Abstract
Soil solution composition is one of the most important factors governing the uptake of nutrients by plants. However, sampling soil solution is usually time and labor consuming. We analyzed soil solutions and water extracts from 126 soil samples including 41 allophanic Andisols for major cations and anions. The multiplication of the concentration of a cation in the water extract by the dilution ratio (i.e. the ratio of the water content at water extraction to that at soil solution separation) always overestimated its concentration in soil solution. For soils in which the soil solutions were unsaturated with respect to gypsum, there were highly significant linear correlations between the concentration in soil solution and the concentration in water extract multiplied by the dilution ratio for Ca, Mg, K and Na. The linear regression analyses gave the following equations for predicting cation concentration in soil solution: [Ca]ss = 0.8553 r d [Ca]wex − 0.9282, [Mg]ss = 0.9346 r d [Mg]wex − 0.6645, [K]ss = 0.4978 r d [K]wex − 0.8984, [Na]ss = 0.7092 r d [Na]wex − 0.5283, where [ ] refers to the concentration in mmol L−1, the subscripts ss and wex are soil solution and water extract, respectively, and r d is the dilution ratio. We also proposed a practical criterion to evaluate the saturation status of soil solution with respect to gypsum.
Key words:
INTRODUCTION
Soil solution composition is one of the most important factors governing the uptake of nutrients by plants (CitationCameron 1911). Keeping nutrient concentrations in appropriate ranges is crucial to achieve sustainable crop production in intensive agriculture. Crop yield declines under insufficient nitrate concentrations, but excessive nitrate concentrations lead to groundwater pollution. CitationItokawa (1997) reported that monitoring the nitrate concentration in soil solution is useful for preventing leaching of applied nitrate. CitationTaira et al. (2004) demonstrated through a series of pot experiments that the concentrations of Ca, Mg and K in soil solution are good indices of the bioavailability of these nutrients and highly useful in diagnosing an imbalance in these cations.
It is not difficult to analyze a soil solution for major cations and anions with portable analyzers once the soil solution has been collected. However, sampling soil solution is usually time consuming for soils at field moisture contents (CitationRhoades 1996). In contrast, it is much easier to obtain water extracts of soils at a soil to solution ratio of, for example, 1 kg to 5 L (CitationRhoades 1996) and it would be of practical importance to be able to estimate the soil solution composition from the water extract. A water extract of a soil is basically a diluted soil solution. In general, the ionic concentrations in the soil solution cannot be correctly predicted by multiplying the concentrations in the water extract by the dilution ratio (i.e. the ratio of the water content at water extraction to that at soil solution separation) because cation exchange equilibria shift (CitationWada and Ootani 1998) to a significant extent and some salts may dissolve during extraction (CitationRhoades 1996). Therefore, to estimate the soil solution composition from the water
Table 1 Range of selected chemical and mineralogical properties of the soil samples used
An exception is the nitrate concentration in soil solution. CitationWada and Furmura (1996) reported that the nitrate concentration in soil solution could be fairly accurately predicted from the water extract by multiplying by the dilution ratio. CitationOdahara et al. (2005) found that this method is also applicable to the chloride concentration in soil solution. In the present study, the major cation concentrations in water extracts from 126 soil samples were multiplied by the dilution ratio and compared with the measured concentrations in soil solutions. It was found that the calculated concentration did not agree with the measured one; however, there were highly significant linear correlations between them. On the basis of this observation, regression equations relating the cation concentrations in water extracts to the concentrations in soil solution are proposed.
MATERIALS AND METHODS
Eighty-five non-Andisol samples were collected from the Ap horizon of agricultural fields in Fukuoka prefecture, Japan. In addition, 41 Andisol samples were collected in Kumamoto and Kagoshima prefectures, Japan. Of the 85 samples from Fukuoka prefecture, 67 samples were taken in greenhouses raising green vegetables and tomatoes and the remaining samples were collected in fields growing wheat, maize and lettuce. Of the Andisols, 15 samples were collected in fields where horse bean was raised and the remaining samples were taken in greenhouses raising watermelons, tomatoes and flowers. The allophane content of the Andisols was determined using selective dissolution (CitationParfitt and Wilson 1985). Some selected chemical and mineralogical properties of the samples are listed in .
