Abstract
Effects of parent rocks and types of land use on soil physical and chemical properties and the phosphorus (P) accumulation in surface soil were examined in a small watershed. The soil developed from granite with a coarse texture, and felsic chemical and mineralogical compositions showed a coarser texture, lower contents of organic matter, water-soluble cations and anions and a lower cation exchange capacity (CEC) than soil developed from andesite. Farmland soils exhibited higher pH and electrical conductivity values, a lower ignition loss and CEC and a coarser texture compared with forest soils from the same parent rocks. The farmland soils contained greater amounts of total P, water-soluble reactive P, organic P and adsorbed inorganic P, and showed a higher degree of P saturation (DPS) than the forest soils in the following order: upland field > orchards > paddy fields > forest. The DPS of the farmland soils (28.2–93.1%) indicated that the soils showed a high potential to release P in the surface water and that careful management was required to reduce P release for the preservation of surface water quality.
INTRODUCTION
Degradation of soil quality in farmlands has been a worldwide agricultural and environmental concern (CitationWienhold et al. 2004). Soil quality degradation can lead to erosion, a decline in fertility, changes in aeration and moisture content, salinization or changes in the soil flora and fauna (CitationBarrow 1991). The erosion rate of soil increased and the contents of organic carbon (C) and total nitrogen (N) and the cation exchange capacity (CEC) were significantly reduced when grasslands changed to farmlands (CitationWu and Tiessen 2002). CitationMcGrath et al. (2001) studied the nutrient dynamics of soils after the conversion of native forest to agricultural land by slash and burn in the Amazon basin. According to their study, the pH, effective cation exchange capacity (ECEC) and the amount of exchangeable calcium (Ca) in soil increased after land conversion, whereas the contents of total C, N and water-soluble inorganic phosphorus (P) of the soils decreased.
In addition to the degradation of soil quality by cultivation, the transport of applied nutrients for agricultural production from farmlands to surface water leads to the deterioration of water quality (CitationSchroeder et al. 2004). Among the nutrients transported from farmlands to surface water, P has attracted the interest of soil and environmental scientists because it has been recognized as a limiting factor of eutrophication of surface water. Eutrophication can impair water use for drinking, recreation, aquaculture and industry because of the increased growth of undesirable algae and aquatic weeds.
Soil P occurs in various forms, namely water-soluble, and labile and non-labile in solid phase forms, in terms of biological availability (CitationHartkainen 1991). Inorganic P in non-calcareous and acidic soils is dominated by hydrous sesquioxides and aluminium (Al) and iron (Fe) compounds, whereas the Ca compound of P is the predominant phase in calcareous and alkaline soils. The loss of P from agricultural land to surface water occurs via erosion, runoff and subsurface drainage (CitationBorling 2003). The most important aspect of the loss of P from farmlands in terms of surface eutrophication is the
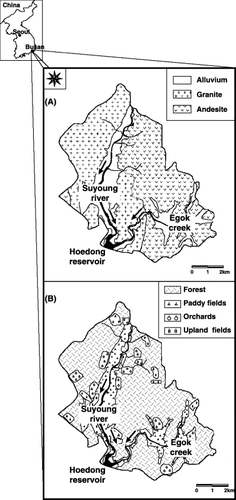
Figure 1 Location, geological (A) and land-use (B) conditions of the study site. Arrows indicate the flow direction of stream water.
Most farmlands in Korea have been cultivated for a considerable period of time and excessive amounts of fertilizers have been applied to the farmlands, particularly during the 1970s to 1990s. Accumulated nutrients in farmland soils are one of the major sources of surface water eutrophication. The objectives of the present study were to examine the changes in soil physical and chemical properties and to examine the P accumulation in surface soil in relation to parent rocks and land-use types in a small watershed.
MATERIALS AND METHODS
Study site and sampling
The study site was a small watershed located in the northern part of Busan, Korea (). The stream drained a total catchment area of 66 km2 with an altitudinal range of 45–720 m and flowed to the Hoedong reservoir. Mean annual precipitation was 1490 mm and 50–60% of the precipitation occurred during summer (July–August). Mean annual air temperature was 14.4°C and mean annual soil temperature at a 10 cm depth was 15.3°C. Seventy percent of the surface area was used for forest, 15% for paddy fields, 8% for orchards, 6% for upland fields and 1% for residential areas and roads. The forest consisted of a mixture of pine trees and deciduous trees. The major crops were sesame and green perilla, pepper, carrot and Chinese cabbage for the upland fields, and plum and peach for the orchards. The bedrock of the study site consisted of Cretaceous granite and andesite. The soil series were designated as followings: Samgag for forest, Pungcheon for paddy fields and Anryong for upland fields and orchards (granite); Seogto for forest, Pangog for paddy fields and Chilgog for upland fields and orchards (andesite). All the soil series were classified as Inceptisols (CitationNational Institute of Agricultural Science and Technology and Rural Development Administration, Korea 2000).
