Abstract
The vertical distribution of the content of the chloroform-extractable green fraction (CEGF) in the soil profiles of four Japanese Andosols, one Japanese Cambisol and one Nepalese Cambisol was examined using a colorimetric method with an ultraviolet and visible spectrophotometer. In addition, the relationship between the CEGF content and the soil chemical properties was examined by statistical analysis. CEGF was detected in all the soil samples, and the content ranged from 0.02 to 1.16 g kg−1. The CEGF content in the surface A horizon of the Andosol tended to be higher than in the Cambisol. The vertical distribution of the CEGF content in three soil profiles (two Andosol profiles and one Nepalese Cambisol) revealed maximum values in the subsurface horizon. In the Japanese Cambisol, the content of the CEGF was highest in the surface horizon. In the remaining two Andosol profiles containing a buried humus horizon, the content of the CEGF was highest in the buried humus horizon and the distribution was considerably different from that of organic carbon. Statistical analysis showed that the content of the CEGF displayed a significant positive correlation (P < 0.001, n = 36) with the contents of dithionite-citrate-bicarbonate-extractable Al (Ald), and with oxalate-oxalic acid-extractable Fe and Al (Feo and Alo, respectively). Furthermore, the content of the CEGF showed a significant positive correlation with the content of Alo (r = 0.944, P < 0.01, n = 6) in the soil with the highest content of CEGF in the profile. It was, therefore, suggested that CEGF is actively produced in humus-rich soils like Andosols, and that Ald, Feo and, in particular, Alo were associated with the vertical distribution of CEGF in the soil profiles.
INTRODUCTION
In a previous paper, a colorimetric method developed for the estimation of the content of the chloroform-extractable green fraction (CEGF) in soils was described (CitationKobayashi et al. 2005). The CEGF, which exhibits strong absorption maxima at 454, 440 and 418 nm in chloroform, is considered to be of microbial origin and one of the components of, or a closely related substance to, the green fraction of humic acid (HA) called Pg (CitationKumada 1987; CitationWatanabe et al. 1996) as previously reported (CitationKobayashi et al. 2005). It is important to note that in a previous paper (CitationKobayashi et al. 2005), CEGF was found to occur in various soils and that the method for estimating its content could be applied to various soils. Pg is distributed worldwide in various soils (CitationKumada 1987; CitationZancada et al. 2003) and numerical studies on the distribution, origin (CitationKumada and Hurst 1967; CitationSato 1976b; CitationValmaseda et al. 1989) and chemical properties (CitationKumada and Sato 1980; CitationWatanabe et al. 1996) of Pg have been conducted in view of the importance of Pg, as one of the components of HA (CitationKumada 1987), and of its role in the mobilization and transport of iron through its chelating ability (CitationNakabayashi and Wada 1991). Furthermore, it has been reported that a Pg-like pigment occurs in the soil fulvic acid fraction (CitationSchnitzer and Skinner 1968), humin fraction (CitationTsutsuki and Kuwatsuka 1989) and in dark-colored soil fungi (CitationSato 1976b; CitationValmaseda et al. 1989). On the basis of these studies, it is assumed that both Pg and the Pg-like pigment are distributed worldwide in various soils. However, the quantitative distribution of these soil green pigments is poorly documented because of the lack of appropriate methods for determining the content, except for Pg, and because the method for estimating Pg content in soil cannot be applied to humus-rich soils (CitationSato 1974). It was, therefore, concluded that the factors described above could not be well established without quantitative determination of Pg and Pg-like pigments in various soils.
Although CEGF is only a closely related substance to Pg, it is, at present, the only fraction that enables the estimation of the content of green pigments in various soils. Therefore, studies on the quantitative distribution of CEGF in various soils should contribute to studies on soil green pigments including Pg. The purpose of the present study was to investigate the vertical distribution of the CEGF content in four Andosol profiles and two Cambisol profiles using the method described by CitationKobayashi et al. (2005). In addition, the relationship between the CEGF content and several soil chemical properties was examined.
