Abstract
To investigate the effect of low phosphorus (P) conditions on phytosiderophore (PS) release and mineral nutrient status in graminaceous plants, an experiment was conducted in a phytotron with barley plants grown in iron-deficient (Fe0) nutrient solutions with four P levels (0.5, 5, 50, 500 [control]µmol L−1) at pH 6.5 for 21 days after treatment (DAT). The results showed that the growth and chlorophyll index of plants cultured under low P conditions (0.5, 5 and 50 µmol L−1) were higher than those of the control plants. The accumulation amount (mg or µg/plant) of mineral nutrients in shoots was higher for potassium, iron and copper in plants in the low P treatment than in the control plants. The accumulation of PS in roots and the amount of PS released from the roots at 14 DAT were lower in plants in the Fe0 and low P treatments. These results indicated that low P depressed PS release from roots and PS accumulation in roots in Fe0 barley. This might result from the higher Fe content in shoots and the alleviation of Fe chlorosis with low P treatment of plants. These results showed that low P treatment enhanced the growth, chlorophyll index and Fe mobilization to shoots in Fe0 barley. Low P conditions alleviated Fe-deficiency symptoms, suggesting that P is physiologically competing with Fe in plant tissues.
INTRODUCTION
Phosphorus (P) and iron (Fe) are essential mineral elements for both animals and plants. Except for strains of lactobacilli, bacteria found in milk, which are unique organisms capable of living without Fe, neither plants nor animals can grow without P and Fe (CitationBrady and Weil 2002). Phosphorus is the energy currency of the living cell in the form of adenosine triphosphate (ATP) and the seat of genetic inheritance DNA and RNA, which control protein synthesis in both plants and animals (CitationBrady and Weil 2002). Iron is involved in the reduction of O2, CO2 and N2 (CitationNeilands 1994). Plants need Fe for the development of their photosynthetic apparatus. Iron has already been identified as a constituent of blood, and the content of hemoglobin Fe in an adult human is approximately 80 mmol (CitationNeilands 1994).
Iron chlorosis occurs in plants growing in every region of the world (CitationNeilands 1994). The problem of P deficiency in practical agriculture mainly occurs because the P compounds commonly found in soils are mostly unavailable for plant uptake because they are highly insoluble (CitationBrady and Weil 2002). The fixation of P is characterized by the formation of insoluble complexes with Fe, aluminium (Al) and manganese (Mn) under acidic soil conditions and with calcium (Ca) under alkaline soil conditions (CitationMengel et al. 2001). Therefore, combined Fe and P deficiency may potentially occur under both acidic and alkaline conditions, particularly in calcareous soils where, because of high pH values, Fe as well as P is unavailable for plant uptake.
Plant tissues have developed several physiological and biochemical mechanisms to withstand Fe- or P-deficient conditions. For instance, CitationMarschner et al. (1986) reported that plants are divided into two groups with respect to their strategies for the uptake of sparingly soluble Fe under Fe-deficient conditions, namely strategy I plants (non-graminaceous monocots and dicots) and strategy II plants (graminaceous monocots). The latter group is characterized by the synthesis of the mugineic acid family of phytosiderophores (PS), the release of PS from roots and the absorption of the PS–Fe (III) complex by roots for Fe acquisition in the rhizosphere (CitationMarschner et al. 1986, CitationTakagi 1976). Under P-deficient conditions, some crops show a multiplication of roots and root hairs and the secretion and release of organic acids to take up sparingly soluble P in the growth medium (CitationMengel et al. 2001). In Stylosanthes hamata grown under P-deficient conditions, shoot growth decreased rapidly, whereas the roots continued to grow not only by retaining most of the P, but also by translocating P from shoots to roots (CitationSmith et al. 1990). Rhizosphere acidification has been reported to be a widespread response to P deficiency. Depending on the plant species, acidification was brought about by proton or organic acid release. For example, in P-deficient tomato plants, increased net H+ efflux occurred as a consequence of depressed nitrate uptake (CitationHeuwinkel et al. 1992). A number of plants such as rape (CitationHoffland et al. 1989) and leguminous species (CitationOhwaki and Hirata 1992) released organic acids, particularly citric acid, under P-deficient conditions (CitationMengel and Kirkby 2001). In general, the numerous reactions for the synthesis and release of PS and for the absorption of mineral elements by plants are ATP-dependent reactions. The deficiency of P in the growth medium of strategy II plants should potentially affect the activity of roots, including PS release and the absorption of PS or PS-Fe3+, and the mineral nutrition of plants grown under Fe-deficient conditions. The effect of Fe-deficiency on PS production of strategy II plants has been well documented. However, the effect of combined Fe and P deficiency in the growth medium, which is likely to occur under natural conditions, on PS and the growth of these plants remains to be investigated.
