Abstract
To evaluate the role of cytokinin in the source–sink relationship, panicles of rice were cut from the stem at the panicle emergence stage. Xylem sap exudates were collected using the stem cut method and the cytokinin concentration in the collected sap was determined by bioassay and further analysis using enzyme-linked immunosorbent assay. The rate of cytokinin translocation from roots to shoots decreased continuously after panicle initiation, whereas, when the panicle was removed, the rate increased by up to 1.5-fold, at which time no cytokinin was found in the plants with panicles. Retardation of leaf senescence was not observed and nitrogen concentration in the leaves continued to decrease after panicle removal, irrespective of cytokinin (mainly dihydrozeatin riboside and trans-zeatin riboside) level. Thus, leaf autonomy is regulated by an endogenous program of nitrogen translocation from the leaf regardless of cytokinin level in the xylem.
INTRODUCTION
Cytokinins have been considered to be signal compounds that provide signals from the roots to the shoots and function by directing information on the absorption of nitrogen by the roots (CitationSakakibara et al. 1998), the level of cytokinin decrease when plants are starved of nitrogen (CitationSamuelson and Larsson 1993; CitationTakei et al. 2001, Citation2002). Cytokinins are known to be synthesized mainly in the root tips, and are translocated to the shoot meristematic cells to enhance growth (CitationBernier et al. 1977) and retard leaf senescence (CitationBuchanan-Wollaston et al. 2003; CitationGan and Amasino 1995). Although many plant parts are known to have the ability to produce cytokinin (e.g. CitationLetham 1994), the major place of production is considered to be the roots. As nitrogen is absorbed through the roots, the focus has been on the role of cytokinins. Nitrogen uptake rate per unit root dry weight has been used as an indicator of root activity (CitationOsaki et al. 1996;Citation Shinano et al. 1994). In rice, studies have shown that keeping the specific absorption rate of nitrogen (SARN) high after flowering leads to higher productivity (CitationOsaki et al. 1996). Comparisons of high-yielding and standard-yielding varieties have shown a higher retention of nitrogen and chlorophyll content in leaves in the former (CitationHe et al. 2002; CitationOsaki et al. 1993; CitationSoejima et al. 1992), particularly in the flag leaf (CitationSoejima et al. 1995). By comparing two cultivars of different yield potential, CitationSoejima et al. (1992) demonstrated a higher level of cytokinins in root exudates of the high-yielding cultivar, and concluded that the retardation of leaf senescence observed in high-yielding cultivars was causally related to the higher level of cytokinins in the xylem sap exudate. That is, the level of cytokinin is considered to be a determinant factor in the regulation of leaf senescence. The relationship between the level of cytokinin in the leaves and leaf senescence has been studied in leaves of soybean at different ages (CitationSingh et al. 1992) and by directly painting cytokinin onto the leaves of Nicotiana rustica (CitationMothes and Engelbrecht 1961) and of soybean (CitationGarrison et al. 1984). CitationSmart et al. (1991) constructed a transgenic tobacco that over-expressed iso-pentenyl transferase, which catalyzes the synthesis of the cytokinin precursor iso-pentenyl adenine riboside. The level of endogenous cytokinin in the transformant was 10-fold higher than the levels in the untransformed plant, and leaf senescence was retarded in the transgenic plant. CitationHe et al. (2005) compared hormonal concentration of different senescence types of maize and found that the concentration of cytokinins (trans-zeatin riboside [tZR], dihydrozeatin riboside [DHZR] and iso-pentenyl adenosine [iPA]) was higher in stay-green type.
When panicles were removed from rice plants at the flowering stage, the levels of rubisco, chlorophyll and cytochrome f remained higher than normal, and sucrose phosphate synthase and ADP-glucose pyrophosphorylase activities were also higher than normal (CitationNakano et al. 1995). Thus, the potential carbon-synthesizing activity of these plants might also be high. CitationOsaki et al. (1995) reported that, in cereals, the removal of a sink (panicles) caused dry matter to accumulate in stems and plant productivity did not decrease as a result of sink removal. Thus, at least in the case of rice plants, sink removal does not affect leaf photosynthetic traits or carbon allocation. We evaluated the effect of a reduced sink size (panicle removal) on the cytokinin levels in root exudates and investigated the relationship between cytokinin level and nitrogen uptake ability and leaf senescence retardation in rice.
MATERIALS AND METHODS
Experiment 1. Effect of panicle removal on sap exudation, cytokinin content and nutrient uptake
Cultivation methods
The rice (Oryza sativa L.) cultivar Michikogane was cultivated in a paddy field at Hokkaido University, Japan. Seeds were sown on 19 April 2002 and transplanted on 30 May. Planting density was 30 cm × 15 cm and two seedlings were planted per hill. One hundred kilograms N, 100 kg P2O5 as superphosphate and 100 kg K2O were applied. Half of the nitrogen was applied as a slow-release fertilizer (Long 70, Chisso Asahi, Japan) and the other half was applied as ammonium sulfate.
Panicle removal was carried out after 4 August at the panicle emergence stage, and treated plants were then checked at 2-day intervals to remove any panicles that appeared later.
Four to six plants were sampled at 2-week intervals and separated into live leaves, dead leaves, stems (including sheaths) and panicles (if present). Each sample was dried in an air-forced oven for at least 48 h at 80°C, weighed, ground and passed through a 1-mm mesh for subsequent analysis. To obtain roots, soil to a depth of 20 cm was sampled from two hills and soaked in tap water overnight to make washing easier. The roots were washed with tap water and collected on a 2-mm mesh net. Roots were also dried and ground. Three replicate samples were taken.