MATERIALS AND METHODS
Soil samples
The soil profiles used in the present study were as follows: three Japanese Silic Andosols, namely Sugadaira-G (SGG), Sugadaira-F (SGF) and Soni (SN), one Japanese Umbric Andosol, Gyosei (GS), and one Japanese Dystric Cambisol, namely Kuroiwa (KI), where G and F stand for grassland and forest, respectively. In addition, one Nepalese Dystric Cambisol profile, Jiri-F (JR), was used. Although the JR profile contained hydroxyanthraquinone pigments at the same level as the Japanese Cambisol (CitationSuzuki et al. 1999b), the soil environment was very different from that of the Japanese soil. Detailed information about the sites and soil properties was presented in CitationSuzuki et al. (1999a). Soil samples were taken from each horizon of the soil profile. The sample was air-dried and passed through a 2-mm mesh sieve. A portion of the sample was ground to pass through a 0.5-mm mesh sieve.
Soil chemical analyses
Exchangeable Al (Ex. Al) of the soil samples was extracted with 1 mol L−1 KCl (CitationBertsch and Bloom 1996) and the concentration was subsequently determined using Shimadzu ICPS-1000III (Shimadzu Co., Kyoto, Japan). The remaining chemical data for the soil samples were from CitationSuzuki et al. (1999b).
Classification of HAs and estimation of Pg content in soils
The HAs of the soil samples were prepared according to the method described by CitationOba (1964). The HAs were dissolved in 0.1 mol L−1 NaOH and used for the classification of HAs and for the estimation of the Pg content in soil. The log (A 400/A 600) (= Δlog K, A λ: absorbance at λ nm in alkaline solution) value of the HA solutions was determined using the method of CitationOba (1964). The volume of 0.02 mol L−1 KMnO4 consumed by the HA solutions (30 mL), except for the HAs from the JR profiles, was determined using the method of CitationOba (1964). Subsequently, the volume was transformed into the organic carbon concentration using a conversion factor (0.0157) described by CitationIkeya and Watanabe (2003). For the JR profiles, the organic carbon concentration of the HA solution was determined using a dissolved C analyzer (Shimadzu TOC-VCPH), according to the method of CitationIkeya and Watanabe (2003). The classification of HAs was carried out using the A 600/C-log (A 400/A 600) diagram (A 600/C; the absorbance at 600 nm mg−1 C mL−1 of HA in 0.1 mol L−1 NaOH) described by CitationIkeya and Watanabe (2003). Based on their log (A 400/A 600) and A 600/C values, HAs were classified into 4 types, Type A, Type B, Type P and Type Rp, and their degree of humification increased in the order of Type A, Type B and Type Rp (CitationKumada 1987). Type P HA is a group with absorption maxima near 615, 570 and 450 nm in alkaline solution because of the presence of Pg (CitationKumada 1987). Subdivision of HAs was carried out using the method of CitationKumada et al. (1967). In this method, Type P HA is subdivided into Type P±, P+, P++ and P+++, depending on the intensities of the absorption maxima near 615, 570 and 450 nm because of the presence of Pg. In cases where the same absorption maxima were found in the HA except for Type P, they were also subdivided into Type P HA (e.g. A± and Rp++).
The Pg content in the HA solutions prepared was estimated according to the method developed by CitationSato (1974). This method is a geometrical method based on the absorption curve of Type P HA in alkaline solution.
Estimation of CEGF content in soils
The CEGF of each soil sample (0.5–2 g) was extracted three times with HCl-dimethylsulfoxide (DMSO) solvent (DMSO in 0.2 mol L−1 HCl) at a soil/solvent ratio of 1/20 (w/v) for 24 h in the first extraction, 1/20 (w/v) for 6 h in the second extraction and 1/10 (w/v) for 30 s in the third extraction. In the present study, the ratio of extractant was twofold that indicated in our previous paper (CitationKobayashi et al. 2005) because it was considered that CEGF could not be completely extracted from the
Figure 1 Visible spectra of chloroform-extractable fraction in chloroform from (a) Andosol (Sugadaira-G [SGG]) and (b) Cambisol (Kuroiwa [KI]) profiles.
![Figure 1 Visible spectra of chloroform-extractable fraction in chloroform from (a) Andosol (Sugadaira-G [SGG]) and (b) Cambisol (Kuroiwa [KI]) profiles.](/cms/asset/0e7036db-991e-4ad1-b047-58593ab8da8c/tssp_a_10382377_o_f0001g.gif)
Ultraviolet and visible spectral analysis
The ultraviolet and visible (UV–Vis) spectra of HAs in 0.1 mol L−1 NaOH and of CEFs in chloroform were measured using a JASCO V-530 spectrophotometer (Jasco Co., Tokyo, Japan).