Therefore, the objective of the present experiment was to investigate the combined effect of Fe deficiency and low P concentration in the medium on the growth, PS activity and mineral nutrition of barley plants.
MATERIALS AND METHODS
Plant growth
Procedures for culture under Fe0 (without the addition of Fe source) and low P conditions were modified based on the method of CitationKawai et al. (1993). The seeds of barley (Hordeum vulgare L. cv. Minorimugi) were surface sterilized with 2% chlorinated lime for 30 min, then rinsed with tap water and kept soaked between two moistened towels covered with plastic wrapping paper at 25°C for 1 day. It is generally recognized that this cultivar releases mugineic acid and 2′-deoxymugineic acid, compounds belonging to PS (CitationKawai et al. 1993). The germinating seeds were transferred to a plastic net covering a bucket filled with a solution of 2 mmol L−1 CaCl2. The seed box was covered with wrapping aluminium foil and kept in a phytotron (day/night, 14/10 h; temperature, 17/10°C; light intensity, 280 µmol m−2 s−1). The wrapping foil was removed after 2 days and the seedlings were maintained in the solution for 7 days. Then, the growth medium was replaced with a 1/5-strength modified Hoagland–Arnon solution (CitationHoagland and Arnon 1950) containing 4.0 µmol L−1 Fe-Ethylene Diamine Tetraacetic Acid (EDTA). The seedlings were grown in this solution until their second leaf reached approximately 20% of the size of the first leaf. At this growth stage, the seedlings were transferred in bunches of three plants, and 16 bunches were placed in each of the plastic buckets (10 L) filled up with 1/2-strength modified Hoagland–Arnon solution containing 10 µmol L−1 Fe-EDTA, whereas the plants were suspended through holes of a plastic lid cover put over the bucket of the nutrient solution. The plants were allowed to grow in this +Fe medium for 48 h.
The roots were carefully washed with deionized water and transferred to the −Fe 1/2-strength modified Hoagland–Arnon solution (CitationTakagi 1993) with the following P levels, 0.5, 5, 50 and 500 µmol L−1 (control) supplied as NaH2PO4. In the present study, these media were designated as Fe0 media. The plants were allowed to grow for 21 days after transfer (DAT). The pH of the nutrient solution was monitored daily and adjusted to 6.5. The nutrient solution was renewed at 7-day intervals.
Phytosiderophores released from roots
Roots from one bunch in triplicate for each treatment were soaked in 500 mL beakers filled with deionized water just before the onset of light and were allowed to release PS for 4 h at 7, 14 and 21 DAT. Then, the plants were returned to the growth medium. Approximately 10 mg of thymol (Kanto Chemical Company, Tokyo, Japan) was added to each beaker with the root washing solutions to prevent microbial degradation of PS. The root washings were filtered and passed through a column (1.5 cm internal diameter × 13 cm) of Amberlite IR-120 cation exchange resin (Rohm and Hass Company, Philadelphia, USA), and the resin was washed with deionized water. The PS adsorbed to the resin was eluted with 125 mL of 1 mol L−1 NH4OH. The ammonium solution containing PS was condensed under vacuum and kept in a freezer until analysis. The amount of PS released from the roots was determined using the method of CitationTakagi (1976).