Approximately 100–200 g portions of the samples were placed into plastic bags, aggregates were broken by pinching the bags and gravels larger than approximately 2 mm were removed where possible by hand picking because sieving of moist soil samples was impractical. Water content was determined by oven-drying at 105°C. Water extraction was carried out at a soil:solution ratio of approximately 1 kg:5 L by shaking approximately 10 g of a soil sample with a calculated volume of deionized water for 1 h, followed by centrifugation at 980 g. Soil solution was separated using centrifugation at 2050–37,700 g, depending on the water content. If a soil sample was too dry to obtain a sufficient amount of soil solution for analysis, deionized water was added to bring the water content to approximately 60% of the maximum water holding capacity and the sample was homogenized before centrifugation. The range of the water content of the soil samples at soil solution separation and at water extraction is listed in .
The anion concentrations in the water extracts and soil solutions were determined using ion chromatography. The cation concentrations were determined using atomic absorption spectroscopy. All extractions were carried out in duplicate and the results were averaged.
RESULTS AND DISCUSSION
A major process that occurs during water extraction is the dilution of the soil solution. If no adsorption–desorption and precipitation–dissolution reactions occurred, the concentration of an ion in soil solution could be estimated by multiplying its concentration in the water extract by the dilution ratio. The concentrations of Ca, Mg, K and Na in water extracts were multiplied by the dilution ratio and plotted against the measured concentrations in soil solution (). Hereafter, the concentration of the cation i that is calculated by multiplying the concentration in water extract by the dilution ratio will be designated as DCC i . shows that DCC was a relatively good estimate of the concentration in soil solution for Mg and Na, but that the simple correction with regard to the dilution generally overestimated the concentrations in soil solution. The DCCK was also significantly higher than the measured K concentration in almost all the soil samples. Although the DCC did not agree with the measured cationic
Figure 1 Ion concentrations predicted using multiplication by the dilution ratio plotted against the measured concentrations in soil solution. (○) non-Andisols; (•) Andisols.
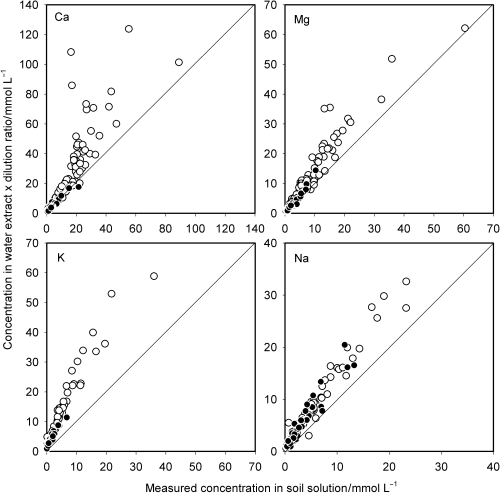
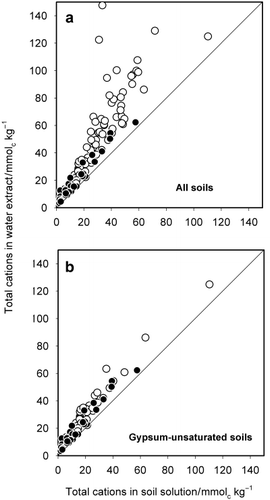
The fact that the DCC was almost always higher than the measured concentration in soil solution for all cations suggests that there was a net release of cations from the solid phase during water extraction. To estimate the extent of the release, the total amount of cations in the water extract was calculated and plotted against the amount in the soil solution (). As expected, the total amount of cations in the water extract was significantly greater than the amount in the soil solution, and this trend was more conspicuous for non-Andisols than for Andisols. The scatter pattern of the plots resembled that observed in the graph for Ca in , suggesting that Ca salts dissolved. Ion speciation in the soil solutions using a computer code developed by CitationWada and Seki (1994) showed that the soil solutions from many non-Andisol samples were saturated with respect to gypsum. This finding suggests that gypsum contained in the soils dissolved during water extraction. The total amount of dissolved cations in the water extract was re-plotted against the amount in the soil solution () for soils with saturation index (SI) values < −0.2. The SI is the logarithm of the ratio of the product of Ca and SO4 ion activities to the solubility product of gypsum. The plot points fell near the diagonal line except for one, indicating that the large upward deviation primarily resulted from the dissolution of gypsum.