Statistical analysis
All statistical analyses were carried out using SPSS for Windows (ver. 11.5 J, SPSS Inc., Tokyo, Japan). Correlations were obtained using the Pearson correlation coefficient. P < 0.05 was considered to be significant.
RESULTS
Classification of HAs and Pg content in soils
shows the CEGF and Pg contents, soil chemical properties and the properties of HAs in the soil samples. In all Andosol profiles (SGG, SGF, SN and GS), most of the HAs were classified into Type A. In the Cambisol profiles, HAs from the KI profile were classified into Type P or Type Rp, and those from the JR profile were classified into Type P.
Although Pg was detected in all samples (), the method for estimating the content of Pg in the soil could only be applied for the Type P HA soil (CitationSato 1974). It was, therefore, impossible to estimate the content of Pg in soils except for the JR profiles and the remaining soils with Type P HA (). In the JR profile, the Pg content, which was high in the A1 and AB horizons (0.17 and 0.16 g kg−1, respectively), markedly decreased, thereafter, with depth ().
UV–Vis spectral analysis
The visible spectra of the chloroform solution of CEF from the Andosol (SGG) and Cambisol (KI) profiles are shown in . Characteristic absorption maxima near 583, 565, 542, 454, 440 and 418 nm were detected in all horizons of the two profiles. These absorption maxima were also detected in all horizons of the remaining four profiles (SGF, SN, GS and JR), and they were almost identical to those of CEGF reported in CitationKobayashi et al. (2005). The relative absorption intensity at 454, 440 and 418 nm, however, differed among the soil horizons (). Therefore, the ratios of E 440CEGF to E 454CEGF and of E 418CEGF to E 454CEGF (E λCEGF : absorbance at λ nm due to CEGF) from the SGG and KI profiles were calculated using the method described in CitationKobayashi et al. (2005), and the ratios are presented in . The ratios were different among the soil horizons from the SGG and the KI profiles and this difference was also observed in the remaining soil profiles.
Distribution of CEGF in soil profiles
The CEGF was detected in all soil samples and the content ranged from 0.02 to 1.16 (g kg−1), as shown in .
Table 1 Chloroform-extractable green fraction (CEGF) and Pg contents, soil chemical properties and properties of humic acids
Table 2 Values of E440CEGF/E454CEGF and E418CEGF/E454CEGF ratios calculated from the visible spectra of the chloroform-extractable fraction (CEF) from the Sugadaira-G (SGG) and Kuroiwa (KI) profiles
shows the vertical distribution of the contents of CEGF and organic carbon (O-C) in the soil profiles. The content of O-C tended to decrease with depth in all soil profiles except for the A2 horizon of the SGG profile.
In the SGG, SGF and JR profiles (, respectively), the content of CEGF was highest in the subsurface horizon (1.16, 1.04 and 0.52 g kg−1, respectively) and it decreased with depth in the same way as the O-C content. The distribution of the CEGF and Pg contents in the JR soil profile differed from each other (, , respectively). In the KI profile, the distribution of the content of CEGF showed a similar pattern to that of O-C (). In contrast, the distribution of the CEGF content in the SN and GS profiles, which contained a buried humus horizon, tended to increase up to the buried A horizon (the 3A4 horizon of SN and the 2A4 horizon of GS) and then decreased with depth. In these two profiles, the distribution differed considerably from that of O-C (, respectively).