Phytosiderophores accumulated in roots
Three bunches of plants were sampled in each treatment just before the onset of light on day 14 (14 DAT) because the PS concentration in roots was highest at that time (CitationKawai et al. 1993). The roots were washed with deionized water and lyophilized and homogenized in 80% ethanol using a mortar and pestle. The resulting paste was diluted with 80% ethanol, filtered and concentrated using a vacuum evaporator. The condensed solution was diluted to 100 mL with deionized water and introduced to a column of Amberlite cation resin, similar to the method used for the release of PS. The amount of PS extracted from the roots was also determined using the method of CitationTakagi (1976).
Chlorophyll index of leaves
The chlorophyll index, which represents the chlorophyll content of the 4th leaves of three bunches of plants, was measured at 14 DAT using a SPAD-502 chlorophyll meter (Minolta Camera Company, Tokyo, Japan).
Chemical analysis of plant materials
Three bunches of plants were collected at 14 DAT for mineral element analysis, washed with deionized water and oven-dried at 55°C for 24 h continuously. The plants were separated into shoots and roots, weighed and digested in a mixture of nitric-perchloric acid for minerals other than N (CitationPiper and Piper 1950).
For the analysis of N, another group of triplicate samples was collected at 14 DAT, washed carefully with deionized water and lyophilized for 24 h continuously. These samples were also separated into roots and shoots, weighed and digested in a mixture of sulfuric acid–hydrogen peroxide (Jones et al. 1991).
Phosphorus content in the plants was determined using a method in which vanadomolybdophosphoric yellow color was measured based on the absorbance at a wavelength of 420 nm using a spectrophotometer (UV-Mini 1240, UV-Vis Spectrophotometer; Shimazu Corp., Kyoto, Japan) (CitationBarton 1948; CitationJackson 1958). Determination of the N content in the plants was carried out using the Kjeldahl method (CitationAlam et al. 1991). Measurement of the other mineral elements was carried out using an atomic absorption spectrophotometer (170-30 Atomic Absorption Spectrophotometer; Hitachi, Ltd, Tokyo, Japan).
Statistical analysis
The experimental design consisted of a completely randomized block with 3 replicates. Data were subjected to an anova (CitationSAS Institute 1988). The means were compared according to the Ryan–Einot–Gabriel–Welsch Multiple Range Test (P = 0.05) using the computer “Origin 5” at Iwate University.
RESULTS AND DISCUSSION
Visual symptoms
Iron-deficiency symptoms were observed visually in the control plants (500 µmol L−1 P), and started with interveinal chlorosis 4 DAT that developed into whitish young leaves at 14 DAT. Slight interveinal chlorosis of young leaves was also observed in plants in the 50 µmol L−1 P treatment. However, no Fe-deficiency symptoms were observed in plants in the lower P treatments (5 and 0.5 µmol L−1), whereas P-deficiency symptoms, characterized by dark green leaves and bronze-violet discoloration of the leaf edges, were observed from the beginning of the growth period. After 14 DAT, the control plants began to wither from damage associated with the severity of the Fe-deficiency symptoms, whereas plants in the low P (50 and 5 µmol L−1) treatments were still healthy at 21 DAT. Plants in the lowest P (0.5 µmol L−1) treatment developed severe P-deficiency symptoms. The symptoms of P-deficiency in the plants in the lower P (5 and 0.5 µmol L−1) treatments progressed to old leaf senescence, starting from the tip of the leaf blade and developing to complete senescence of the oldest leaf.
Iron-deficiency symptoms were considerably reduced in plants in the 50 µmol L−1 P treatment and alleviated in plants in the lower P (5 and 0.5 µmol L−1) treatments. This indicates that in barley plants grown in a Fe-deficient nutrient solution with low P, Fe-deficiency symptoms could be alleviated, attenuated or masked by treatments with low levels of P. In other words, it was considered that the expression of Fe-deficiency symptoms in the control plants might be induced by the high P concentration in the growth medium.
Dry matter production
Plants in the Fe0 and low P (50, 5 and 0.5 µmol L−1) treatments displayed a higher dry matter yield in shoots and roots than the control plants (). Among the three low P treatments, the highest dry weight value was obtained when the P concentration of the medium was 50 µmol L−1 P. In addition, growth of the plants subjected to 5 and 0.5 µmol L−1 P was more vigorous than that of plants in the 500 µmol L−1 P treatment, but was less vigorous than that of plants in the 50 µmol L−1 P treatment. It was considered that the inferior growth of plants in the 500 µmol L−1 P treatment resulted from the high P concentration of the medium, indicating that retardation of growth in the control plants depended on the P concentration in the growth medium. These results suggested that low P supply to barley plants grown under Fe0 conditions alleviated Fe-deficiency symptoms and enhanced growth without being related to the severity of the P-deficiency symptoms.