Relative growth rate (RGR) was calculated as 1/W × dW/dt, where W is the dry weight of the plant (g) and t is the time (days). The specific absorption rate of nitrogen (SARN) was calculated as ΔN × RW−1, where ΔN is the nitrogen absorption rate per day of the whole plant (g N m−2 day−1) and RW is root dry weight (g m−2). To calculate RGR, ΔN, SARN, as the experiment was done by using one field, all the data obtained at each sampling were averaged and used for the calculation.
Chemical analysis
Nitrogen concentration was determined using the semi-micro Kjeldahl method with three replications for each sample (CitationHind 1993).
Collection of xylem sap
Xylem sap exudate was collected overnight from 20.00 to 08.00 hours with five replications. Collections were carried out only when the weather was clear. Xylem sap was collected by placing de-fatted cotton on the cut surface area of the rice base (approximately 10 cm in height from the top of the water level), which was then wrapped with a polyethylene bag and covered with a paper bag to avoid desiccation of the cotton. The amount of root exudate was determined by the change in weight of the cotton. Diurnal change in cytokinin has been recorded in barley (CitationKurapov et al. 2000), carrot (CitationPaasch et al. 1997) and tobacco (CitationNovákováet al. 2005). As the cytokinin level was rather stable at night and during the day, we did not consider the effect of light in this experiment.
Separation of cytokinins
Collected sap was recovered from the cotton using 100% ethanol and dried with a rotary evaporator at 40°C, and resuspended in 1 mL of distilled water. After centrifugation at 16 300 g for 7 min at 4°C, the supernatant was applied to a Sep-Pak C18 cartridge column (Waters, Milford, USA), which was pretreated with 99% methanol followed by distilled water. The column was washed with 1 mL of distilled water and 20 mL of 55% methanol was added to elute the cytokinins. The eluted solution was evaporated and dissolved in a phosphate buffer (pH 6.3, 1/60 mol L−1). This solution was used for subsequent bioassays of total cytokinin activity.
Bioassay of cytokinin activity
Combined extracts were purified as described in CitationSoejima et al. (1992). Cytokinin activity was determined using the Amaranths beta-cyanine bioassay as described by CitationBiddington and Thomas (1973). Amaranthus tricolor cv. bicolor (commercial name Flying Colors) was used and cytokinin activity in the sample was compared with amaranths treated with benzyl adenine. Seeds of amaranth were germinated in a glass Petri dish (21 cm diameter) with three sheets of filter paper that were wetted with water (No. 2, Advantec Toyo, Tokyo, Japan). The glass Petri dish was placed in the dark at 27°C for 48 h, and then for 27 h at 32°C. The seed coat and roots were removed from germinated plants to give a plantlet of approximately 1 cm length, consisting of the cotyledon and stem. A sample extract (0.4 mL) obtained as above and 0.1 mL of tyrosine solution (2.5 g L−1) were mixed and placed in a plastic Petri dish (3.5 cm diameter). One sheet of filter paper (No. 2, Advantec Toyo) was placed in the plastic Petri dish and 18 plantlets were added. After 18 h at 27°C under dark conditions, the plantlets were transferred to a polypropylene tube with 3 mL of distilled water. To extract beta-cyanine from the plantlets, the polypropylene tube was frozen and thawed seven times, and the difference in the absorbance at 620 nm and 542 nm was determined. To obtain a standard curve, 5 × 10−8, 1 × 10−8, 5 × 10−7, 1 × 10−7, 5 × 10−6 and 1 × 10−6 mol L−1 solutions of benzyl adenine were made up with phosphorus buffer (pH 6.3, 0.017 mol L−1). Although it is difficult to determine the exact cytokinin concentration by bioassay, it can be useful in comparing the same species when using the same collection method. Data were expressed as benzyl adenine equivalents (BAeq).
Fractionation of cytokinins
Purified cytokinins were separated by high performance liquid chromatography (HPLC) using the methods described by CitationSoejima et al. (1995). Samples were collected from the control treatment (those plants whose sinks were not removed) and the sink removal treatment on 29 August (at mid-maturation stage). After being concentrated as described above, each cytokinin fraction was separated by HPLC (D-ODS-10-A, 250 mm × 20 mm; YMC, Kyoto, Japan) with 50% methanol at 6.0 mL min−1. trans-Zeatin riboside (tZR), zeatin (Z), iso-pentenyl adenosine (iPA) and iso-pentenyl adenine (iP) were used as standard compounds to determine the retention times of cytokinins. These were 16.4, 18.11, 50.79 and 57.18 min, respectively. Thus, cytokinins were separated into six fractions, as Rt: 0–14.0, 14.0–17.4, 17.4–22.0, 22.0–47.0, 47.0–54.5 and 54.5–65.0 min. Each fraction was considered to contain conjugated cytokinins, tZR (and/or dihydrozeatin riboside [DHZR]), tZ (and/or dihydrozeatin [DHZ]), isop-entenyladenine-9-glucoside (iPG), iPA and iP, respectively.
