Abstract
Rhizomes of Curcuma alismatifolia Gagnep. were planted in a soil-less culture medium composed of sand : perlite mixed at a ratio of 1:1. Three groups of plants were fed with a 15N (15NO− 3 40 mg N L−1+ 15NH+ 4 10 mg N L−1)-labeled culture solution over three different periods: (1) Period 1: from root emergence (2 weeks after planting [WAP]) to the two expanded leaf stage (Stage 1: 6 WAP), (2) Period 2: from Stage 1 to the first floret opening stage (Stage 2: 9 WAP), (3) Period 3: from Stage 2 to harvest during dormancy (Stage 3: 24 WAP). Plants received the same composition of non-labeled N from 2 WAP to 24 WAP, except for the 15N-labeling period. The fourth group of plants was cultured in a nitrogen-free solution and the fifth group was cultured in a complete solution (referred to as control treatment) from planting until harvest and sampled to determine the changes in total N content. Nitrogen content and 15N abundance were determined at different stages of growth. Average absorption of 15N from the culture solution was 23.5 mg N plant−1 during Period 1, 97.8 mg N plant−1 during Period 2 and 78.7 mg N plant−1 during Period 3. Daily N absorption rates per plant were 0.84 mg N during Period 1, 4.66 mg N during Period 2 and 0.75 mg N during Period 3. A large amount of absorbed nitrogen was translocated to the compact spike, particularly in Period 2. At harvest the nitrogen accumulation was partitioned almost equally between new rhizomes and storage roots. Most of the absorbed nitrogen in the new rhizomes and storage roots was derived from that received during Period 3.
INTRODUCTION
Curcuma alismatifolia Gagnep. or “Siam Tulip” belongs to the Zingiberaceae family. It is native to Indochina (Burma, Cambodia and Thailand) and is distributed in all regions of Thailand, at altitudes up to 900 m a.s.l. It is a valuable crop for the cut flower industry and for potted plant production. Thailand exports approximately two million rhizomes per year to Japan, the European Union and the USA (CitationWichailux 2003). Seasonal growth starts from April to May by planting stubbed rhizomes with 4–5 storage roots. Flowering occurs from July to August during the rainy season in Thailand, when the weather is humid and hot. Plants reach a height of approximately 35–40 cm with 5–6 leaves per plant by the flowering period. Growing C. alismatifolia in soil sometimes results in bacterial (Ralstonia solanacearum) disease. According to the Good Agricultural Practices (GAP) formulated by the Department of Agricultural Academic, Ministry of Agriculture, Bangkok, Thailand, growers should supply a high level of nitrogen fertilizer (15 g per plant of N-P-K [15–0−0] or N-P-K [21–7−14] compound fertilizers) once per month from the two-leaf fully expanded stage until the flowering stage, and then change the fertilizer ratio to N-P-K 13–13–21 and apply it at the same rate. Curcuma alismatifolia plants are occasionally grown in soil-less bag cultures using a mixture of sand : rice-husk at a ratio of 1:1 to produce good-quality rhizomes for export. In soil-less culture plants develop nutrient deficiency symptoms if the fertilizer application program is insufficient, particularly nitrogen. Nitrogen is an important nutrient in plants and nitrogen deficiency in C. alismatifolia results in stunted growth and leaf yellowing. The leaf area decreases and flower quality is reduced (CitationRuamrungsri and Apavatjrut 2003). The objective of the present study was to determine the uptake of nitrogen using 15N, to analyze nitrogen utilization by this plant in soil-less culture, and to develop a nitrogen fertilizer regime.
MATERIALS AND METHODS
15N feeding
Rhizomes of C. alismatifolia were grown in 8-inch pots using a sand and perlite mixed medium at a ratio of 1:1. Culture solution (300 mL) was periodically supplied once per week during Period 1 and twice per week during Periods 2 and 3. The complete solution consisted of (in mg L−1) NO− 3-N 40, NH+ 4-N 10, P 25, K 50, Ca 25, Mg 25, Fe 5, Mn 0.5, Zn 0.025, Cu 0.015, Mo 0.015 and B 1.0. Planted rhizomes with storage roots were sampled on planting day (referred to as Stage 0). Two weeks after planting (2 WAP) when the shoots had sprouted and roots had emerged, the plants were separated into five groups. In Group 1, 15N-labeled solution was fed to plants during Period 1 from 2 WAP until two leaves had expanded (Stage 1: 6 WAP). In the second group, the 15N-labeled solution was fed to plants during Period 2 from Stage 1 until the first floret opened (Stage 2: 9 WAP). In the third group, the 15N-labeled solution was fed to plants during Period 3 from the flowering Stage (Stage 2, first floret opened) until dormancy (Stage 3: 24 WAP). The 15N abundance of the stock solution was 10 atom% and the N concentrations of Na15NO3 and (15NH4)2SO4 were 40 and 10 mg L−1, respectively. For these plants, the same concentration of non-labeled nitrogen was used during the period when the 15N-labeled solution was not supplied. The fourth group of plants was cultured in a nitrogen-free solution (referred to as N-free solution). The fifth group was cultured in complete solution (referred to as control treatment) from planting until harvest and sampled to determine changes in total N content.