Hoedong reservoir, to which all the streams drained in the watershed, had been used for the supply of drinking water to the Busan metropolitan area and the city authorities strictly controlled the contamination sources in the watershed to preserve the surface water quality. However, the Hoedong reservoir experiences a serious algal blooming every summer, which is one of the current environmental issues in Busan. Recently, environmental scientists and city authorities recognized that the eutrophication of the Hoedong reservoir was mainly associated with the transport of nutrients from the farmlands to the reservoir in the watershed.
One hundred and twenty eight samples of surface soil (< 30 cm in depth) were collected from the study site. Approximately 15 samples were collected for each land-use type, including forest, paddy fields, orchards and upland fields for the two parent rocks in early March before sowing to minimize temporal variations in nutrient status. The collected samples were air-dried and gently ground with a rubber stopper to pass through a 2 mm mesh sieve. Three fresh rock samples for each parent rock were collected from outcrops in the watershed. The collected rock samples were finely ground using an agate mortar and pestle for chemical and mineralogical analyses. Three ground rock samples for each parent rock were mixed at the same ratio before analysis.
Analysis of samples
The chemical composition of the parent rocks was determined using an X-ray fluorescence spectrometer (XRF-1700 Shimadzu, Tokyo, Japan). The clay fraction of the soil samples was separated by wet sieving and centrifugation after removal of organic matter, carbonates and iron oxides (CitationJackson 1956). Mineralogical composition of the parent rocks, bulk soil samples and clay fractions was determined using an X-ray diffractometer (MXP 18A Rint-2500 MAC Science, Billerica, MA, USA). Ignition loss of the bulk soil samples as an indicator of organic matter content was determined after heating at 550°C for 1 h.
Five grams of soil and 50 mL of distilled water were shaken in a centrifuge tube for 1 h and the suspension was centrifuged and filtered using a 0.45 µm membrane filter. The pH and electrical conductivity (EC) of the filtrates were determined using an Orion pH meter and an Orion EC meter, respectively (Orion, Beverly, MA, USA). The concentrations of Cl−, F−, NO− 3 and SO2− 4 in the filtrates were determined using an ion chromatograph (Dionex DX-120 Sunnyvale, CA, USA). The concentrations of K+, Na+, Ca2+ and Mg2+ in the filtrate were determined with an inductively coupled plasma atomic emission spectrometer (ICP-AES) (JOBIN YVON JY70plus Geoplasma BJY70 Longjumeau Ceder, France). The CEC of the soil samples was determined using the Ca–Mg method (CitationJackson 1956).
Organic P in the soils was determined using the ignition method (CitationPage et al. 1982). Concentration of organic P in the soils was calculated by subtraction of the P content extracted using 1 N H2SO4 from the unheated sample from the P content extracted using 1 N H2SO4 from the heated sample at 550°C for 2 h. Inorganic P in the soil samples was fractionated into various forms, namely water-soluble reactive, adsorbed, carbonate, occluded in Fe oxides and residual forms using a sequential extraction method (CitationPage et al. 1982): (1) distilled water for the water-soluble reactive form, (2) 0.1 N NaOH−1 mol L−1 NaCl for the adsorbed form, (3) 0.3 mol L−1 Na citrate−1mol L−1 NaHCO3 for the carbonate form, (4) 0.3 mol L−1 Na citrate–Na2S2O4 for the occluded form in Fe-oxides, (5) 1 N HCl for the residual form. Phosphorus concentration in the solutions was determined using the ascorbic acid method (CitationWatanabe and Olsen 1965).