Dissolution of gypsum would have kept the Ca concentration in the water extract higher than that expected in the absence of gypsum. The higher Ca concentration may have shifted the exchange reactions between Ca and Mg, and between K and Na, which, in turn, resulted in the release of these cations. This implies that the upward deviation of the plots for Mg, K and Na may result, at least in part, from the release of these cations in the exchange reactions. The soil samples in which the soil solution was unsaturated with respect to gypsum were selected and the DCC was again plotted against the measured concentration in the soil solution (). For Ca, the plot points that fell well beyond the 1:1 line were mostly removed (). The deviated points were also removed from the graph for Mg and many points fell very close to the diagonal line. These results indicate that the upward deviation of DCCCa and DCCMg from the measured concentrations in the soil
Figure 2 Total amount of cations in water extract plotted against the total amount of cations in soil solution for (a) all soils and (b) soils in which the soil solution was unsaturated with respect to gypsum. (○) non-Andisols; (•) Andisols.
According to the thermodynamics of cation exchange, the exchange equilibrium between monovalent and divalent cations shifts to the direction in which divalent cations are adsorbed and monovalent cations are released (CitationSposito 1981) when the equilibrium solution is diluted with water. This was experimentally confirmed by CitationMoss (1963a,Citationb), who added water to soils and observed an increase in the fractions of monovalent cations. CitationWada and Ootani (1998) showed that DCCK and DCCNa might be higher than the actual concentrations in soil solution by 50% through a series of numerical simulations. These facts and the data in and suggest that the upward deviation of DCCK and DCCNa resulted, for the most part, from a shift in the cation exchange equilibria.
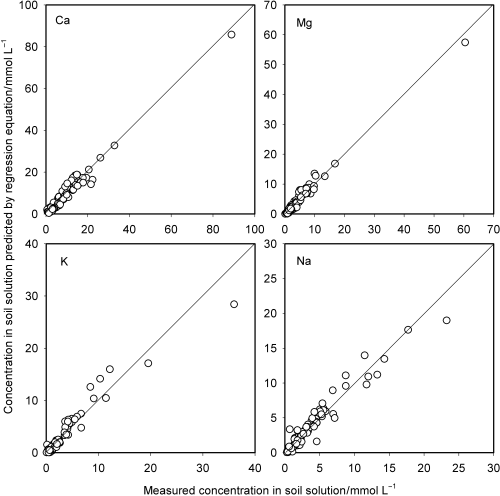
If shifts in the cation exchange equilibria are the major cause of the upward deviations of DCCK and DCCNa (), a natural way to predict the concentrations of K and Na in soil solution is to carry out cation equilibrium calculations in which soil specific selectivity coefficients are needed. However, this is not practical because not only is the value of the selectivity coefficient different for different soils, it also varies with exchangeable cation composition. In particular, this applies to the coefficients for the exchange reactions involving K (CitationWada and Odahara 1993). However, all the plots in are linear and it might be possible to approximate them using straight lines. The measured concentration was regressed against the DCC and the regression equations obtained are listed in . The concentrations of the four cations in soil solution were calculated using these equations and plotted against the measured concentrations (). The predicted concentration was well correlated with the measured concentration over the whole range for all cations.
