Abstract
Two hundred and seventy five bacterial strains were isolated from the floodwater of a Japanese paddy field during rice cultivation and the cells from 205 of these strains were negative for Gram stain. One hundred and nineteen isolates negative for Gram stain were randomly chosen and subjected to sequencing of partial 16S rDNA to compare with DNA clones obtained from floodwater using the polymerase chain reaction-denaturing gradient gel electrophoresis (PCR-DGGE) method. The isolates belonged to α-, β-, and γ-Proteobacteria and the Cytophaga–Flavobacterium–Bacteroides (CFB) group as well as high-GC and low-GC Gram-positive bacteria, with proportions of 47.9, 7.6, 11.8, 13.4, 4.2 and 15.1%, respectively. Although their DNA sequences did not match the sequences of the DNA clones obtained from the floodwater of a neighboring paddy field, some isolates showed identical DNA sequences to DNA clones from rice straw in soil and rice roots. Phylogenetic distribution of the isolates was wider than that of the DNA clones obtained from the floodwater using the DGGE method. As the CFB cluster consisted exclusively of the isolates and the DNA clones obtained from the floodwater and from microcrustaceans, and the other isolates shared the α-, β- and γ-Proteobacterial clusters with the DNA clones associated with rice straw in soil, microcrustaceans, percolating water and rice roots, bacterial communities in the floodwater were estimated to include floodwater-specific members as well as members associated with other habitats in the paddy fields.
INTRODUCTION
Paddy fields are characterized by floodwater overlying the field surface during the period of rice cultivation. Although floodwater is a site where organic matter is actively produced by phytoplankton, algae and submerged weeds, it is also an area where organic matter is consumed by zooplankton and aquatic animals and decomposed by microorganisms.
There are few studies examining bacterial populations in the floodwater of paddy fields. Culturable bacterial populations were in the orders of 108 and 106 c.f.u. L−1 for bacteria and actinomycetes, respectively, in a Madras paddy field in India (CitationRangaswami and Narayanaswami 1965), 105−107 c.f.u. L−1 for aerobic bacteria and 104−106 c.f.u. L−1 for anaerobic bacteria in a Miyagi paddy field in Japan (CitationWakao 1973), and 108−109 c.f.u. L−1 for aerobic bacteria in Tokyo Metropolis and Miyagi paddy fields (CitationFurusaka 1978; CitationSuzuki 1967). In addition, CitationMatsuguchi et al. (1976) enumerated N2-fixing bacteria in the floodwater of 40 Thai rice fields belonging to 9 different soil groups using the dilution frequency method, and recorded values ranging from < 1 to 103 c.f.u. L−1 for the Azotobacter members, from 101 to 104 c.f.u. L−1 for the Clostridium members and from 101 to 103 c.f.u. L−1 for the non-sulfur purple bacterial members. However, no phylogenetic studies have been conducted on bacterial populations in floodwater.
In general, culture methods can only detect 5% or less of the total bacterial inhabitants in natural environments. For example, CitationOkabe et al. (2000a) detected 8.4 × 108 to 1.7 × 109 bacterial cells L−1 and 1.5 × 107 to 8.5 × 107 c.f.u. L−1 of bacteria in the floodwater of a Japanese rice field in Aichi Prefecture. Thus, the proportion of culturable bacteria to total bacteria was 1–5% in floodwater, although soil bacteria detected by culture methods were reported to have accounted for 5–52% of the phylogenetic types of environmental clones (CitationHengstmann et al.1999).
To overcome the drawback of culturing limited members and the bias introduced by the culture methods, molecular biological and biochemical methods, such as polymerase chain reaction-denaturing gradient gel electrophoresis (PCR-DGGE), followed by sequencing (CitationAmann et al. 1995), phospholipid fatty acid (PLFA) pattern analysis (CitationZelles 1997) and quinone profile analysis (CitationKatayama and Fujie 2000), which do not depend on culture, have been devised for identifying the predominant microorganisms in environmental communities. Phospholipid fatty acid pattern analysis revealed that Gram-negative bacteria predominated in the floodwater of Japanese paddy fields (CitationOkabe et al. 2000a,Citationb; CitationShimizu et al. 2002). CitationShibagaki-Shimizu et al. (in press) also reported that the Cytophaga–Flavobacterium–Bacteroides (CFB) group, β-Proteobacteria and actinobacteria predominated in the floodwater of a Japanese paddy field using the PCR-DGGE method followed by sequencing of the characteristic DGGE bands.