Sampling
The experiment was conducted using a completely randomized design. Four replicate plants were sampled (one plant per replication) in each group at each stage. Plant height, number of leaves per plant and number of new shoots per plant were measured. The plants were washed in deionized water and separated into leaves, compact spikes, old (original) rhizomes, new rhizomes, storage roots and fibrous roots. Samples were freeze-dried and ground into a powder. Nitrogen concentration was determined using the modified Kjeldahl method (CitationOhyama et al. 1985, Citation1991). The amount of original-N (ON) was determined in the rhizomes and storage roots before planting. Total nitrogen content in various organs of the plants (i.e. old rhizomes, new rhizomes, storage roots, fibrous roots, leaves, compact spikes) was determined. The 15N abundance (atom% excess) was analyzed by emission spectrometry using a JASCO (Japan Spectroscopic Co. Ltd., Tokyo, Japan) N-150 15N analyzer, according to the method of CitationOhyama and Kumazawa (1979) and CitationOhyama et al. (2004).
RESULTS AND DISCUSSION
Plant growth and development
Growth of C. alismatifolia was restricted when the plants were cultured in nitrogen-free solution. The number of leaves per plant and the number of new shoots per plant were reduced, although plant height was similar to that of the control plants with continuous N supply (). The nitrogen content of the plants cultured in the nitrogen-free solution was lower than the control plants (). CitationRuamrungsri and Apavatjrut (2003) also reported that the values of parameters related to flower quality, such as the number of coma bracts, length of flower stalks and width of spikes in the plants, subjected to N-deficient treatment were lower than those in control plants.
Table 1 Effect of nitrogen (N) supply on plant growth and development at 20 weeks after planting
Table 2 Mean nitrogen (N) content of plants in N-free solution compared with control plants
Nitrogen distribution in different organs
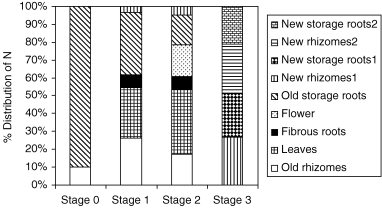
The underground parts of C. alismatifolia comprise two storage organs. The first is an ovoid-rhizome with buds, nodes and internodes, called a stubbed rhizome that will produce new shoots in the next season. The other consists of storage roots bearing thick roots with a round ball shape at the end. The nitrogen contents of the stubbed rhizomes and storage roots were 10.0 and 90.9 mg plant−1, respectively. Approximately 90% of the total nitrogen was present in the storage roots (), indicating that they are important organs and provide a source of nitrogen for new shoot growth. CitationRoh and Lawson (1993), CitationZhang et al. (1995) and CitationHagiladi et al. (1997) also reported that storage roots were important organs for the development of this species. In plants originating from rhizomes with a large amount of storage roots, the yield and quality of the spikes were higher. After two leaves fully expanded at 6 WAP (Stage 1), approximately 28% and 7% of nitrogen were distributed between the leaves and the fibrous roots, respectively, and the amounts of nitrogen remaining in the rhizomes and storage roots were approximately 26% and 35%, respectively. When the first floret opened (9 WAP: Stage 2), 17% of the nitrogen had accumulated in the compact spikes and 37% remained in the leaves. At harvest during the period of dormancy (24 WAP: Stage 3), two of the new rhizomes were harvested and approximately 26–28% of the nitrogen had accumulated in the new rhizomes, whereas approximately 20–28% was present in the new storage roots ().
Uptake of absorbed nitrogen by plants
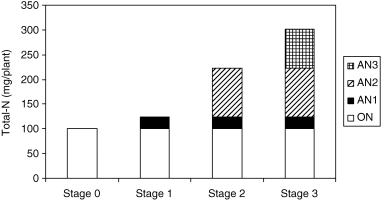
The total nitrogen content in the planted rhizomes at Stage 0 was 101 mg plant−1 at the time of planting and was referred to as “original-N (ON)”. Absorbed nitrogen (AN)1, AN2 and AN3 refer to the amount of N absorbed during Periods 1–3, respectively. The amounts
Figure 2 Utilization of nitrogen (N) at different stages of growth. AN, absorbed nitrogen; ON, original nitrogen.
Translocation of N in the plants
After the solution was fed to the plants, 0.77 mg of AN1 and 2.17 mg of AN2 were translocated into the old rhizomes. The amounts of ON increased in the old rhizomes from Stage 1 to Stages 2 and 3, indicating that some ON from the original storage roots was translocated to the old rhizomes during growth and development ().