Relationship between CEGF content and soil chemical properties
The CEGF content exhibited a significant positive correlation with the O-C content (P < 0.001, n = 36) in all soil samples. However, the distribution of the CEGF content in the SN and GS profiles, which contained a buried humus horizon, differed considerably from that of O-C. Correlation analyses between the contents of CEGF and O-C in the soil profiles was, therefore, carried out separately in soil profiles that contained a buried humus horizon and in those without one. As a result, the content of CEGF did not show any significant relationship with that of O-C (n = 13) in the former two profiles (SN and GS). In contrast, there was a significant relationship between the content of CEGF and O-C (r = 0.922, P < 0.001, n = 23) in the latter four soil profiles (SGG, SGF, KI and JR). shows the relationship between the content of CEGF and dithionite-citrate-bicarbonate-extractable Fe and Al (Fed and Ald, respectively), and CEGF and oxalate-oxalic acid-extractable Fe and Al (Feo and Alo, respectively) in all soil samples. A significant positive correlation (P < 0.001, n = 36) was found between the content of CEGF and Ald, Feo and Alo (, respectively) in all soil samples. As the content of O-C showed a significant correlation with Ald (P < 0.01), Feo (P < 0.05) and Alo (P < 0.05) in all soil samples, a partial correlation analysis between the CEGF content and Ald, Feo and Alo was carried out to exclude the influence of O-C. As a result, the CEGF content showed a significant correlation with Ald (r = 0.693, P < 0.001), Feo (r = 0.504, P < 0.01) and Alo (r = 0.535, P < 0.01). In contrast, there was no significant relationship between the content of CEGF and Fed (), or with the pH (H2O) value or the concentration of Ex. Al in all soil samples (n = 36). In addition, we determined whether a relationship between the CEGF content and the chemical properties of the soil with the highest content of CEGF in the profile could be found. As a consequence, the content of CEGF showed a significant correlation with Alo at P < 0.01 (r = 0.944, n = 6: the content of Alo did not show a significant relationship with O-C), as shown in , but not with the remaining parameters.
DISCUSSION
Distribution of CEGF in soil profiles
The CEGF content in the surface A horizon of the Andosol tended to be higher than the content in the Cambisol. In general, Andosol is a humus-rich soil with a higher organic carbon content than other soils. Therefore, CEGF, which is assumed to be of microbial origin (CitationKobayashi et al. 2005), may be actively produced in a humus-rich soil like Andosol. CitationSato (1976a) pointed out that humus-rich soils might promote the activity of Pg-producing fungi. Although CEGF is only a closely related substance to Pg, this may support our concept.
Based on the results of the distribution of CEGF content, it appears that the vertical distribution of CEGF
Figure 2 Vertical distribution of chloroform-extractable green fraction (CEGF) (•) and organic carbon (◊) contents in the soil profiles. (a) Sugadaira-G (SGG), (b) Sugadaira-F (SGF), (c) Soni (SN), (d) Gyosei (GS), (e) Kuroiwa (KI) and (f) Jiri-F (JR).
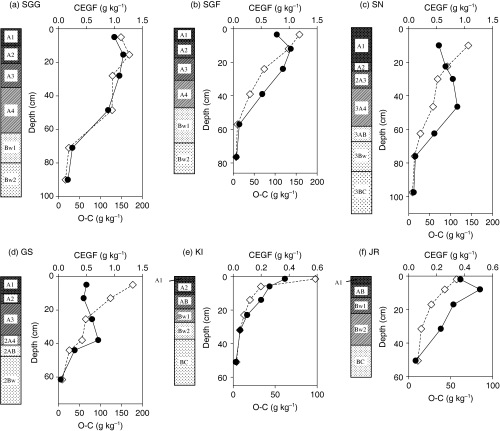
Figure 3 Relationship between the chloroform-extractable green fraction (CEGF) content and the contents of (a) Fed, (b) Ald, (c) Feo and (d) Alo. Parentheses indicate partial correlation coefficient excluding the influence of organic carbon (O-C). (–•–) all soil samples (n = 36); (–○–) soil samples with the highest content of CEGF in each soil profile (○, SGG [A2 horizon]; ▵, SGF [A2 horizon]; ◊, SN [3A4 horizon]; ▿, GS [2A4 horizon]; □, KI [A1 horizon]; , JR [AB horizon]). **P < 0.01; ***P < 0.001.