Molecular biological approaches such as the PCR-DGGE method followed by sequencing are not perfect as intrinsic bias occurs because of the differences in the efficiencies of DNA extraction and PCR amplification among bacteria (CitationJosephson et al. 1993; CitationSuzuki and Giovannoni 1996; CitationWang and Wang 1997). Large differences in the phylogenetic distribution of bacteria in the pinyon rhizosphere and neighboring soil were observed using culture-identification methods and DNA sequencing methods (CitationDunbar et al. 1999). Therefore, it is necessary to confirm, using culture methods, whether bacteria that display the same DNA sequences as those found in environmental DNA predominate in floodwater samples.
In the present study, we first determined partial 16S rDNA sequences in a variable region (V3; Escherichia coli positions between 357 and 517) of 119 bacterial strains that were isolated from the floodwater of a Japanese paddy field, and revealed their phylogenetic positions. Partial sequences of this region were sufficient to determine phylogenetic positions at the genus level (CitationKurisu 1999). Second, we examined the phylogenetic relationships between the isolates and the DNA clones that were investigated by CitationShibagaki-Shimizu et al. (in press), and we compared the diversity of the isolates with that of the DNA clones in the floodwater.
MATERIALS AND METHODS
Study site
The study field was located at the Aichi-ken Anjo Research and Extension Center, Central Japan (E2 field; latitude 34°48′N, longitude 137°30′E). The soil of the field was an Anthraquic Yellow Soil (Oxyaquic Dystrudept). Some of the soil properties are as follows: total C content 13.3 g kg−1; total N content 0.9 g kg−1; pH (H2O) 6.0; pH (KCl) 4.9. Rice plants (Oryza sativa L. cv. Koshihikari) were cultivated using conventional methods. Detailed field management was previously described by CitationOkabe et al. (2000a). Basal application of fertilizer was carried out on 21 April 1997 at the rates of 21 kg N ha−1, 24 kg P2O5 ha−1 and 21 kg K2O ha−1. Topdressing of fertilizer was carried out on 7 July and 16 July at rates of 16 kg N ha−1 and 16 kg K2O ha−1. Irrigation water was introduced from the Meiji irrigation canal.
Sampling of floodwater
Sampling of the floodwater was carried out six times during the rice cultivation in 1997 on 6 May (13 days after flooding), 23 May (30 days after flooding), 12 June (one day before mid-season drainage), 4 July (10 days after mid-season drainage), 28 July and 16 August (11 days before harvest). Sampling was done between 10:30 am and 11:30 am. Two-liter water samples were collected carefully to avoid disturbing the surface soil with a measuring cup from five points in the field. A 10 L composite sample was passed through a 46-m mesh sieve in the field to remove large aquatic organisms and particles. The floodwater sample was transported to the laboratory in ice water and centrifuged at 8,000 g for 10 min. The supernatant was subjected to bacterial isolation immediately.
Isolation of bacteria
The floodwater sample was mixed well and an appropriate amount of the sample was diluted 103-fold with 0.85% of sterilized physiological saline solution. One loopful dilution was spread onto 1/100 strength nutrient broth (NB) agar in a Petri dish (1/100 NB; beef extract 0.03 g, peptone 0.1 g, NaCl 0.05 g L−1 distilled water, pH 7.0) and the plates were incubated for 10 days at 30°C. Bacterial colonies that appeared on the agar medium were randomly transferred to 1/100 NB agar using a sterile needle and inoculated. The bacterial isolates were purified by streaking again on 1/100 NB. Approximately 50 isolates were obtained from each sampling time. Finally, 275 bacterial isolates were successfully purified and preserved in a 20% glycerol solution at −80°C.
Gram staining
Gram staining was conducted according to Hucker's modified method (CitationSociety of American Bacteriologists 1957).