Experiment 2. Determination of plant hormones using enzyme-linked immunosorbent assay
Cultivation method
In 2003, to confirm the effect of sink removal on the composition of plant hormones in the bleeding sap, another experiment was carried out. The rice cultivar Michikogane was cultivated in a 12 L pot with soil obtained from the paddy field. Nitrogen as ammonium sulfate, phosphorus as superphosphate and potassium as potassium sulfate were fertilized as 1.5 g N, 1.5 g P2O5, and 1.5 g K2O per pot. Seeds were sown on 28 April 2003 and transplanted on 3 June. Panicle removal treatment was carried out on 7 August at the panicle emergence stage, and treated plants were then checked every day to remove panicles that appeared later. Sap was collected as described above on 5–6 September with 5 replications.
Extraction of hormone and quantification
Cytokinins were further analyzed for those compounds known to have high activity; tZR, DHZR and iPA were tested. The methods for extraction and purification were modified from those described by CitationHe et al. (2005). After sap was collected and evaporated as described in Experiment 1, it was dissolved in 3 mL of 80% MeOH. Extracts were purified by passing them through Sep-Pak Plus C18 cartridge (Waters, Milford, MA, USA). The cartridge was previously eluted with 10 mL of 99% MeOH and passed through 5 mL of 80% MeOH. After the sample was passed to the cartridge, 2 mL of 80% MeOH was used to wash the cartridge. Combined elutes were evaporated and dissolved in 1.0 mL of TBS buffer (tris-buffered saline; 50 mmol L−1 Tris, pH 7.8, 1 mmol L−1 MgCl2, 10 mmol L−1 NaCl, 0.1% tween, 0.1% gelatin). DHZR, tZR and iPA contents were determined by enzyme-linked immunosorbent assay (ELISA) using monoclonal antibodies (Phytodetek, Agdia, Elkhart, IN, USA) following the protocol provided by the manufacturer. As the antibodies of cytokinins also recognize free bases, nucleotides and 9N-glucosides, the sum of free basis, ribosides, nucleotides and 9N-glucosides of corresponding cytokinins was measured. The amount of DHZR also includes DHZ, and tZR includes tZ.
RESULTS
Plant growth
Panicle emergence occurred on 4 August (66 days after transplanting) and harvest was on 4 October (122 days after transplanting). In the panicle removal treatment, the number of tillers increased from 500 tillers m−2 to 1100 tillers m−2, whereas, in the controls the number of tillers decreased gradually to 450 tillers m−2 (data not shown). However, the new tillers in the panicle removal treatment contributed little to the dry matter or nitrogen nutrient status of the plant because of their small size.
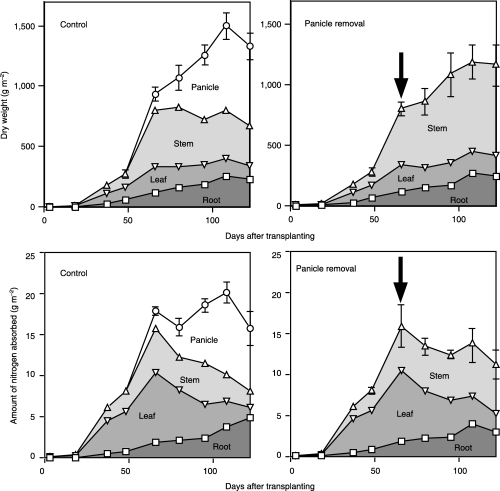
The dry weight of the whole plant increased exponentially until the panicle emergence stage, and then increased gradually until 2 weeks before harvest (). When the sink was removed, the increase in dry weight stopped temporarily and then increased again. In the panicle removal treatment, the dry weight of the stem increased and the leaf dry weight remained constant until harvest ().
The root dry weight increased until 108 days after transplanting and was not affected by removal of the sink ().
Figure 1 Successive changes in dry weight and in the amount of nitrogen absorbed in various parts of the plant. Data are cumulatively accumulated and the top value is equal to the whole plant dry weight. Bars indicate standard error (SE; n = 3) of the whole plant dry weight. Symbols without bars mean that the SE is so small that it is covered by the symbol. Arrows indicate the date of panicle removal.
Accumulation of nitrogen
Nitrogen accumulated in the leaves until the panicle emergence stage, and then decreased regardless of treatment (). More nitrogen accumulated in the stem in the panicle removal treatment than in the control after the panicle emergence stage, indicating that the stem has a role as an alternative nitrogen sink.
After panicle emergence, nitrogen increased in the control, whereas the accumulated nitrogen gradually decreased when the panicles were removed ().
Nitrogen and chlorophyll concentration
Nitrogen concentration in the leaves decreased rapidly after the panicle emergence stage (). Chlorophyll concentration increased until 88 days after transplanting, and then decreased rapidly (). There was no difference between the control and the panicle removal treatment in the concentrations of either nitrogen or chlorophyll. The nitrogen concentration in the roots decreased gradually until the mid-maturation stage, when the concentration increased. Nitrogen concentration in the roots increased at harvest if the panicle was removed ().
Nitrogen uptake and specific absorption rate of nitrogen
The nitrogen uptake rate (ΔN) increased until 48 days after transplanting, and then decreased. The uptake rate was suppressed by panicle removal. The specific absorption rate of nitrogen (SARN) showed a tendency similar to ΔN (). SARN also decreased in the panicle removal treatment.