Chlorophyll index
Plants grown under Fe0 and low P conditions exhibited a higher chlorophyll concentration than the control plants, although no significant differences were detected among plants in the 3 low P treatments (50, 5 and
Figure 1 Dry matter weight of shoots and roots of barley plants grown in iron-deficient nutrient solutions with different levels of phosphorus (P) at 14 days after treatment. Different letters at the top of each bar indicate significant differences (P < 0.05) according to the Ryan–Einot–Gabriel–Welsch Mutiple Range Test.
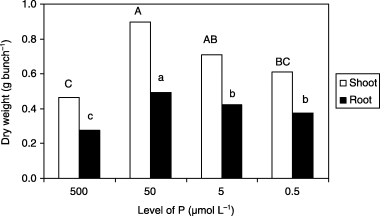
Figure 2 Chlorophyll index (SPAD value) of new leaves of barley plants grown in iron-deficient nutrient solutions with different levels of phosphorus (P) at 14 days after treatment. Different letters at the top of each bar indicate significant differences (P < 0.05) according to the Ryan–Einot–Gabriel–Welsch Mutiple Range Test.
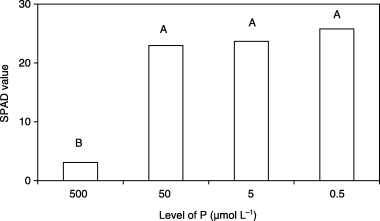
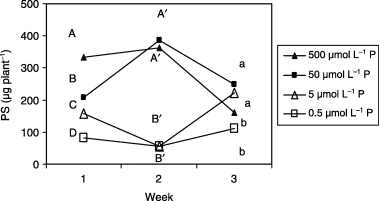
Figure 3 Phytosiderophore (PS) release from roots of barley plants grown in iron-deficient nutrient solutions with different levels of phosphorus (P). Different letters at the top of each drawn line indicate significant differences (P < 0.05) according to the Ryan–Einot–Gabriel–Welsch Mutiple Range Test.
Phytosiderophore release from roots and accumulation in roots
The amount of PS released and its variation pattern were affected by the low P treatments (). The order of the variations was as follows: 7 DAT: 500(control) > 50 > 5 > 0.5 µmol L−1 P. This release pattern indicated that PS release decreased with decreasing P concentration in the growth medium. (14 DAT: 500 = 50 > 5 = 0.5 µmol L−1 P). Tthe amount of PS released decreased significantly under lower P (5 and 0.5 µmol L−1) conditions. The amount of PS released in plants in the 50 µmol L−1 P treatment was similar to that of the control plants. (21 DAT: 50 = 5 > 500 = 0.5 µmol L−1 P). Even in plants in the lowest P (0.5 µmol L−1) treatment, the amount of PS released reached a similar level to that of the control plants.
In plants grown under less severe P-deficient conditions (50 µmol L−1), the amount of PS released at 14 DAT was similar to that of the control plants. These findings are not always consistent with the assumption that the amount of PS released varies depending on the severity of Fe-deficiency (CitationMori 1994; CitationGries et al. 1995) because the Fe-deficiency symptoms appearing as chlorosis () were mild or not severe in the plants in the 50 µmol L−1 P treatment. These results showed that greening of leaves was not a reliable indicator for predicting the amount of PS released. It has been suggested that the mechanisms of PS release are genetically controlled and may be absent or uncontrollable in special cases (CitationJolley and Brown 1994). The activity of the production of PS in roots may be responsible for the amount of PS released, which might be physiologically regulated by the P and Fe status in shoots and roots in Gramineae.
Figure 4 Phytosiderophore (PS) accumulation in roots of barley plants grown in iron-deficient nutrient solutions with different levels of phosphorus (P) at 14 days after treatment. Different letters at the top of each bar indicate significant differences (P < 0.05) according to the Ryan–Einot–Gabriel–Welsch Mutiple Range Test.