After two leaves fully explanded at 6 WAP, an average of 27.3 mg of ON was translocated to the shoots and 15.6 mg was absorbed from the nutrient solution (AN1). At the flowering stage (Stage 3), the shoots accumulated approximately 24.7 mg of ON, and AN1 and AN2 from the nutrient solution amounted to 21.6 mg and 43.2 mg, respectively. Average amounts of 13.2 mg of ON, 6.95 mg of AN1 and 22.3 mg of AN2 accumulated in the compact spikes ().
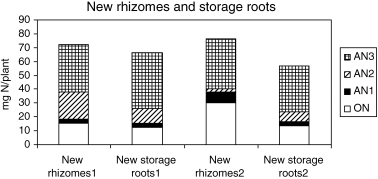
After dormancy, two of the new rhizomes with new storage roots were harvested from underground. During the first period of dormancy, nitrogen that accumulated in the new rhizomes with new storage roots was partitioned almost equally between the two new
Figure 3 Original nitrogen (ON), absorbed nitrogen (AN)1 and AN2 fractions in (a) old rhizomes, (b) shoots and (c) compact spikes at different stages of growth.
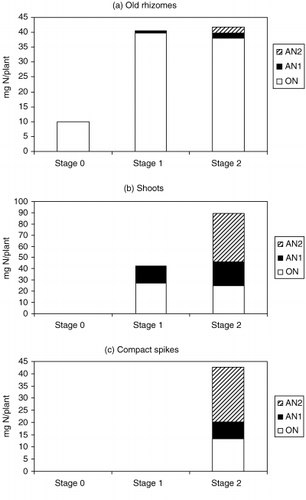
Figure 4 Original nitrogen (ON) and absorbed nitrogen (AN) fractions in new rhizomes and new storage roots at harvest (Stage 3: 24 weeks after planting).
ACKNOWLEDGMENTS
We thank the Hitachi Scholarship Foundation for funding part of the experiment in Japan and we thank H.M. the King's Initiative Center for Flower and Fruit Propagation for funding some materials.
REFERENCES
- Ohyama , T , Ikarashi , T and Baba , A . 1985 . Nitrogen accumulation in the roots of tulip plants (Tulip gesneriana) . Soil Sci. and Plant Nut. , 31 : 581 – 588 .
- Wichailux , O . Patumma . Proceedings of Thai ormental plant to international markets seminar . 26 March . pp. 45 – 9 . Bangkok : Department of Agricultural Extenstion . 2005 (in Thai)
- Ohyama , T , Ito , M Kobayashi , K . 1991 . Analytical procedure of N, P, K contents in plants and manure materials using H2SO4–H2O2Kjeldahl digestion method . Bull. Fac. Agric., Niigata Univ. , 43 : 111 – 120 .
- Ruamrungsri , S and Apavatjrut , P . Effect of nutrient deficiency on the growth and development of Curcuma alismatifoliaGagnep . Proceedings of the 3rd Symposium on the family Zingiberaceae . pp. 98 – 04 . Thailand : Khon Kaen .
- Ohyama , T , Tewari , K Abdel-Latif , S . 2004 . Direct analysis of 15N abundance of Kjeldahl digested solution by emission spectrometry . Bull. Fac. Agric., Niigata Univ. , 57 : 33 – 40 .
- Ohyama , T and Kumazawa , K . Assimilation and transport of nitrogenous compounds originating from 15N2fixation and 15NO3absorption . Proceedings of the third international conference . pp. 329 – 35 . London : Academic press .
- Roh , MS and Lawson , RH . 1993 . Curcuma. Growers Notebook. A step-by-step guide to success . Greenhouse Manager , 12 : 10
- Zhang , JX , Huang , JP and Lin , LM . 1995 . A new favorite in flower markets: cultivation technique and regulation of flowering of Curcuma alismatifolia . Taiwan Flower Industry , 92 : 36 – 40 .
- Hagiladi , A , Umiel , N and Gilad , Z . 1997 . Curcuma alismatifoliaI. Plant morphology and the effect of tuberous root number on flowering date and yield of inflorescences . Acta Hort. , 430 : 747 – 753 .
- Ruamrungsri , S , Ruamrungsri , S , Ikarashi , T and Ohyama , T . 1997 . Uptake, translocation and fractionation of nitrogen in Narcissusorgans by using 15N . Acta Hort. , 430 : 73 – 78 .
- Ruamrungsri , S , Ohtake , N , Sueyoshi , K , Suwanthada , C , Apavatjrut , P and Ohyama , T . 2001 . Changes in nitrogenous compounds, carbohydrates and abscisic acid in Curcuma alismatifoliaGagnep. during dormancy . J. Hort. Sci. Biotech. , 76 : 48 – 51 .
- Ruamrungsri , S , Ohtake , N , Sueyoshi , K , Suwanthada , C , Ohyama , T and Apavatjrut , P . 2005 . Effect of nitrogen and potassium on growth and development of Curcuma alismatifoliaGagnep . Acta Hort. , 673 : 443 – 448 .