PCR amplification
16S rDNA was amplified with PCR using the primer set for eubacteria, 357f-GC clamp (E. coli position: 341–357, 5′-CGC CCG CCG CGC GCG GCG GGC GGG GCG GGG GCA CGG GGGG CCT ACG GGA GGC AGC AG-3′, the underlined sequence corresponded to the GC clamp) and 517r (5′-ATT ACC GCG GCT GCT GG-3′, E. coli position: 517–534) (CitationMuyzer et al. 1993). DNA template solution used in this reaction was prepared by suspending one loopful of colony into autoclaved ultra-pure water (50 µl). PCR was carried out in a total reaction solution of 50 µL in a 200 µL-microtube that contained 1 µL of each primer (50 pmol each), 4 µL of 2.5 mmol L−1 dNTP mixture, 5 µL of 10X Ex Taq buffer (20 mMg2+ Takara, Tokyo, Japan), 0.25 µL of Ex Taq DNA polymerase (Takara) and 38.75 µL of DNA templates.
Sequencing
The PCR products were sequenced using the Thermo Sequenase TM Dye Terminator Cycle Sequencing Kit (Amersham, Tokyo, Japan), according to the manufacturer's instructions, by using the set of two primers, 357f (no GC clamp) and 517r, with a 373S DNA sequencer (ABI, Urayasu, Japan).
Phylogenetic analysis
The closest relatives corresponding to the 16S rDNA sequences of the bacterial isolates were determined using the BLAST search program and the database of the DNA Data Bank of Japan (DDBJ) (http://www.ddbj.nig.ac.jp/). A phylogenetic tree was constructed by 100-fold bootstrap analysis according to the neighbor-joining method using the nj plot and the Tree ViewPPC (version 1.6.6.Developmental) software.
Nucleotide sequence accession number
The 16S rDNA partial sequences obtained in the present study are available in the DDBJ databases under the accession numbers of AB202126–AB202244. Strain name was designated with the date and number of the isolate (e.g. strain 052301 isolate No.1 obtained on 23 May).
RESULTS AND DISCUSSION
Gram staining of isolated bacteria
Cells from 205 of the 275 bacterial isolates from the floodwater were Gram-negative. The proportion of isolates negative for Gram stain (hereafter referred to as “Gram-negative isolates” was significantly higher than that of Gram-positive isolates (P < 0.01) and ranged from 53% to 95% during the period of rice cultivation. These findings indicated the predominance of Gram-negative bacteria in the floodwater.
Phylogenetic positions of bacterial isolates and their relationships with the DNA clones obtained from the floodwater
As the Gram-negative bacteria predominated in the floodwater, phylogenetic positions of the Gram-negative isolates were determined by sequencing of the 16S rDNA of the isolates. Partial 16S rDNA sequences were determined for 119 isolates that were randomly selected from the 205 Gram-negative isolates. The isolates belonging to α-, β- and γ-Proteobacteria and the Cytophaga–Flavobacterium–acteroides (CFB) group (Gram-negative bacteria) accounted for 80.7% of the total, with 47.9, 7.6, 11.8 and 13.4%, respectively. The other isolates consisted of 4.2% and 15.1% of low-GC and high-GC Gram-positive bacteria, respectively. As some of the isolates showed identical DNA sequences with those of other isolates, 38 out of 57 strains, 6 out of 9 strains, 15 out of 16 strains and 13 strains eventually displayed different DNA sequences from those of α- and β-Proteobacteria, CFB group, and γ-Proteobacteria, respectively. CitationShibagaki-Shimizu et al. (in press) identified only the β-Proteobacteria and CFB group as Gram-negative bacteria and did not detect α- and γ-Proteobacteria from the clone analysis, whereas the present culture method enabled isolation of α- and γ-Proteobacteria in addition to β-Proteobacteria and CFB group from the floodwater.