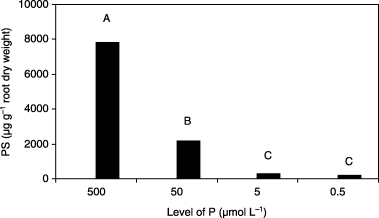
The pattern of PS accumulation in roots at 14 DAT was different from that of PS release from roots (). The amount of accumulated PS decreased with decreasing P level in Fe0 medium. These results indicated that a decrease in P concentration in Fe0 medium decreased PS accumulation in roots. It was apparent that higher P conditions induced higher PS accumulation in roots. However, it is possible that low P conditions damaged the plasma membrane of the roots and enabled the leakage of PS that had accumulated in the roots. This possibility should be examined in future studies.
Relative ratio of phytosiderophore release from roots and accumulation in roots
The relative ratio of PS release from roots and PS accumulation in roots at 14 DAT was higher in plants in the Fe0 and low P treatments compared with the control plants (). The reason why a higher P concentration in the Fe0 media reduced the relative ratio of PS release/PS accumulation has not been determined. High P concentration might affect the site of PS release on the root membrane and decrease PS release. Otherwise, a high P concentration might enhance the re-absorption of released PS.
Furthermore, approximately 50% of the PS accumulated in the roots of the control plants was released (). In plants in the Fe0 and low P treatments the relative ratio of PS exceeded 100%, indicating that the amount of PS released was greater than the accumulated PS under low P conditions. These results were repeatedly obtained. These new phenomena were observed only under low P conditions. Based on these results, the origin of released PS remains to be elucidated. It is possible that potential PS, which was not extracted with 80% ethanol, may occur in the roots of plants grown under Fe0 and low P conditions.
Figure 5 Relative ratio of phytosiderophore (PS) release from roots and PS accumulation in roots of barley plants grown in iron-deficient nutrient solutions with different levels of phosphorus (P) at 14 days after treatment. Different letters at the top of each bar indicate significant differences (P < 0.05) according to the Ryan–Einot–Gabriel–Welsch Mutiple Range Test.
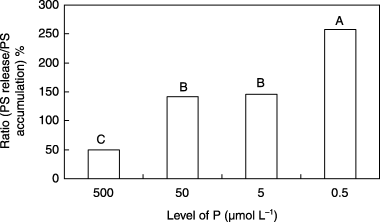
Further investigations on the ratio of PS release from roots and PS accumulation in roots should be conducted to determine the role of P in PS release and accumulation in the roots of graminaceous plants.
Mineral nutrition of plants
The accumulation (mg plant−1) and concentration (mg g−1 dry weight) of macronutrients in roots and shoots are shown in and . The accumulation of N in shoots and roots in plants in the 50 µmol L−1 P treatment was higher compared with the control plants (). This higher accumulation of N in plants in the 50 µmol L−1 P treatment may result from the higher Fe concentration in shoots and to the less severe P deficiency symptoms because Fe and P are necessary for N uptake and metabolism, which require ATP and ferredoxin. Obviously, the accumulation and concentration of P in shoots and roots decreased with decreasing P concentration in the medium.
The accumulation of K in shoots and roots was higher in plants in the low P (50 and 5 µmol L−1) treatments than in the control plants. However, K accumulation in the shoots and roots of plants in the lowest P (0.5 µmol L−1) treatment was lower than that of the control plants. The accumulation of K in shoots and roots was highest in plants in the 50 µmol L−1 P treatment. This higher K accumulation may be one of the factors responsible for the vigorous growth of these plants. The concentration of K in shoots and roots was not appreciably affected by the low P treatment compared with the control plants. Therefore, K concentration may not be responsible for the greening of the leaves of plants in the low P treatment.