Soil (0.5 g) and 20 mL of pH 3 ammonium oxalate were reacted in a centrifuge tube in a dark room for 2 h, afterwhich the suspension was filtered with a 0.45 mm membrane filter (CitationPote et al. 1996). Concentrations of Al, Fe and P in the solutions were determined using ICP-AES. The degree of P saturation (DPS) of the soil samples was calculated using the following equation:
where α is a constant for the soil P adsorption capacity determined in a P adsorption experiment (a ranged from 0.3 to 0.7 for most soils). We used a = 0.5 in the absence of a P adsorption experiment in the present study (CitationPautler and Sims 2000). All chemicals used in this study were reagent grade and the chemical and physical analyses of the soil samples were conducted in duplicate.
To evaluate differences in the soil characteristics associated with the parent rock and land-use types, soil chemical data including ignition loss, pH and EC values, concentrations of cations, anions, inorganic P, organic P and total P, and DPS for the parent rocks and land-use types were subjected to two-way anova to calculate the Least Significant Difference (LSD) at the 95% confidence level (CitationSAS Institute 1989).
RESULTS AND DISCUSSION
Characteristics of soils
The chemical and mineralogical composition of the parent rocks is shown in . Andesite contained larger amounts of Fe2O3 and MgO2 and a smaller amount of SiO2 than granite. In addition, andesite contained a significant amount of mafic minerals such as amphibole and chlorite. Based on visual observation, andesite displayed a finer texture than granite. Selected physical,
Table 1 Chemical and mineralogical composition of the parent rocks
Table 2 Selected physical, chemical and mineralogical properties of the soils
The physical, chemical and mineralogical properties of the parent rocks were directly reflected on the soil properties. Mafic minerals are known to be more weatherable than felsic minerals during the soil genesis process (CitationDrever 1984). Therefore, the soil developed from andesite with finer texture and significant amounts of mafic minerals showed finer texture and a higher EC value than the soil developed from granite with a coarser texture and felsic minerals. The higher CEC of the soil developed from andesite might result from the higher content of organic matter, indicated by the ignition loss and the presence of vermiculite and interstratified mineral of vermiculite and mica as weathering products of mica.
Farmland soils, including soils from paddy fields, orchards and upland fields, showed a higher pH value, lower ignition loss and CEC, and a coarser texture than forest soils (). The higher pH of the farmland soils was the result of higher Ca concentration associated with fertilizer application. The cultivation and harvest of farmlands might lead to acceleration in the oxidation of organic matter and to a reduction of the input of organic matter into soil, resulting in a lower content of organic matter (CitationWu et al. 2004). Tillage and low vegetation coverage of the farmlands also led to a higher erosion rate than that observed for forest soils (CitationWu and Tiessen 2002). In addition, the reduced organic matter content and the erosion of fine particles of farmland soils were responsible for their lower CEC.
The soils from the orchards and upland fields displayed higher pH and EC values, and higher contents of cations and NO− 3 than the paddy field soils. The paddy fields were flooded during the rice-growing season for approximately 6 months. Flooding led to leaching of cations and anions from the surface soil to the subsurface, as well as to reduced conditions in the surface soil resulting in a reduction in NO− 3.
The EC values (0.085–0.337 dS m−1) of the farmland soils in the watershed in the present study indicated that accumulated salts may not be a cause of serious salt stress for the crops, although the farmlands had been cultivated and had received an excessive amount of fertilizer over a long period of time (CitationNissen and Wander 2003; CitationShani and Dudley 2001). This might be because of relatively high precipitation in the watershed (mean annual precipitation of 1490 mm). The high precipitation may decrease the salt accumulation in surface soils by leaching and runoff. The high concentration of
Table 3 Total and fractions of phosphorus (P) in the soils
Table 4 Contents of oxalate-extractable aluminium (Al), iron (Fe) and phosphorus (P), and the degree of P saturation (DPS) of the soils
Phosphorus accumulation
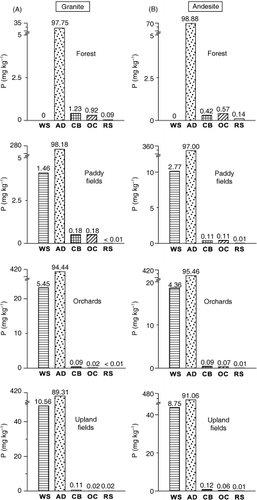
The forest soil developed from andesite contained a greater amount of total P than the soil developed from granite (), which corresponded to the P content of the parent rocks (). No water-soluble reactive P was detected in the forest soils. The amounts of water-soluble reactive P, adsorbed P, organic P and total P in the soils were in the following order: upland fields > orchards > paddy fields > forest. The content of organic P in the soils ranged from 43% to 82% of total soil P. Adsorbed P determined by sequential extraction was the dominant form of inorganic soil P, accounting for more than 97% of the inorganic P (). The higher P content of the farmland soils compared with the forest soils might be because of the addition of P in the chemical and organic fertilizers. Accumulated P in the soils associated with fertilizer application remained in organic and adsorbed forms (, ).