Figure 2 Successive changes in root and leaf nitrogen concentration and in the chlorophyll concentration in green leaves of plants with the panicle removed and control plants (no treatment). Arrows indicate the date of panicle removal. Bars indicate standard error. An asterix above the bar indicates that the difference between the control and the panicle removal treatment is significant using a t-test (P < 0.05).
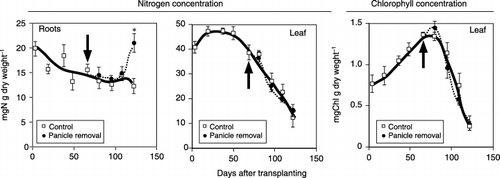
Figure 3 Successive changes in the relative growth rate (RGR; g g−1 day−1), nitrogen uptake rate (ΔN; g N m−2 day−1) and the specific absorption rate of nitrogen (SARN; g N g RW−1 day−1). To calculate RGR, ΔN and SARN, because the experiment was done using only one field, all data obtained at each sampling were averaged and used to calculate the subsequent changes in dry weight and the accumulated amount of nitrogen. Thus, the data are not replicated. Arrows indicate the date of panicle removal.
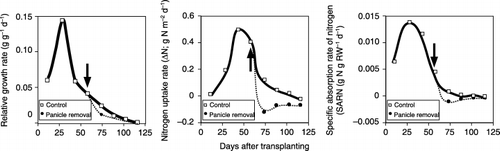
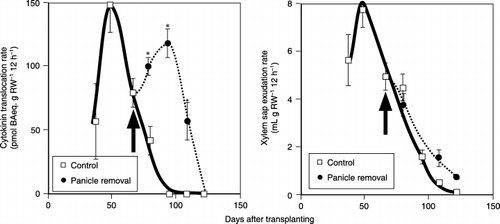
Figure 4 Successive changes in the xylem sap exudation rate (mL g RW−1 12 h−1) and the cytokinin translocation rate (pmol bAeq. g RW−1 12 h−1). The cytokinin level is indicated as benzyl adenine (BA) eqiuivalents. Arrows indicate the date of panicle removal. Bars indicate standard error (SE) and symbols without bars mean that the SE is so small that it is covered by the symbol. An asterix above the bar indicates that the difference between the control and the panicle removal treatment is significant using a t-test (P < 0.05).
Cytokinin translocation rate and xylem sap exudation rate
The cytokinin translocation rate increased until the booting stage, and then decreased quickly. When the panicle was removed at the panicle emergence stage, the cytokinin translocation rate increased rapidly for approximately 5 weeks and then decreased rapidly after the mid-ripening stage ().
The exudation rate of xylem sap was high before flowering, and then decreased sharply during the reproductive stage (). When the panicle was removed, the xylem sap exudation rate became higher than that in the control treatment.
Composition of cytokinins in the xylem sap
The effect of sink removal on the composition of cytokinins is shown in . The cytokinin activity was mainly derived from a large amount of activity occurring in chromatographic fractions where ZR, tZ and iPG standards occurred. This activity may correspond to ZR, tZ and iPG, but definitive identification will require mass spectrometry (MS) analysis with heavy isotope labeled standards or other critical identification techniques.
Determination of cytokinins using enzyme-linked immunosorbent assay
In iPA, DHZR and tZR, a significant increase was observed by sink removal in the level of tZR (). Although the level of iPA decreased with sink removal, the change was not significant.
Figure 5 Cytokinin activity using the Amaranthus beta cyanine bioassay in fractions separated using high performance liquid chromatography and identified using standards that separated into each fraction. Bars indicate standard error (SE) and symbols without bars mean that the SE is so small that it is covered by the symbol. An asterix above the bar indicates that the difference between the control and the panicle removal treatment is significant using a t-test (P < 0.05).
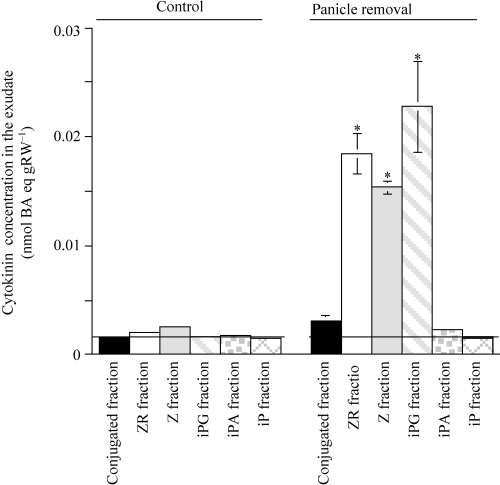
Table 1 Concentration of cytokinins determined using enzyme-linked immunosorbent assay
DISCUSSION
Leaf nitrogen status and cytokinin
Sink activity is sometimes believed to be detrimental to the regulation of the balance of the source–sink. Dry matter production is assumed to be regulated by the activity of the sink on the basis of the following results: (1) when the sink was removed, the photosynthetic rate decreased markedly in wheat (CitationKing et al. 1967), (2) when varieties with different source–sink relationships were grafted, the photosynthetic rate varied with the strength of the sink in sweet potato (CitationHahn 1977) and in beet (CitationThorne and Evans 1964). However, in recent studies, photosynthetic rate or dry matter production of the whole plant did not decrease, even after the sink organs (panicles in cereals and pods in legumes) were completely removed (CitationKoide and Ishihara 1992a,Citationb; CitationOsaki et al. 1995). CitationOsaki et al. (1995) observed that nitrogen translocation from the leaves of various cereal crops was not affected by panicle removal. This observation indicates that the destruction and translocation of nitrogen compounds in leaves was not regulated by sink demand, but that there was a physiological autonomy in the leaves. In the current paper, leaf autonomy in terms of nitrogen translocation during the ripening stage of the rice plant was confirmed because: (1) the nitrogen and chlorophyll concentrations in the leaves were not affected by panicle manipulation (), (2) even in the panicle removal treatment, nitrogen decreased in the leaves, and then either accumulated in the stems or was lost from the plants entirely (). This indigenous regulation of nitrogen translocation from the leaves is observed in cereals; however, in legume crops, leaf senescence and nitrogen translocation from the leaves are depressed by sink manipulation (CitationNoodén and Letham 1993; CitationOsaki et al. 1995).