The accumulation and concentration of Ca in shoots were barely affected by the low P treatment. The
Table 1 Accumulation of nutrients in shoots and roots of barley plants grown in iron-deficient nutrient solutions with different levels of phosphorus at 14 days after treatment
Table 2 Concentration of nutrients in shoots and roots of barley plants grown in iron-deficient nutrient solutions with different levels of phosphorus at 14 days after treatment
The accumulation of magnesium (Mg), a component of chlorophyll, in shoots was not affected by the low P treatment. The concentration of Mg in the shoots of plants in the low P treatments (50, 5 and 0.5 µmol L−1) was lower than that of the control plants. The increase in the chlorophyll index in plants in the low P (50, 5 and 0.5 µmol L−1) treatments () was not accompanied by a high Mg concentration in the shoots of the plants. Therefore, Mg may not be responsible for the greening of the leaves in plants in the low P treatments.
The accumulation (µg plant−1) and the concentration (µg g−1 dry weight) of micronutrients in shoots and roots are shown in and . The accumulation of Fe in the shoots of plants in the low P (50, 5 and 0.5 µmol L−1) treatments and the concentration of Fe in the shoots of plants in the low P (5 and 0.5 µmol L−1) treatments were higher than the control plants. All the Fe concentrations of the shoots in this experiment were within the range of the critical deficiency level, 30–50 µg g−1 (CitationRömheld and Marschner 1991), at which the leaves should show Fe chlorosis. It is interesting to note that Fe chlorosis did not develop in the leaves of plants in the low P treatment despite the low Fe concentration in shoots. It appears that leaf chlorosis is regulated not only by the Fe concentration but also by the P concentration in shoots.
Total accumulation (shoots + roots) of Fe calculated from the data in and was higher in plants in the low P treatments (50, 5 and 0.5 µmol L−1) than in the control plants. Total accumulation of Fe should be uniform among the treatments because the Fe0 media in all treatments should not contain Fe. This higher Fe accumulation may be derived from marginal contamination with Fe of the Fe0 media, which were prepared with deionized water.
Plants grown under low P conditions were more efficient in the uptake of sparingly contaminated Fe, and it was inferred that low P conditions might facilitate Fe uptake by roots.
Plants in the low P treatments (50, 5 and 0.5 µmol L−1) showed a lower Fe concentration in roots and a higher Fe concentration in shoots than the control plants. This higher Fe concentration in the shoots of plants in the low P treatment may result from the enhancement of internal Fe mobilization within the plant tissues. It appeared that the translocation of Fe from roots to shoots might be enhanced by the low concentration of P in the shoots, although the accumulation of Fe in the roots of the plants in the low P treatment was similar to that in the control plants.
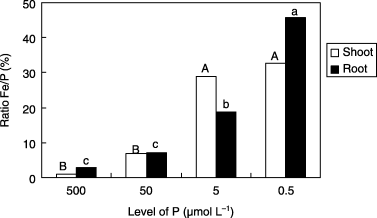
CitationDeKock and Alexander (1955) and CitationPushnik et al. (1984) reported that the severity of Fe chlorosis may be controlled by the Fe/P ratio. The results of the present study showed that the Fe/P ratio in shoots and roots of plants increased with decreasing P concentration in the Fe0 medium (). Our results are consistent with the findings previously reported. The higher concentration of P in the control plants may lead to inactivation of Fe in the plant tissues, appearance of Fe-deficiency symptoms and higher production of PS in roots. However, the mechanism of the inactivation of Fe by high P concentration in plant tissues is yet to be elucidated.
Figure 6 Ratio of iron (Fe) and phosphorus (P) concentrations in shoots and roots of barley plants grown in iron-deficient nutrient solutions with different P levels at 14 days after tratment. Different letters at the top of each bar indicate significant differences (P < 0.05) according to the Ryan–Einot–Gabriel–Welsch Mutiple Range Test.
The accumulation of Cu in shoots was higher in plants grown under low P conditions (5 and 0.5 µmol L−1) than in control plants, whereas the accumulation of Cu in roots was not significantly affected. This higher Cu content in shoots is consistent with a report indicating that P deficiency resulted in a slightly higher Cu accumulation than that of plants grown under adequate P conditions in bush bean (CitationWallace 1984). The concentration of Cu was the highest in the roots of plants in the 5 µmol L−1 P treatment. This phenomenon was repeatedly observed in subsequent studies.