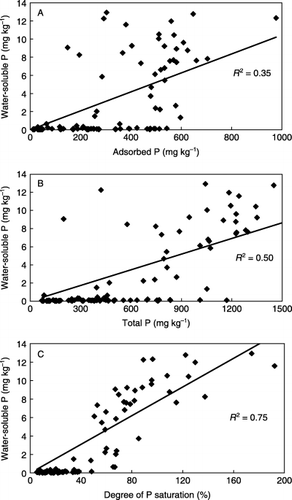
The soils developed from andesite exhibited higher concentrations of oxalate-extractable Al and Fe than those developed from granite, regardless of the land-use types (), also reflecting the chemical composition of the parent rocks. The farmland soils contained a higher concentration of oxalate-extractable P than the forest soils, regardless of parent rocks, in the following order: upland fields > orchards > paddy fields > forest. The values of DPS of the forest and farmland soils ranged from 8.8% to 93.2% in the same order as the oxalate-extractable P content (). In some of the upland soils, DPS exceeded 100% and most of the soils were almost saturated with P. The DPS values did not vary with the parent rock types, but the values of the farmland soils were much higher than the forest soils, namely threefold to 10-fold higher those of the forest soils. In several previous studies (CitationMcDowell and Sharpley 2001; CitationMaguire et al. 2001), it was reported that the content of water-soluble reactive P of soil sharply increased when the soil DPS values reached 20–25%. The DPS data in the present study indicated that the farmland soils, particularly the soils of the orchards and upland fields, displayed a very a high potential for P release to surface water by runoff
Figure 2 Fractions of inorganic phosphorus (P) in soils with different parent rocks, (A) granite and (B) andesite and land-use types (AD, adsorbed; CB, carbonate; OC, occluded in Fe-oxides; RS, residual; WS, water-soluble).
Statistical significance of the effects of parent rocks and land-use types on soil chemical properties
Mean values and LSD of the chemical properties and P concentrations of the soils with different parent rocks and land-use types are listed in . Data from the statistical analysis indicated that the properties of the parent rocks and the land-use types were reflected in soil chemical properties such as pH and EC and in the accumulation of organic matter and nutrients such as P and NO− 3 in the soils. The statistical data also confirmed that soil developed from andesite with finer mafic minerals displayed higher EC and lower pH values and had a larger accumulation of organic matter and nutrients than soils developed from granite. The statistical data clearly showed that P accumulation
Table 5 Effects of parent rocks and land use types on the soil chemical properties and phosphorus accumulation in the soils
Conclusion
The physical, chemical and mineralogical properties of the parent rocks were directly reflected in the soil properties in the small watershed examined in the present study. The forest soil developed from andesite with finer mafic minerals showed a finer texture, higher EC and CEC values, and higher contents of water-soluble cations (Na+, K+, Ca2+ and Mg2+) and anions (Cl− and NO− 3) than the forest soil developed from granite. The farmland soils in the andesite region displayed a greater accumulation of nutrients (NO− 3 and P) than farmland soils in the granite region.
Farmland soils showed higher pH values and concentrations of K+, Ca2+, Mg2+ and P, lower CEC and ignition loss, and a coarser texture compared with the forest soils, regardless of the parent rock types. The upland soils showed the highest concentration of NO− 3 and the highest EC values among the land-use types. However, soils of the forest, paddy fields and orchards could not be statistically differentiated with respect to the concentration of NO− 3 and the EC value. Farmland soils displayed lower concentrations of Cl− and F− than forest soils in the following order: forest > paddy fields > orchards > upland fields. Total P content of the farmland soils was twofold to fivefold higher than that of the forest soils in the following order: upland fields > orchards > paddy fields > forest. Organic and adsorbed inorganic forms of P were the dominant P forms in the soils. Most P accumulation by fertilizer application in the farmland soils remained in adsorbed and organic forms. The DPS values of the farmland soils ranged from 27.6% to 93.2%. Although the DPS of the soils could not be statistically differentiated in terms of parent rocks, the DPS values increased by cultivation in the following order: upland fields > orchards > paddy fields > forest. Among the DPS, total P and adsorbed inorganic P parameters DPS was the best parameter to predict the content of water-soluble reactive P in the soils. The high DPS values of the farmland soils, above 25%, implied that careful management of the farmlands, including decreasing the amount of fertilizer applied and controlling erosion, should be implemented for the reduction of P release to surface water in the watershed.