Twenty-three low-GC and high-GC Gram-positive bacteria were included in the Gram-negative isolates. Therefore, the proportion of Gram-negative bacteria changed from 74.5% (205/275) to 60.1% (205/275 × 96/119). Gram-negative bacteria predominated over Gram-positive bacteria significantly as a whole (P < 0.01). The proportions of Gram-negative bacteria were 63.9% on 6 May, 45.1% on 23 May, 19.4% on 12 June, 58.6% on 4 July, 84.8% on 28 July and 81.8% on 16 August. CitationShibagaki-Shimizu et al. (in press) investigated the bacterial communities of floodwater in paddy fields with a long-term fertilizer trial in the vicinity of the present field using PCR-DGGE methods. The fields were irrigated with water from the Meiji irrigation canal in the same way as the field in the present study. Floodwater samples were collected at the same time in the morning with a similar interval during the rice cultivation period. CitationShibagaki-Shimizu et al. (in press) reported that the proportion of Gram-positive
Table 1 Phylogenetic distribution of 119 Gram-negative isolates at the family or genus level
Identification of low-GC and high-GC Gram-positive bacteria from the Gram-negative isolates might have occurred because the Gram stain dose not enable differentiation of old cells of Gram-positive bacteria, such as Arthrobacter, Agromyces and Microbacterium, as Gram-negative ones (CitationCollins and Bradbury 1992; CitationJones and Keddie 1992) and that some members such as Bacillus circulans are Gram-negative (CitationSmith et al. 1952). A number of Gram-positive bacteria such as Arthrobacter, Agromyces and Microbacterium were included in the Gram-negative isolates.
Phylogenetic positions of the isolates at the family or genus level are shown in . Forty-one isolates were identified as Sphingomonas spp., followed by 7 isolates as Cytophaga, 4 isolates as Rhodobacter, 4 isolates as Ralstonia, 4 isolates as members of Flavobacteriaceae and 3 isolates as Chryseobacterium. Members belonging to Sphingomonas and the CFB group, such as Flavobacterium, Cytophaga and Chryseobacterium, are common inhabitants of aquatic environments (CitationHolmes 1992), and Sphingomonas spp. are known to adapt to oligotrophic and starvation conditions (CitationBalkwill et al. 2003). Many isolates belonging to γ-Proteobacteria are common in the soil environment (CitationJanda 2002; CitationPalleroni 1992; CitationTowner 1992). These results suggest that bacterial members that are common in oligotrophic environments, such as freshwater and soil, also inhabited the floodwater.
Changes in the composition of isolates in the floodwater during rice cultivation
Seasonal changes in the bacterial composition were observed for Gram-negative bacteria (). α-Proteobacteria appeared constantly and tended to predominate during the late period of rice cultivation. All of the isolates of Gram-negative bacteria on 28 July were α-Proteobacteria, and almost all isolates (92.6%) belonged to Sphingomonas. As shown in , some members of Proteobacteria appeared only at specific times: Ralstonia on 6 May and Azospirillum and Luteimonas on 16 August. Different species of γ-Proteobacteria appeared during rice cultivation, except on 28 July (). Although CFB members dominated the floodwater in the study using PCR-DGGE analysis, followed by sequencing of major DNA bands (CitationShibagaki-Shimizu et al. in press), they only appeared on 3 out of 6 sampling occasions.
These findings indicate that the bacterial inhabitants of the floodwater changed during the period of rice cultivation. However, factors responsible for these changes have not been elucidated in the present study. Further studies examining the physiological characteristics of the isolates should be carried out to explain the changes.