![Figure 3 Relationship between the chloroform-extractable green fraction (CEGF) content and the contents of (a) Fed, (b) Ald, (c) Feo and (d) Alo. Parentheses indicate partial correlation coefficient excluding the influence of organic carbon (O-C). (–•–) all soil samples (n = 36); (–○–) soil samples with the highest content of CEGF in each soil profile (○, SGG [A2 horizon]; ▵, SGF [A2 horizon]; ◊, SN [3A4 horizon]; ▿, GS [2A4 horizon]; □, KI [A1 horizon]; , JR [AB horizon]). **P < 0.01; ***P < 0.001.](/cms/asset/786951f7-769c-43b9-8511-86479695f52a/tssp_a_10382377_o_f0003g.gif)
In a previous paper (CitationKobayashi et al. 2005), CEGF was considered to consist of several fractions. The difference in the E 440CEGF/E 454CEGF and E 418CEGF/E 454CEGF ratios among the soil samples (), therefore, may reflect differences in the properties of CEGF, such as the composition of the fractions constituting CEGF. Furthermore, the difference in the ratios might reflect differences in the soil environment. These ratios, however, were not strictly correct because of the interference of a fraction that differed from CEGF (). It will be necessary to develop a separation and purification method for CEGF to investigate the distribution of CEGF content and its properties in detail.
The distribution of the CEGF content was different from that of Pg in the JR profiles. This indicates that CEGF is not identical to Pg. It will be necessary to examine the distribution of the CEGF and Pg contents in soils with Type P HA to clarify their precise relationship.
Occurrence of CEGF and Pg in buried humus horizons
The CEGF content was highest in the buried A horizons of the SN and GS profiles (, respectively). Pg was detected in these buried humus horizons, although the content could not be estimated (). It is known that Pg, which is considered to be of microbial origin (CitationKumada and Hurst 1967), is distributed not only in various surface soils but also in buried humus horizon approximately 100,000 years bp (CitationWatanabe and Kobayashi 2001). CitationWatanabe and Kobayashi (2001) reported that the Pg absorption strength (CitationWatanabe 1992; Pg absorption strength = (A 615-A 700)/(A 600-A 700), A λ: absorbance at λ nm of HA in alkaline solution; Pg showed a characteristic absorption maximum at 615 nm in alkaline solution) might be used as an indicator reflecting the soil environment in the past. However, the use of the Pg absorption strength is not appropriate in the case of humus-rich soils because a brown fraction that differs from Pg strongly interferes with Pg absorption (CitationWatanabe and Kobayashi 2001). In contrast, the method for estimating the content of CEGF in soil can be applied to various soils including humus-rich soils. Although more detailed studies examining the relationship between the contents of CEGF and Pg should be carried out, it might be possible to predict the soil environment in the past more precisely using the CEGF content and Pg absorption strength in combination.
Conclusion
In the present study, the vertical distribution of a soil green pigment in an Andosol profile was reported for the first time quantitatively by determination of the CEGF content. CEGF occurred not only in the A horizon, but also in the buried A and remaining horizons (B and BC horizons). The CEGF content in the A horizon of the Andosol was higher than that in the Cambisol. The content of CEGF was highest in the surface or subsurface horizon in the soil profiles that did not contain a buried humus horizon. In contrast, the maximum content of CEGF was observed in the buried humus horizon when the soil profiles contained this horizon. Some minerals, such as Ald, Feo and, in particular, Alo, are considered to be associated with the distribution of CEGF in the soil profile. In addition, it was revealed that the properties of CEGF differed among the soil samples.
Further studies examining the quantitative distribution of CEGF in soils should be carried out to emphasize the importance of the soil green pigments, including Pg.
ACKNOWLEDGMENT
The authors thank Dr Takeshi Suzuki, Faculty of Agriculture, Kobe University, for classification of humic acids.
REFERENCES
- Kobayashi , T , Asakawa , D Yanagi , Y . 2005 . Method for estimating the content of the chloroform-extractable green fraction (CEGF) in HCl-DMSO extract of soils . Soil SciPlant Nutr , 51 : 779 – 786 .
- Kumada , K . 1987 . Chemistry of Soil Organic Matter , Tokyo : Japan Scientific Society Press .
- Watanabe , A , Fujimori , H , Nagai , Y , Miyajima , T and Kuwatsuka , S . 1996 . Analysis of the green fraction of humic acids . EurJSoil Sci , 47 : 197 – 204 .
- Zancada , MC , Almendros , G and Ballesta , RJ . 2003 . Humus quality after eucalypt reforestations in Asturias (Northern Spain) . SciTotal Environ , 313 : 245 – 258 .