It must be noted that the regression equations shown in , particularly those for Ca and Mg, are valid only when the soil solution is unsaturated with respect to gypsum. Therefore, the saturation status in a soil solution must be predicted from the composition of a water extract before using the equations. Examination of the relationship between the SI value and the ionic composition of the water extract showed that the soil solution is unsaturated with respect to gypsum if the product of DCCCa and DCCSO4 does not exceed 1000.
Thus, the procedure to predict the cationic concentration in the soil solution of a given soil would be as follows: (1) measure the water content of the soil sample, (2) carry out water extraction at a soil to water ratio of approximately five and analyze the extract for cations and SO4, (3) calculate the product of DCCCa and DCCSO4, (4) if the calculated value is > 1000, the application of the present method is not appropriate, (5) otherwise, calculate the ionic concentrations in soil solution using the regression equations in . The practical criterion for the saturation with respect to gypsum used in step (4) was proposed by CitationWada et al. (2006).
The empirical prediction with the regression equations is satisfactory in a statistical sense (). However, the relative deviation of the predicted value from the measured one is as large as 100% for some soils, particularly at low concentrations. The scattering of the data points may result from an analytical error as well as the difference in the cation exchange property of the soils. The prediction using the proposed method should not replace analyses of the actual soil solutions. In addition,
Figure 3 Ion concentrations predicted using multiplication by the dilution ratio plotted against the measured concentrations in soil solution for soils in which the soil solution was unsaturated with respect to gypsum. (○) non-Andisols; (•) Andisols.
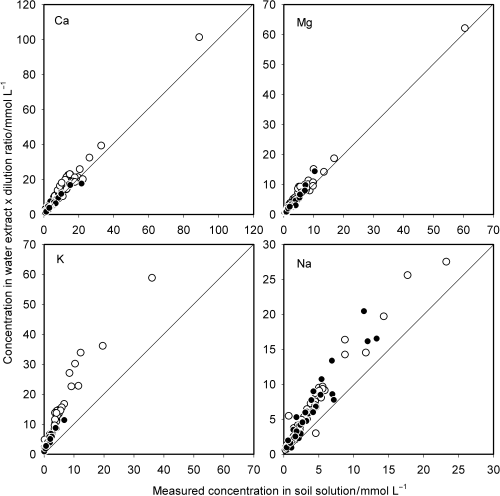
Table 2 Regression equations relating ionic concentration in water extract with the concentration in soil solution
The present study provided not only a practical tool for soil testing, but also important implications for the cation exchange reactions in soils. As already discussed, the upward deviation of DCCK () was caused by a shift in the cation exchange equilibria during water extraction, and cation exchange equilibrium calculation is required for theoretical prediction of the ionic composition of the soil solution from the composition of the water extract. One of the difficulties in predicting cation exchange equilibria in soils is that the value of the selectivity coefficient for an exchange reaction differs greatly among soils and varies depending on the exchangeable cation composition. In particular, this applies to the exchange reactions involving K (CitationWada and Odahara 1993). The soil samples used in the present study were mineralogically diverse, and included smectitic, kaolinitic, mixed kaolinitic–illitic–vermiculitic and allophanic soils. The exchangeable cation composition varied widely. For example, the charge fraction of exchangeable K ranged from 0.005 to 0.241. Undoubtedly, the value of the selectivity coefficient for K-involving exchange reactions in the soil samples differed greatly. Nevertheless, DCCK was linearly correlated with the measured K concentration in soil solution and the relationship could be satisfactorily approximated using a single straight line. This strongly implies that it is possible to carry out approximate cation exchange equilibrium
Figure 4 Ion concentrations predicted by the regression equations plotted against the measured concentrations.