Exogenous application of cytokinin is known to delay leaf senescence by retarding the degradation of chlorophyll and photosynthetic proteins (CitationBadenoch-Jones et al. 1996). Genetic manipulation of tobacco to produce cytokinin under the control of a senescence-driven promoter revealed that expression of cytokinin in the tobacco leaves could delay leaf senescence (CitationGan and Amasino 1995). The production of cytokinin also prolonged the photosynthetic activity of tobacco (CitationJordi et al. 2000; CitationWingler et al. 1998). Cytokinin is known to upregulate several genes, such as the rubisco small subunit (CitationPlumley and Schmidt 1991) and phosphoenolpyruvate carboxylase (CitationSugiharto et al. 1990; CitationSugiharto and Sugiyama 1992). It has been reported that, under nitrogen-limiting conditions, exogenously applied nitrogen could induce several genes that regulate photosynthesis in maize, and this induction can occur with the addition of cytokinin (CitationSuzuki et al. 1994).
The pattern of decreasing chlorophyll concentration in the leaves did not change and leaf senescence was unaffected, regardless of the increase in the cytokinin level in the xylem sap (). In general, it is assumed that leaf senescence is regulated by sink demand (panicle formation) and cytokinin concentration; however, at least in rice plants, the data obtained suggest that leaf senescence is not regulated by its endogenous program, or by sink (panicle) demand for nitrogen or by cytokinin level directly (). From the comparison between different senescent type maize, CitationHe et al. (2005) demonstrated that the stay-green hybrid showed more vigorous root growth and cytokinin production than the early senescent hybrid. Furthermore, although t-ZR and DHZR contents are greater in the roots of the stay-green hybrid than the early senescent hybrid, the contents of iPA showed the reverse pattern. As a similar situation was observed in the panicle removal treatment, it is suggested that panicle removal induces a large distribution of carbohydrates from leaves to roots, and these carbohydrates keep the root activity for the synthesis of cytokinins high. In contrast, the disturbance of the source–sink balance by panicle removal changed the composition of cytokinins.
Nitrogen demand and absorption
Nitrogen uptake was stopped by the panicle removal treatment (), thus, the uptake of nitrogen by the roots appears to be regulated by the sink requirements regardless of carbohydrate status. Accordingly, nitrogen absorption is regulated by a strong nitrogen requirement. High root activity is important for achieving high productivity; however, the nitrogen absorption activity of the roots must relate to a high nitrogen demand in the shoot. In contrast, the root nitrogen concentration increased and was significantly higher in the panicle removal treatment than in the control (). This indicates that retardation of nitrogen transport occurred by panicle removal.
Xylem sap exudation
It is widely accepted that cytokinins are root-produced phytohormones and that they are transported to the shoot through the xylem. Although the expression of genes encoding the synthesis of cytokinin (ATP/ADP iso-pentenyl transferases) clearly demonstrates that the expression occurs in a wide range of organs and cell types (CitationMiyawaki et al. 2004). Furthermore, it should be noted that the cytokinin obtained in this experiment is not solely derived from the xylem because the occurrence of phloem to xylem transport of cytokinin is reported (CitationEmery et al. 2000). Furthermore, the contribution of rhizobacteria that produce cytokinin that is transported into the plant is also known (CitationGarcia de Salamone et al. 2001). We do not know the contribution of phloem and/or rhizobacteria-derived cytokinin in this experiment; however, in the latter case it is suggested that if the carbohydrate supply from shoots to roots increased after sink removal, which promotes the production of cytokinin by rhizobacteria, this can be incorporated in the role of cytokinin from the root. If more cytokinin was transported from the roots to the shoot, the vigor of the shoot can be assumed to remain high. After panicle removal treatment, a large increase in the amount of xylem sap exuded and the increment of several cytokinin in the xylem sap indicated that higher activity of root was induced (, ). Although we have considered two opposite situations on root activity with the panicle removal treatment, because the amount of xylem sap and cytokinin translocated in the stem increased with panicle removal, it is suggested that the root activity appears to be supported or impressed. However, as dry matter increased and nitrogen uptake decreased with panicle removal, nitrogen uptake by the roots was regulated differently. A large increase in cytokinin by panicle removal appears to create a new destination for the photoassimilates, and this might increase the number of tillers of rice by panicle removal (data not shown), but it was ineffective with regard to panicle production. The cytokinin concentration in xylem exudate increased after decapitation of the bean crop (CitationBangerth 1994). Although cytokinin has recently been considered to be a signal compound that responds to nitrogen in maize roots (CitationSakakibara et al. 1998), pod removal of soybean (CitationNoodén et al. 1990) and rice (this report) or decapitation in the bean (CitationBangerth 1994) also induce a large increase of cytokinin in the stem exudate. Cytokinin biosynthesis appears to be regulated by more than just nitrogen and the observed leaf autonomy for nitrogen was not affected by increased levels of cytokinin. This result is also supported by the study of cytokinin deficient Arabidopsis (CitationWerner et al. 2003), where the mutant did not show any promotion of senescence.