Phylogenetic relationships between isolates from the floodwater and DNA clones obtained from the paddy field ecosystem
shows the phylogenetic positions of the Gram-negative isolates in the present study and their relationships with DNA clones obtained from floodwater (CitationShibagaki-Shimizu et al. in press), rice straw in soil
Figure 1 Seasonal changes in the composition of Gram-negative bacteria in floodwater during the rice cultivation period. Figures at the top of the columns indicate the number of Gram-negative bacteria examined for their 16S rDNA sequences. (![]()
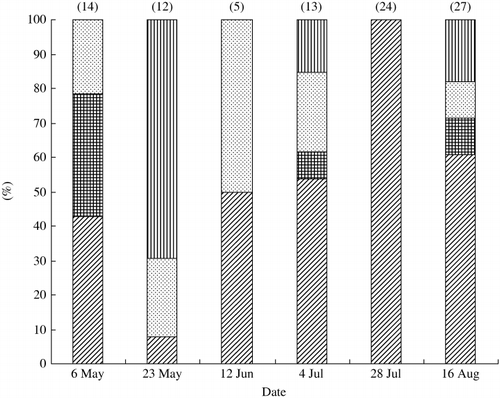
Table 2 Changes in the phylogenetic composition of bacterial isolates during the rice cultivation period
No isolates displayed a 16S rDNA sequence identical to that of the DNA clones from the floodwater. The DNA clones were selected from DGGE bands with a strong intensity and/or with continuous presence during the growth period of rice by estimating their predominance among the bacterial communities in floodwater (CitationShibagaki-Shimizu et al. in press). The absence of isolates with an identical 16S rDNA sequence to that of the DNA clones was ascribed to the fact that very few members among the predominant bacterial species could be cultured in floodwater. In addition, the medium used for isolation (1/100 NB) enabled very few bacterial members among the floodwater inhabitants to grow selectively because most of the DNA clones in floodwater were closely related to uncultured bacteria (CitationShibagaki-Shimizu et al. in press). In contrast, two isolates showed an identical 16S rDNA sequence to that of the environmental clones, one obtained from rice straw in soil (a clone of α-Proteobacteria; CitationSugano et al. 2005) and the other from rice roots (a clone of β-Proteobacteria; CitationIkenaga et al. 2003). In addition, a number of isolates showed a high similarity to the DNA clones obtained from the paddy field components mentioned above (similarity values above 95% are shown in ). These findings indicate that it is possible to isolate bacteria with identical 16S rDNA sequences to those of the DNA clones in the paddy field ecosystem on an individual basis.
Although only a few members of the total bacterial community can, in general, be isolated from environments
Figure 2 Phylogenetic relationships between Gram-negative isolates from floodwater and DNA clones obtained from various sites in paddy fields. (•) isolates (present study); CP, composting process of rice straw; FW, floodwater; MC, microcrustaceans; PW, percolating water; RS, rice straw; RR, rice roots. The scale bar represents 0.1 substitutions per nucleotide. Bootstrap values are shown at branch points when the values exceed 50%.
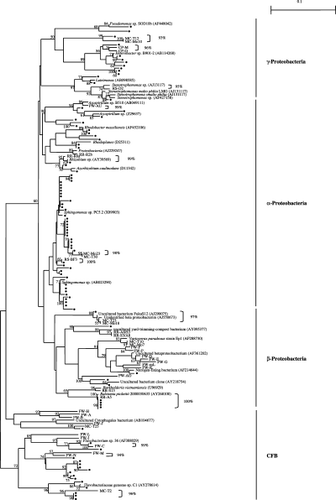
The CFB clusters consisted exclusively of isolates and DNA clones obtained from the floodwater and microcrustaceans (), indicating that members of the CFB group were characteristic of the bacterial communities in the floodwater. In contrast, many isolates and DNA clones obtained from rice straw in soil, microcrustaceans, percolating water and rice roots shared the same α-, β- and γ-Proteobacterial sub-clusters. Therefore, the bacterial communities in floodwater are considered to include members specific to floodwater as well as individuals associated with other habitats in paddy fields.
Notes
Present addresses: Home, 151-1, Kitanominami, Gifu 501-2516, Japan.
Graduate School of Bio-Applications and Systems Engineering, Tokyo University of Agriculture and Technology, Koganei 184-8588, Japan.
REFERENCES
- Rangaswami , G and Narayanaswami , R . 1965 . Studies on the microbial population of irrigation water in rice fields . IntRice CommNewsl , 14 : 35 – 42 .
- Wakao , N . 1973 . Ecological studies on sulfate-reducing bacteria in paddy-field soil . BullInstAgrResTohoku Univ , 24 : 177 – 224 . (in Japanese with English summary)
- Furusaka , C . 1978 . “ Microbiology in paddy soils ” . In Soil Sciences in Paddy Fields , Edited by: Kawaguchi , K . 201 – 227 . Tokyo : Kodansha . (in Japanese)
- Suzuki , T . 1967 . Characteristics of microorganisms in paddy field soils . JARQ , 2 : 8 – 12 .