The accumulation and the concentration of Mn in shoots were not significantly affected by the low P concentration in the medium. The accumulation of Mn in roots was not significantly affected, except for plants in the 5 µmol L−1 P treatment.
The accumulation of zinc (Zn) in shoots was higher in plants in the low P (5 and 0.5 µmol L−1) treatment, indicating a Zn–P antagonistic effect (CitationMarschner and Cakmak 1986) under Fe-deficient conditions. However, Zn accumulation in roots of plants in the low P treatment was similar to control plants. The concentration of Zn in the shoots was lower in plants in the 50 µmol L−1 P treatment, but was not affected by the other low P treatments, compared with the control plants. The concentration of Zn in the roots of plants in the low P (50, 5 and 0.5 µmol L−1) treatments was much lower than the control plants.
In conclusion, our results indicated that low P conditions alleviated Fe-deficiency symptoms, such as Fe chlorosis and higher release of PS by the barley roots, despite the low Fe concentration (in the range of critical deficiency level) of shoots. Furthermore, it was found that the chlorophyll index was not reliable for predicting the amount of PS released. The results obtained suggested that P is physiologically competing with Fe in plant tissues. It was also suggested that the depression of chlorophyll synthesis and loss of chlorophyll under Fe-deficient conditions did not result from the low concentration of Mg or Fe, but rather from the high P concentration that may repress the translocation of Fe from roots to shoots. It is considered that the lower ratio of Fe/P in plants grown under 500 µmol L−1 P (control) and Fe0 conditions may be a major factor in the induction of Fe-deficiency symptoms. Therefore, further studies focusing on the mechanism of inactivation of Fe by P in plant tissues should be carried out.
ACKNOWLEDGMENT
The first author thanks the Ministry of Education, Science and Culture of the Japanese Government for providing him with the scholarship that made possible this study.
Notes
Present address: Department of Biotic Environment, Faculty of Agriculture, Iwate University, Ueda 3-18-8, Morioka 020-8550, Japan.
REFERENCES
- Alam , MD , Imamul Huq , SM , Rahman , MS and Anam , K . 1991 . “ Major available nutrients in soils ” . In A Handbook on Chemical Analyses of Soil, Plant and Water , Edited by: Shamsuddin , AP . 60 – 69 . Dhaka : Modhumoti Mudranalaya .
- Brady , NC and Weil , RR . 2002 . “ Soil phosphorus and potassium ” . In The Nature and Properties of Soils , Edited by: Helba , S and Carey , MR . 592 – 635 . New Jersey : Pearson Education .
- Barton , CJ . 1948 . Photometric analysis of phosphate rock . AnalChem , 20 : 1068 – 1073 .
- DeKock , PC and Alexander , H . 1955 . The phosphorus–iron relationship in genetical chlorosis . Plant Physiol , 30 : 293 – 296 .
- DeKock , PC and Mitchell , RL . 1957 . Uptake of chelated metals by plants . Soil Sci , 84 : 55 – 62 .
- Foyer , C and Spencer , C . 1986 . The relationship between phosphate status and photosynthesis in leaves. Effects of intracellular orthophosphate distribution, photosynthesis and assimilate partitioning . Planta , 167 : 369 – 375 .
- Gries , D , Brunn , S , Crowley , DE and Parker , DR . 1995 . Phytosiderophore release in relation to micronutrient metal deficiencies in barley . Plant Soil , 172 : 299 – 308 .
- Heuwinkel , H , Kirkby , EA , Lebot , J and Marschner , H . 1992 . Phosphorus deficiency enhances molybdenum uptake by tomato plants . JPlant Nutr , 15 : 549 – 568 .
- Hoagland , RR and Arnon , DI . 1950 . The water culture methods for growing plants without soil . CalAgricExpSTCirc , 347 : 1 – 32 .
- Hoffland , E , Findenegg , GR and Nelemans , JA . 1989 . Solubilization of rock phosphate by rape. II. Local root exudation of organic acids as a response to P-starvation . Plant Soil , 113 : 161 – 165 .
- Jackson , ML . 1958 . “ Total elemental phosphorus ” . In Soil Chemical Analysis , Edited by: Jackson , ML . 175 – 176 . New Jersey : Prentice-Hall .