Changes in cytokinin composition by panicle removal
In the present study, cytokinin activity in the root exudates was separated into different types of cytokinin fraction using HPLC. CitationMurofushi et al. (1983) reported the existence of cis-zeatin, cis-ribosyl zeatin, tZR and zeatin glucoside (conjugated fraction) in rice root exudates. CitationSoejima et al. (1992) reported the existence of N6-isopentenyladenosine, tZR, tZ and conjugated zeatin, with conjugated zeatin appearing in the highest amount in rice. Thus, these researchers judged conjugated zeatin to be important in cytokinin transport from roots to shoots in rice. In the control plants, results from our chromatographic fraction suggest a similar tendency. Although detailed cytokinin-related compounds might be evaluated by using other techniques (e.g. liquid chromatography-mass spectropmetry (LC-MS)), it is still important to consider the major cytokinin bioassay for evaluating the role of cytokinin in plants.
In the present study, the presence of tZR, tZ and iPG in the exudates of control plants was suggested by chromatographic behavior. After removing the panicles, we observed increased activity not only in the tZR and tZ fractions, but also in the iPG fraction. Although the increase in the concentration in the xylem sap of the active form of cytokinin is only observed in tZR from ELISA (), panicle removal increased significantly the amount of all of the active cytokinin forms (iPA, tZR, DHZR) in the xylem sap exudation (). It is suggested that panicle removal affects the overall expression of cytokinins synthesis. Although glucosylated cytokinins are categorized as inactive, a number have shown high activity, as was observed in oat, radish, Chinese cabbage (CitationLetham and Palni 1983), sweet potato (CitationSugiyama and Hashizume 1989; CitationSugiyama et al. 1983) and petunia (CitationAuer and Cohen 1993). Changes in the relative strength of RZ, tZ and iPG fraction to iPA and iP fraction from the data of HPLC, or the relative strength of DHZR and tZR to iPA from the data of ELISA, suggest that these ratios could be a result of source–sink balance changes in rice plants. As recent analytical development to determine the cytokinin metabolite more precisely (e.g. CitationNovákováet al. 2005), and not only the synthesis of cytokinin but also degradation of cytokinin by cytokinin oxydase/dehydrogenase also play an important role in the regulation of rice productivity (CitationAshikari et al. 2005), further study is required to determine why the cytokinin composition changes with panicle removal.
Recent findings have clarified cytokinin signaling within a cell (e.g. CitationHowell et al. 2003), and the importance of cytokinin on the regulation of shoot development is widely accepted (e.g. CitationSchmülling 2001). However, in the present study, although the cytokinin level in the xylem sap changed with panicle removal, retardation of leaf senescence was not observed. This indicates that the actual role of cytokinin appears to be different at the cellular level compared with the individual level. If we wish to use cytokinin for the regulation of rice plant growth, we need to understand long-distance cytokinin signaling more precisely.
Conclusion
Panicle removal from rice plants resulted in a large increase in cytokinin level (both in the amount and activity) in the xylem sap. However, leaf senescence was not delayed from the viewpoint of nitrogen and chlorophyll concentration change. Thus, it should be noted that a single cytokinin level does not regulate leaf senescence; rather the role of cytokinin on leaf senescence appears to change with plant (or leaf) developmental stage. These findings are particularly important in considering the role of cytokinin on rice plant growth regulation.
REFERENCES
- Sakakibara , H , Suzuki , M , Takei , K , Deji , A , Taniguchi , M and Sugiyama , T . 1998 . A response-regulator homologue possibly involved in nitrogen signal transduction mediated by cytokinin in maize . Plant J , 14 : 337 – 344 .
- Samuelson , ME and Larsson , C-M . 1993 . Nitrate regulation of zeatin riboside levels in barley roots: effects of inhibitors of N assimilation and comparison with ammonium . Plant Sci , 93 : 77 – 84 .
- Takei , K , Sakakibara , H , Taniguchi , M and Sugiyama , T . 2001 . Nitrogen-dependent accumulation of cytokinins in root and the translocation to leaf: implication of cytokinin species that induce gene expression of maize response regulator . Plant Cell Physiol , 42 : 85 – 93 .
- Takei , K , Takahashi , T , Sugiyama , T , Yamaya , T and Sakakibara , H . 2002 . Multiple routes communicating nitrogen availability from roots to shoots: a signal transduction pathway mediated by cytokinin . JExpBot , 53 : 971 – 977 .
- Bernier , G , Kinet , JM , Jacqmard , A , Havelange , A and Bodson , M . 1977 . Cytokinin as a possible component of the floral stimulus in Sinapis alba . Plant Physiol , 60 : 282 – 285 .
- Buchanan-Wollaston , V , Earl , S Harrison , E . 2003 . The molecular analysis of leaf senescence–A genomics approach . Plant BiotechJ , 1 : 3 – 22 .
- Gan , S and Amasino , RM . 1995 . Inhibition of leaf senescence by autoregulated production of cytokinin . Science , 270 : 1986 – 1988 .