Another important soil chemical implication of the present study is that the net release of cations from soils in which the soil solution is unsaturated with respect to gypsum occurs during water extraction. This is clearly observed in , which shows that the total amount of cations in water extract was greater than that in soil solution for all soils. Of these soils, the Andisol samples did not contain detectable amounts of carbonates. The only possible mechanism underlying the observed ion release is the simultaneous desorption of cations and anions from surface complexes. According to CitationWada (1984), this reaction can be expressed as:
where SOH refers to surface hydroxyl groups on soil minerals and M and A are monovalent cations and anions, respectively. The value of a quantitative model of the above reaction for more accurate predictions of the composition of soil solutions using water extracts is indisputable. However, to be practical, the model must preferably work without soil specific parameters.
Notes
Present address; Astec Corporation, Iwabana cho 107-4, Himeji 670-0028, Japan.
REFERENCES
- Cameron , FK . 1911 . The Soil Solution: The Nutrient Medium for Plants , London : The Chemical Pub. Co. .
- Itokawa , S . 1997 . Movement of fertilizer nitrogen in greenhouse field and nitrate nitrogen concentration of groundwater . JpnJSoil SciPlant Nutr , 68 : 327 – 330 . (in Japanese)
- Taira , K , Fujita , N and Sorin , T . 2004 . A trial for soil diagnosis method of accumulated cation availabilities in a soil solution . JpnJSoil SciPlant Nutr , 75 : 15 – 20 . (in Japanese with English summary)
- Rhoades , JD . 1996 . “ Salinity: Electrical conductivity and total dissolved solids ” . In Methods of Soil AnalysisPt3 – Chemical Methods , Edited by: Sparks , DL . 417 – 435 . Madison : Soil Science Society of America .
- Wada , S-I and Ootani , T . 1998 . Changes in cationic composition of soil solution associated with changes of water content: A numerical study . Soil SciPlant Nutr , 44 : 237 – 244 .
- Wada , S-I and Furumura , S . 1996 . Prediction of nitrate ion concentration in soil solution from that in 1:5 water extract . JpnJSoil SciPlant Nutr , 67 : 180 – 182 . (in Japanese)
- Odahara , K , Kuroyanagi , N , Fujita , A , Kaneko , A and Wada , S-I . 2005 . Prediction of major ion concentration of soil solution from those of water extract (Abstract) . JpnSocSoil SciPlant Nutr , 51 : 132 (in Japanese)
- Parfitt , RL and Wilson , AD . 1985 . Estimation of allophane and halloysite in three sequences of volcanic soils . Catena Suppl , 7 : 1 – 8 .
- Wada , S-I and Seki , H . 1994 . Compact computer code for ion speciation in aqueous solutions based on a robust algorithm . Soil SciPlant Nutr , 40 : 165 – 172 .
- Sposito , G . 1981 . The Thermodynamics of Soil Solutions , New York : Oxford University Press .
- Moss , P . 1963a . Some aspects of the cation status of soil moisture. Pt. I: The effect of dilution and calcium ions on the release of potassium . Plant Soil , 18 : 99 – 113 .
- Moss , P . 1963b . Some aspects of the cation status of soil moisture. Pt. II: The ratio law and soil moisture content . Plant Soil , 18 : 114 – 123 .
- Wada , S-I and Odahara , K . 1993 . Potassium–calcium exchange in five Ap soils from paddy fields and its effect on potassium concentration in soil solution . Soil SciPlant Nutr , 39 : 129 – 138 .
- Wada , S-I , Odahara , K , Gunjikake , N and Chishaki , N . 2006 . A water extraction-based practical method for identification of gypsum in soils . Jpn JSoil SciPlant Nutr , 77 : 205 – 206 . (in press) (in Japanese)
- Wada , S-I . 1984 . A mechanism of apparent salt adsorption in Andosols . Soil SciPlant Nutr , 30 : 77 – 83 .
- Present address; Astec Corporation, Iwabana cho 107-4, Himeji 670-0028, Japan.