- Matsuguchi , T , Bunharn , T and Somchai , P . 1976 . Flora and N2-fixing activities of free-living N2-fixing bacteria in Thai paddy fields . Soil Microorganisms , 18 : 7 – 19 . (in Japanese)
- Okabe , A , Oike , H , Toyota , K and Kimura , M . 2000a . Comparison of phospholipid fatty acid composition in floodwater and plow layer soil during the rice cultivation period in a Japanese paddy field . Soil SciPlant Nutr , 46 : 893 – 904 .
- Hengstmann , U , Chin , KJ , Janssen , PH and Liesack , W . 1999 . Comparative phylogenetic assignment of environmental sequences of genes encoding 16S rRNA and numerically abundant culturable bacteria from an anoxic rice paddy soil . ApplEnvironMicrobiol , 65 : 5050 – 5058 .
- Amann , RI , Ludwig , W and Schleifer , KH . 1995 . Phylogenetic identification and in situ detection of individual microbial cells without cultivation . MicrobiolRev , 59 : 143 – 169 .
- Zelles , L . 1997 . Phospholipid fatty acid profiles in selected members of soil microbial communities . Chemosphere , 35 : 275 – 294 .
- Katayama , A and Fujie , K . 2000 . “ Characterization of soil microbiota with quinone profile ” . In Soil Biochemistry , Edited by: Bollag , JM and Stotzky , G . Vol. 10 , 303 – 347 . New York : Marcel Dekker .
- Okabe , A , Toyota , K and Kimura , M . 2000b . Seasonal variations of phospholipid fatty acid composition in the floodwater of a Japanese paddy field under a long-term fertilizer trial . Soil SciPlant Nutr , 46 : 177 – 188 .
- Shimizu , M , Nakajima , Y , Matsuya , K and Kimura , M . 2002 . Comparison of phospholipid fatty acid composition in percolating water, floodwater, and the plow layer soil during the rice cultivation period in a Japanese paddy field . Soil SciPlant Nutr , 48 : 595 – 600 .
- Shibagaki-Shimizu , T , Nakayama , N , Nakajima , Y , Matsuya , K , Kimura , M and Asakawa , S . Phylogenetic study on a bacterial community in the floodwater of a Japanese paddy field estimated by sequencing 16S rDNA fragments after denaturing gradient gel electrophoresis . BiolFertilSoils , DOI: 10.1007/s00374-005-0041-x
- Josephson , KL , Gerba , CP and Pepper , IL . 1993 . Polymerase chain reaction detection of nonviable bacterial pathogens . ApplEnvironMicrobiol , 59 : 3513 – 3515 .
- Suzuki , MT and Giovannoni , SJ . 1996 . Bias caused by template annealing in the amplification of mixtures of 16S rRNA genes by PCR . ApplEnvironMicrobiol , 62 : 625 – 630 .
- Wang , GC and Wang , Y . 1997 . Frequency of formation of chimeric molecules as a consequence of PCR coamplification of 16S rRNA genes from mixed bacterial genomes . ApplEnvironMicrobiol , 63 : 4645 – 4650 .
- Dunbar , J , Takala , S , Barns , SM , Davis , JA and Kuske , CR . 1999 . Levels of bacterial community diversity in four arid soils compared by cultivation and 16S rRNA gene cloning . ApplEnvironMicrobiol , 65 : 1662 – 1669 .
- Kurisu , F . 1999 . “ Microbial community structure analysis for the thermophilic contact oxidation process ” . Japan : University of Tokyo . (PhD thesis)
- Society of American Bacteriologists . 1957 . Manual of Microbiological MethodSociety of American Bacteriologists , New York : McGraw Hill .
- Muyzer , G , Waal , ECD and Uitterlinden , AG . 1993 . Profiling of complex microbial populations by denaturing gradient gel electrophoresis analysis of polymerase chain reaction-amplified genes coding for 16S rRNA . ApplEnvironMicrobiol , 59 : 695 – 700 .
- Kimura , M , Shibagaki , T , Nakajima , Y , Matuya , K and Ikenaga , M . 2002 . Community structure of the microbiota in the floodwater of a Japanese paddy field estimated by restriction fragment length polymorphism and denaturing gradient gel electrophoresis pattern analyses . BiolFertilSoils , 36 : 306 – 312 .