- Kumada , K and Hurst , HM . 1967 . Green humic acid and its possible origin as a fungal metabolite . Nature , 214 : 631 – 633 .
- Sato , O . 1976b . A green pigment similar to the Pg fraction of P type humic acids and related compounds produced by litter-decomposing fungi . Soil SciPlant Nutr , 22 : 269 – 275 .
- Valmaseda , M , Martinez , AT and Almendros , G . 1989 . Contribution by pigmented fungi to P-type humic acid formation in two forest soils . Soil BiolBiochem , 21 : 23 – 28 .
- Kumada , K and Sato , O . 1980 . Characteristics of the green fraction of P type humic acid . Soil SciPlant Nutr , 26 : 309 – 316 .
- Nakabayashi , K and Wada , H . 1991 . Factors controlling Pg content in a dark brown forest soil . Soil SciPlant Nutr , 37 : 93 – 99 .
- Schnitzer , M and Skinner , SIM . Gel filtration of fulvic acid, a soil humic compound . Isotopes and Radiation in Soil Organic Matter Studies. Proc. Symp . pp. 41 – 55 . Vienna : International Atomic Agency .
- Tsutsuki , K and Kuwatsuka , S . 1989 . Degradation and stabilization of humus in buried volcanic ash soils. I. Humus composition, molecular size distribution of humic acids, and sugar composition of soils . Soil SciPlant Nutr , 35 : 207 – 216 .
- Sato , O . 1974 . Methods for estimating Pg content in P type humic acid and for calculating Δlog K of its Pb fraction . Soil SciPlant Nutr , 20 : 343 – 351 .
- Suzuki , T , Fujitake , N , Ueda , Y and Oji , Y . 1999b . Vertical distribution of main soil hydroxyanthraquinones in soil profiles . Soil SciPlant Nutr , 45 : 551 – 561 .
- Suzuki , T , Fujitake , N and Oji , Y . 1999a . Horizontal distribution of main anthraquinones in soil . Soil SciPlant Nutr , 45 : 297 – 306 .
- Berstch , PM and Bloom , PR . 1996 . “ Aluminum ” . In Methods of Soil AnalysisPart 3Chemical Methods, Soil Science Society of America, Book Series: 5 , Edited by: Sparks , D , Page , A Helmke , P . 517 – 550 . Madison : Soil Science Society of America .
- Oba , Y . 1964 . Methodology for the study of soil humus. II. Kobo and Oba's method . Pedologist , 8 : 108 – 116 . (in Japanese)
- Ikeya , K and Watanabe , A . 2003 . Direct expression of an index for the degree of humification of humic acids using organic carbon concentration . Soil SciPlant Nutr , 49 : 47 – 53 .
- Kumada , K , Sato , O , Ohsumi , Y and Ohta , S . 1967 . Humus composition of mountain soils in central Japan with special reference to the distribution of P type humic acid . Soil SciPlant Nutr , 13 : 151 – 158 .
- Sato , O . 1976a . The distribution of Pg (green fraction of P type humic acid) and the degree of humification of Pb (brown fraction of P type humic acid) in soils of central Japan . Soil SciPlant Nutr , 22 : 159 – 167 .
- Dahlberg , A , Jonsson , L and Nylund , JE . 1997 . Species diversity and distribution of biomass above and below ground among ectomycorrhizal fungi in an old-growth Norway spruce forest in south Sweden . CanJBot , 75 : 1323 – 1335 .
- Watanabe , M , Kado , T , Ohta , H and Fujitake , N . 2002 . Distribution and development of sclerotium grains as influenced by aluminum status in volcanic ash soils . Soil SciPlant Nutr , 48 : 569 – 575 .
- Nakabayashi , K , Wada , H and Takai , Y . 1982 . Distribution patterns of Pg fraction of P-type humic acid and iron in a dark brown forest soil . Soil SciPlant Nutr , 28 : 217 – 223 .
- Watanabe , M and Kobayashi , T . 2001 . Existing state and forming environment of humic acid Pg fraction in tephra soil sequence of southern Kyushu, Japan . QuatRes , 40 : 19 – 28 . (in Japanese with English summary)
- Watanabe , M . 1992 . Environmental changes clarified by humus properties of volcanic ash soils and their shift of zonal distribution . SciTotal Environ , 117/118 : 293 – 304 .