ACKNOWLEDGMENTS
This study was supported by the Korea Institute of Geoscience and Mineral Resources (KIGAM) and the Korea Ministry of Environment.
REFERENCES
- Wienhold , BJ , Andrews , SS and Karlen , DL . 2004 . Soil quality: a review of the science and experiences in the USA . EnvironGeochemHealth , 26 : 85 – 95 .
- Barrow , CJ . 1991 . Land degradation: development and breakdown of terrestrial environments , New York : Cambridge University Press .
- Wu , R and Tiessen , H . 2002 . Effect of land use on soil degradation in alpine grassland soil, China . Soil SciSocAmJ , 66 : 1648 – 1655 .
- McGrath , DA , Smith , CK , Gholz , HL and Oliveira , FA . 2001 . Effects of land-use change on soil nutrient dynamics in Amazonia . Ecosystems , 4 : 625 – 645 .
- Schroeder , PD , Radcliff , DE , Cabrera , ML and Belew , CD . 2004 . Relationship between soil test phosphorus in runoff: Effects of soil series variability . JEnvironQual , 33 : 1452 – 1463 .
- Hartkainen , H . 1991 . Potential mobility of accumulated phosphorus in soil as estimated by the indices of Q/I plots and by extractant . Soil Sci , 152 : 204 – 209 .
- Borling , K . 2003 . Phosphorus sorption, accumulation and leaching (Dissertation) , Uppsala : Swedish University of Agricultural Sciences .
- McDowell , RW and Sharpley , AN . 2001 . Approximating phosphorus release from soils to surface runoff and subsurface drainage . JEnvironQual , 30 : 508 – 520 .
- Pote , DH , Daniel , TC , Sharpley , AN , Moore , PA , Edward , DR and Nicols , DJ . 1996 . Relating extractable soil phosphorus to phosphorus losses in runoff . Soil SciSocAmJ , 60 : 855 – 859 .
- National Institute of Agricultural Science and Technology and Rural Development Administration, Korea . 2000 . Taxonomical Classification of Korean Soils , Suwon : NIAST and RDA .
- Jackson , ML . 1956 . Soil Chemical Analysis-Advanced Course , Madison : Department of Soils, University of Wisconsin .
- Watanabe , FS and Olsen , SR . 1965 . Test of an ascorbic acid method for determining phosphorus in water and NaHCO3 extracts from soil . Soil SciSocAmProc , 29 : 677 – 678 .
- Pautler , MC and Sims , JT . 2000 . Relationships between soil test phosphorus and soluble phosphorus saturation in Delaware soils . Soil SciSocAmJ , 64 : 765 – 773 .
- SAS Institute . 1989 . SAS/STAT Users Guide Version 6 , 4th edn , Vols 1 and 2 , Cary : SAS Institute .
- Drever , JI . 1984 . The chemistry of weathering D , Boston : Reidel Publishing Co. .
- Wu , T , Schoenau , JJ Li , F . 2004 . Influence of cultivation and fertilization on total organic carbon and carbon fractions in soils from the loess plateau of China . Soil Tillage Res , 77 : 59 – 68 .
- Nissen , TM and Wander , MM . 2003 . Management and soil-quality effects on fertilizer-use efficiency and leaching . Soil SciSocAmJ , 67 : 1524 – 1523 .
- Shani , U and Dudley , LM . 2001 . Field studies of crop response to water and salt stress . Soil SciSocAmJ , 65 : 1522 – 1528 .
- Maguire , RO , Foy , RH , Bailey , JS and Sims , JT . 2001 . Estimation of the phosphorus sorption capacity of acidic soils in Ireland . EurJSoil Sci , 52 : 479 – 488 .
- Page , AL , Miller , RH and Keeney , DR . 1982 . Methods of Soil Analysis Part 2: chemical and microbial properties , Madison, Wisconsin, , USA : ASA and SSSA .