- Letham , DS . 1994 . “ Cytokinins as phytohormones—sites of biosynthesis, translocation, and function of translocated cytokinin ” . In Cytokinins: Chemistry, Activity, and Function , Edited by: Mok , DWS and Mok , MC . 57 – 80 . Boca Raton : CRC Press .
- Osaki , M , Matsumoto , M , Shinano , T and Tadano , T . 1996 . A root–shoot interaction hypothesis on high productivity of root crops . Soil SciPlant Nutr , 42 : 289 – 301 .
- Shinano , T , Osaki , M , Yamada , S and Tadano , T . 1994 . Comparison of root growth and nitrogen absorbing ability between Gramineae and Leguminosae . Soil SciPlant Nutr , 40 : 485 – 495 .
- He , P , Osaki , M , Takebe , M and Shinano , T . 2002 . Changes of photosynthetic characteristics in relation to leaf senescence in two maize hybrids with different senescent appearance . Photosynthetica , 40 : 547 – 552 .
- Osaki , M , Morikawa , K , Matsumoto , M , Shinano , T , Iyoda , M and Tadano , T . 1993 . Productivity of high-yielding crops III. Accumulation of ribulose-1,5-bisphosphate carboxylase/oxygenase and chlorophyll in relation to the productivity of high yielding crops . Soil SciPlant Nutr , 39 : 399 – 408 .
- Soejima , H , Sugiyama , T and Ishihara , K . 1992 . Changes in cytokinin activities and mass spectrometoric analysis of cytokinins in root exudates of rice plant (Oryza sativaL.) . Plant Physiol , 100 : 1724 – 1729 .
- Soejima , H , Sugiyama , T and Ishihara , K . 1995 . Changes in chlorophyll contents of leaves and in levels of cytokinins in root exudates during ripening of rice cultivars Nipponbare and Akenohoshi . Plant Cell Physiol , 36 : 1105 – 1114 .
- Singh , S , Letham , DS and Palni , LMS . 1992 . Cytokinin biochemistry in relation to leaf senescence. VII. Endogenous cytokinin levels and exogenous application of cytokinins in relation to sequential leaf senescence of tobacco . PhysiolPlant , 86 : 388 – 397 .
- Mothes , K and Engelbrecht , L . 1961 . Kinetin-induced directed transport of substances in excised leaves in the dark . Phytochemistry , 1 : 58 – 62 .
- Garrison , FR , Brinker , AB and Noodén , LD . 1984 . Relative activities of xylem-supplied cytokinins in retarding soybean leaf senescence and sustaining pod development . Plant Cell Physiol , 25 : 213 – 224 .
- Smart , CM , Scofield , SR , Bevan , MW and Dyer , TA . 1991 . Delayed leaf senescence in tobacco plants transformed with tmr, a gene for cytokinin production in Agrobacterium . Plant Cell , 3 : 647 – 656 .
- He , P , Osaki , M , Takebe , M , Shinano , T and Wasaki , J . 2005 . Endogenous hormones and expression of senescence-related genes in different senescent types of maize . JExpBot , 56 : 1117 – 1128 .
- Nakano , H , Makino , A and Mae , T . 1995 . Effects of panicle removal on the photosynthetic characteristics of the flag leaf of rice plants during the ripening stage . Plant Cell Physiol , 36 : 653 – 659 .
- Osaki , M , Yamada , S and Tadano , T . 1995 . Effect of sink manipulation on nitrogen accumulation and distribution among organs of Gramineae and Leguminosae . Soil SciPlant Nutr , 41 : 33 – 44 .
- Hind , G . 1993 . “ Thylakoid components and processes ” . In Photosynthesis and Production in a Changing Environment: A Field and Laboratory Manual , Edited by: Hall , DO , Scurlock , JMO , Bolhar-Nordenkampf , HR , Leegood , RC and Long , SP . 283 – 298 . London : Chapman & Hall .
- Kurapov , PB , Skorobogatova , Sal’nikova IV , Sorkina , EI and Siusheva AG , GL . 2000 . Diurnal courses of endogenous phytohoromones in barley . Izvestiya Akademii Nauk Seriya Biologicheskaya , 1 : 108 – 114 .
- Paasch , K , Lein , C , Nessiem , M and Neumann , HK . 1997 . Changes in the concentration of some phytochromes in cultured root explants and in intact carrot plants (Daucus carotaL.) during the day . Angewanddte Botanik , 71 : 85 – 89 .
- Nováková , M. , Motyka , V. , Dobrev , PI , Malbeck , J , Gaudinová , A and Vanková , R . 2005 . Diurnal variation of cytokinin, auxin and abscisic acid levels in tobacco leaves . JExpBot , 56 : 2877 – 2883 .
- Biddington , NL and Thomas , TH . 1973 . A modified Amaranthusbetacyanin bioassay for the rapid determination of cytokinins in plant extracts . Planta , 111 : 183 – 186 .
- King , RW , Wardlaw , IF and Evans , LT . 1967 . Effect of assimilate utilization on photosynthetic rate in wheat . Planta , 77 : 261 – 276 .
- Hahn , SK . 1977 . A quantitative approach to source potentials and sink capacities among reciprocal grafts of sweet potato varieties . Crop Sci , 17 : 559 – 562 .
- Thorne , GN and Evans , AF . 1964 . Influence of tops and roots on net assimilation rate of sugar-beet and spinach beet and grafts between them . AnnBot , 28 : 499 – 508 .