- Collins , MD and Bradbury , JF . 1992 . “ The genera Agromyces, Aureobacterium, Clavibacter, Curtobacterium, and Microbacterium ” . In The Prokaryotes: A Handbook on the Biology of Bacteria: Ecophysiology, Isolation, Identification, Applications , 2nd edn , Edited by: Balows , A , Trüper , HG , Dworkin , M , Harder , W and Schleifer , KH . Vol. 2 , 1355 – 1368 . New York : Springer-Verlag .
- Jones , D and Keddie , RM . 1992 . “ The genus Arthrobacter ” . In The Prokaryotes: A Handbook on the Biology of Bacteria: Ecophysiology, Isolation, Identification, Applications , 2nd edn , Edited by: Balows , A , Trüper , HG , Dworkin , M , Harder , W and Schleifer , KH . Vol. 2 , 1283 – 1299 . New York : Springer-Verlag .
- Smith , NR , Gordon , RE and Clark , FE . 1952 . Aerobic spore-forming bacteria , Monograph No.16 US Department of Agriculture .
- Holmes , B . 1992 . “ The genera Flavobacterium, Sphingobacterium, and Weeksella ” . In The Prokaryotes: A Handbook on the Biology of bacteria: Ecophysiology, Isolation, Identification, Applications 2nd edn , Edited by: Balows , A , Trüper , HG , Dworkin , M , Harder , W and Schleifer , KH . Vol. 4 , 3620 – 3630 . New York : Springer-Verlag .
- Balkwill , DL , Fredrickson , JK and Romine , MF . 2003 . “ Sphingomonasand related genera ” . In The Prokaryotes , 3rd edn , Edited by: Dworkin , M . New York : Springer-Verlag . release 3.14., http://141.150.157.117:8080/prokPUB/chaprender/jsp/showchap.jsp?chapnum=314
- Janda , JM . 2002 . “ New members of the family Enterobacteriaceae ” . In The Prokaryotes , 3rd edn , Edited by: Dworkin , M . New York : Springer-Verlag . release 39, http://141.150.157.117:8080/prokPUB/chaprender/jsp/showchap.jsp?chapnum=375
- Palleroni , NJ . 1992 . “ Introduction to the family Pseudomonadaceae ” . In The Prokaryotes: A Handbook on the Biology of Bacteria: Ecophysiology, Isolation, Identification, Applications , 2nd edn , Edited by: Balows , A , Trüper , HG , Dworkin , M , Harder , W and Schleifer , KH . Vol. 3 , 3074 – 3085 . New York : Springer-Verlag .
- Towner , K . 1992 . “ The genus Acinetobacter ” . In The Prokaryotes: A Handbook on the Biology of Bacteria: Ecophysiology, Isolation, Identification, Applications , 2nd edn , Edited by: Balows , A , Trüper , HG , Dworkin , M , Harder , W and Schleifer , KH . Vol. 4 , 3137 – 3143 . New York : Springer-Verlag .
- Sugano , A , Tsuchimoto , H , Tun , CC , Asakawa , S and Kimura , M . 2005 . Succession and phylogenetic profile of eubacterial communities in rice straw incorporated into a rice field: Estimation by PCR-DGGE analysis . Soil SciPlant Nutr , 51 : 51 – 60 .
- Ikenaga , M , Asakawa , S , Muraoka , Y and Kimura , M . 2003 . Bacterial communities associated with nodal roots of rice plants along with the growth stages: Estimation by PCR-DGGE and sequence analyses . Soil Sci Plant Nutr , 49 : 591 – 602 .
- Niswati , A , Murase , J and Kimura , M . 2005 . Comparison of bacterial communities associated with microcrustaceans from the floodwater of a paddy field microcosm: Estimation based on DGGE pattern and sequence analyses . Soil SciPlant Nutr , 51 : 281 – 290 .
- Murase , J , Shimizu , M , Hayashi , M , Matsuya , K and Kimura , M . 2005 . Vertical changes in bacterial communities in percolating water of a Japanese paddy field as revealed by PCR-DGGE . Soil SciPlant Nutr , 51 : 83 – 90 .
- Present addresses: Home, 151-1, Kitanominami, Gifu 501-2516, Japan.
- Graduate School of Bio-Applications and Systems Engineering, Tokyo University of Agriculture and Technology, Koganei 184-8588, Japan.