- Jolley , VD and Brown , JC . 1994 . “ Genetically controlled use of iron uptake by plants ” . In Biochemistry of Metal Micronutrients in the Rhizosphere , Edited by: Manthey , JA , Crowley , DE and Luster , DG . 251 – 266 . Boca Raton : CRC Press .
- Kawai , S , Itoh , K and Takagi , S . 1993 . Incorporation of 15N and 14C of methionine into the mugineic acid family of phytosiderophores in iron-deficient barley roots . Physiol Plant , 88 : 668 – 674 .
- Marschner , H , Römheld , V and Kissel , K . 1986 . Different strategies in higher plants in mobilization and uptake of iron . JPlant Nutr , 9 : 695 – 713 .
- Marschner , H and Cakmak , I . 1986 . Mechanisms of phosphorus induced zinc deficiency in cotton. II Evidence of impaired shoot control of phosphorus uptake and translocation under zinc deficiency . PhysiolPlant , 68 : 491 – 496 .
- Mengel , K and Kirkby , EA . 2001 . “ Phosphorus and iron ” . In Principles of Plant Nutrition , 5th edn , Edited by: Mengel , K . 453 – 570 . Dordrecht : Kluwer Academic Publishers .
- Mori , S . 1994 . “ Mechanisms of iron acquisition by graminaceous (strategy II) plants ” . In Biochemistry of Metal Micronutrients in the Rhizosphere , Edited by: Manthey , JA , Crowley , DE and Luster , DG . 225 – 249 . Boca Raton : CRC Press .
- Neilands , JB . 1994 . “ Iron in biology ” . In Biochemistry of Metal Micronutrients in the Rhizosphere , Edited by: Manthey , JA , Crowley , DE and Luster , DG . 15 – 27 . Boca Raton : CRC Press .
- Ohwaki , Y and Hirata , H . 1992 . Differences in carboxylic acid exudation among P starved leguminous crops in relation to carboxylic acid contents in plant tissues and phospholipids levels in roots . Soil SciPlant Nutr , 38 : 235 – 243 .
- Omar , MH , Hamdi , AME and Wafik , I . 1971 . Iron and phosphorus interactions in calcareous soils . United Arab RepJSoil Sci , 11 : 245 – 247 .
- Piper , CS and Piper , CS . 1950 . Soil and Plant Analysis , Adelaide : Hassell Press .
- Price , CA . 1968 . Iron compounds and plant nutrition . AnnRevPlant Physiol , 19 : 239 – 248 .
- Pushnik , JC , Miller , GW and Manwaring , JH . 1984 . The role of iron in higher plant chlorophyll biosynthesis, maintenance and chloroplast biogenesis . JPlant Nutr , 7 : 733 – 758 .
- Römheld , V and Marschner , H . 1991 . “ Function of micronutrients in plants ” . In Micronutrients in Agriculture , Edited by: Mortvedt , JJ , Cox , FR Shuman LM , LM . 297 – 328 . Madison : Soil Science Society of America .
- SAS Institute . 1988 . SAS/STAT User's Guide, No1, ANOVA, Version 6 , Cary : Statistical Analysis System Institute .
- Smith , FW , Jackson , WA and van den Berg , PJ . 1990 . Internal phosphorus flows during development of phosphorus stress in Stylosanthes hamata . AustJPlant Physiol , 17 : 451 – 464 .
- Takagi , S . 1993 . “ Production of phytosiderophores ” . In Iron Chelation in Plants and Soil Microorganisms , Edited by: Borton , LL and Hemming , BC . 111 – 131 . New York : Academic Press .
- Takagi , S . 1976 . Naturally occurring iron-chelating compounds in oat- and rice-washings. I. Activity measurement and preliminary characterization . Soil SciPlant Nutr , 22 : 423 – 433 .
- Wallace , A . 1984 . Effect of phosphorus deficiency and copper excess on vegetative growth of bush bean plants in solution culture at two different solution pH levels . JPlant Nutr , 7 : 603 – 608 .
- Present address: Department of Biotic Environment, Faculty of Agriculture, Iwate University, Ueda 3-18-8, Morioka 020-8550, Japan.