- Koide , K and Ishihara , K . 1992a . Effects of sink cut on dry matter production and its partitioning in wheat and barley . JpnJCrop Sci , 61 : 651 – 658 .
- Koide , K and Ishihara , K . 1992b . Effects of sink cut on photosynthesis of the flag leaf during grain filling in wheat . JpnJCrop Sci , 61 : 659 – 667 .
- Noodén , LD and Letham , DS . 1993 . Cytokinin metabolism and signaling in the soybean plant . AustJPlant Physiol , 20 : 639 – 653 .
- Badenoch-Jones , J , Parker , CW , Letham , DS and Singh , S . 1996 . Effect of cytokinins supplied via the xylem at multiples of endogenous concentrations on transpiration and senescence in derooted seedlings of oat and wheat . Plant Cell Environ , 19 : 504 – 516 .
- Jordi , W , Shapendonk , A Davelaar , E . 2000 . Increased cytokinin levels in transgenic PSAG12-IPT tobacco plants have large direct and indirect effects on leaf senescence, photosynthesis and N partitioning . Plant Cell Environ , 23 : 279 – 289 .
- Wingler , A , von Schaewen , A , Leegood , RC , Lea , PJ and Quick , WP . 1998 . Regulation of leaf senescence by cytokinin, sugars, and light. Effects on NADH-dependent hydroxypyruvate reductase . Plant Physiol , 116 : 329 – 335 .
- Plumley , FG and Schmidt , GW . 1991 . Nitrogen-dependent regulation of photosynthetic gene expression . ProcNatl AcadSci , 88 : 4791 – 4795 .
- Sugiharto , B , Miyata , K , Nakamoto , H , Sasakawa , H and Sugiyama , T . 1990 . Regulation of expression of carbon-assimilating enzymes by nitrogen in maize leaves . Plant Physiol , 92 : 963 – 969 .
- Sugiharto , B and Sugiyama , T . 1992 . Effects of nitrate and ammonium on gene expression of phosphoenolpyruvate carboxylase and nitrogen metabolism in maize leaf tissue during recovery from nitrogen stress . Plant Physiol , 98 : 1403 – 1408 .
- Suzuki , I , Crétin , C , Omata , T and Sugiyama , T . 1994 . Transcriptional and posttranscriptional regulation of nitrogen-responding expression of phosphoenolpyruvate carboxylase gene in maize . Plant Physiol , 105 : 1223 – 1229 .
- Miyawaki , K , Matsumoto-Kitan , M and Kakimoto , T . 2004 . Expression of cytokinin biosynthetic isopentenyltransferase genes in Arabidopsis: tissue specificity and regulation by auxin, cytokinin, and nitrate . Plant J , 37 : 128 – 138 .
- Emery , RJN , Ma , Q and Atkins , CA . 2000 . The forms and sources of cytokinins in developing Lupinus albusseeds and fruits . Plant Physiol , 123 : 1 – 12 .
- Garcia de Salamone , IE , Hynes , RK and Neldon , LM . 2001 . Cytokinin production by plant growth promoting rhizobacteria and selected mutants . CanJMicrobiol , 47 : 404 – 411 .
- Bangerth , F . 1994 . Response of cytokinin concentration in the xylem exudate of bean (Phaseolus vulgarisL.) plants to decapitation and auxin treatment, and relationship to apical dominance . Planta , 194 : 439 – 442 .
- Noodén , LD , Singh , S and Letham , DS . 1990 . Correlation of xylem sap cytokinin levels with monocarpic senescence in soybean . Plant Physiol , 93 : 33 – 39 .
- Werner , T , Motyka , V , Laucou , V , Smets , R , Van Onckelen , H and Schmülling , T . 2003 . Cytokinin-deficient transgenic Arabidopsisplants show multiple developmental alterations indicating opposite functions of cytokinins in regulating shoot and root meristem activity . Plant Cell , 15 : 2532 – 2550 .
- Murofushi , N , Inoue , A , Watanabe , N , Ota , Y and Takahashi , N . 1983 . Identification of cytokinins in root exudate of the rice plant . Plant Cell Physiol , 24 : 87 – 92 .
- Letham , DS and Palni , LMS . 1983 . The biosynthesis and metabolism of cytokinins . AnnuRevPlant Physiol , 34 : 163 – 197 .
- Sugiyama , T and Hashizume , T . 1989 . Cytokinins in developing tuberous roots of sweet potato . AgricBiolChem , 53 : 49 – 52 .
- Sugiyama , T , Suye , S and Hashizume , T . 1983 . Mass spectrometric determination of cytokinins in young sweet-potato plants using deuterium-labeled standards . AgricBiolChem , 47 : 315 – 318 .
- Auer , CA and Cohen , JD . 1993 . Identification of a benzyladenine disaccharide conjugate produced during shoot organogenesis in Petunia leaf explants . Plant Physiol , 102 : 541 – 545 .
- Ashikari , M , Sakakibara , H Lin , S . 2005 . Cytokinin oxydase regulates rice grain production . Science , 309 : 741 – 745 .
- Howell , SH , Lall , S and Chen , P . 2003 . Cytokinins and shoot development . Trends Plant Sci , 8 : 453 – 459 .
- Schmülling , T. 2001 . CREam of cytokinin signalling: receptor identified . Trends Plant Sci , 6 : 281 – 284 .