become a valuable fertilizer in reforestation programs.
ACKNOWLEDGMENTS
We thank Dr Takahiro Tateishi, Iwate University, for valuable comments on the study. We also thank Mr Kimio Nakamura and Takumi Mitsuno for their assistance with the work and for helpful suggestions on the study.
REFERENCES
- Akiyama , K , Matsuzaki , K and Hayashi , H . 2005 . Plant sesquiterpenes induce hyphal branching in arbuscular mycorrhizal fungi . Nature , 435 : 824 – 827 .
- Becard , G , Douds , DD and Pfeffer , PE . 1992 . Extensive in vitro hyphal growth of vesicular-arbuscular mycorrhizal fungi in the presence of CO2and flavonols . ApplEnvironMicrobiol , 58 : 821 – 825 .
- Biermann , B and Linderman , RG . 1983 . Effect of container plant growth medium and fertilizer phosphorus on establishment and host growth response to vesicular-arbuscular mycorrhizae . JAmerSocHortSci , 108 : 962 – 972 .
- Brown , RW , Schultz , RC and Kormanik , PP . 1981 . Response of vesicular-arbuscular endomycorrhizal sweetgum seedlings to three nitrogen fertilizers . For. Sci. , 27 : 413 – 420 .
- Daniels , BA and Graham , SO . 1976 . Effect of nutrient and soil extracts on germination of Glomus mosseaespores . Mycologia , 68 : 108 – 116 .
- Daniels , BA and Trappe , JM . 1980 . Factors affecting spore germination of the vesicular-arbuscular mycorrhizal fungus . Phytopathology , 58 : 900 – 908 .
- Dojyo hyojun bunseki sokutei hou iinkai . 1986 . Dojyo hyojun bunseki sokutei hou , Tokyo : Hakuyusya Incorporation . (in Japanese)
- Gerdemann , JW and Nicolson , TH . 1963 . Spores of mycorrhizal Endogone species extracted from soil by wet sieving and decanting . TransBrMycolSoc , 46 : 235 – 244 .
- Giovannetti , M and Mosse , B . 1980 . An evaluation of techniques for measuring vesicular-arbuscular mycorrhizal infection in roots . New Phytol , 84 : 489 – 500 .
- Graham , JH and Timmer , LW . 1984 . Vesicular-arbuscular mycorrhizal development and growth response of rough lemon in soil and soilless media: effect of phosphorus source . JAmerSocHortSci , 109 : 118 – 121 .
- Green , NE , Graham , SO and Schenck , NC . 1976 . The influence of pH on the germination of vesicular-arbuscular mycorrhizal spores . Mycologia , 69 : 929 – 934 .
- Hepper , CM . 1983 . The effect of nitrate and phosphate on the vesicular-arbuscular mycorrhizal infection of lettuce . New Phytol , 92 : 389 – 399 .
- Hepper , CM and Jakoben , I . 1983 . Hyphal growth from spore of the mycorrhizal fungus Glomus caledonius: effects of amino acids . Soil BiolBiochem , 15 : 55 – 58 .
- Hepper , CM and Warner , A . 1983 . Role of organic matter in growth of a vesicular-arbuscular mycorrhizal fungus in soil . TransBrMycolSoc , 81 : 155 – 156 .
- Joner , EJ and Jakobsen , I . 1995 . Growth and extracellular phosphatase activity of arbuscular mycorrhizal hyphae as influenced by soil organic matter . Soil BiolBiochem , 27 : 1153 – 1159 .
- Keeney , DR and Nelson , DW . 1982 . “ Nitrogen-Inorganic Forms ” . In Methods of Soil Analysis Part 2 – Chemical and Microbiological Properties , 2nd edn , Edited by: Page , A.L. , Miller , R.H. and Keeney , D.R. 672 – 676 . Wisconsin : American Society of Agronomy, Soil Science Society of America .
- Koske , RE and Gemma , JN . 1992 . “ Fungal reaction to plants prior to mycorrhizal formation ” . In Mycorrhizal Functioning: An Integrative Plant–fungal Process , Ed. , Edited by: Allen , M.F. 3 – 36 . New York : Chapman & Hall .
- Linderman , RG and Davis , EA . 2003 . Soil amendment with different peatmosses affects mycorrhizae of onion . HortTechnology , 13 : 285 – 289 .
- Miranda , JCC and Harris , PJ . 1994 . Effects of soil phosphorus on spore germination and hyphal growth of arbuscular mycorrhizal fungi . New Phytol , 128 : 103 – 108 .
- Nagahashi , G , Douds , DD and Abney , GD . 1996 . Phosphorus amendment inhibits hyphal branching of the VAM fungus Gigaspora margaritadirectly and indirectly through its effect on root exudation . Mycorrhiza , 6 : 403 – 408 .
- Ponton , F , Piche , Y , Parent , S and Caron , M . 1990 . The use of vesicular-arbuscular mycorrhizae in Boston fern production: I. Effects of peat-based mixes . Hortscience , 25 : 183 – 189 .
- Saito , M . 2001 . MAFF Microorganism Genetic Resources Manual No9Maintenance of Living Culture Collection of Arbuscular Mycorrhizal Fungi , Tsukuba : National Institute of Agrobiological Resources, Ministry of Agriculture, Forestry and Fisheries . (in Japanese)
- Tawaraya , K , Watanabe , S , Yoshida , E and Wagatsuma , T . 1996 . Effect of onion (Allium cepa) root exudates on the hyphal growth of Gigaspora margarita . Mycorrhiza , 6 : 57 – 59 .
- Tommerup , IC . 1983a . Temperature relations of spore germination and hyphal growth of vesicular-arbuscular mycorrhizal fungi in soil . TransBrMycolSoc , 81 : 381 – 387 .
- Tommerup , IC . 1983b . Spore dormancy in vesicular-arbuscular mycorrhizal fungi . TransBrMycolSoc , 81 : 37 – 45 .
- Wang , H , Parent , S , Gosselin , A and Desjardins , Y . 1993 . Vesicular-arbuscular mycorrhizal peat-based substrates enhance symbiosis establishment and growth of three micropropagated species . JAmerSocHortSci , 118 : 896 – 901 .
- Wang , ZHQ , Li , SHG , Hiroshi , K and Yasuo , N . 2001 . “ The distribution, features and evaluation of the resources of peat composed of rotten mosses in China ” . In Practice and Mechanism of Making the Deserts Green With Peat Composed of Rotten Mosses , Edited by: Wang , Z.H.Q. , Li , S.H.G. , Hiroshi , K. and Yasuo , N. 25 – 28 . Beijing : Science Press . (in Chinese)
- Yazaki , H . 1998 . Component and chemical properties of peat In Science of pea , Edited by: Murayi , S . 123 – 144 . Japan Peat Society Tokyo . (in Japanese)