Abstract
Six desulfo-glucosinolates (DS-GSLs), DS-glucoraphanin, DS-4-(β-d-glucopyranosyldisulfanyl)butyl GSL, DS-glucoerucin, DS-glucobrassicin, DS-dimeric 4-mercaptobutyl GSL and DS-4-methoxyglucobrassicin, were isolated from rocket salad (Eruca sativa Mill.) By using high-pressure liquid chromatography (HPLC) and identified by their retention times on HPLC and electrospray ionization mass spectrometry (ESI-MS) techniques. Total GSL contents were 125, 11 and 22 µmol g−1 dry weight (DW) in seeds, leaves and roots, respectively. Two DS-GSLs, namely DS-glucoraphanin and DS-glucoerucin, were the major GSLs in seeds and roots, while DS-glucoraphanin, DS-glucoerucin and DS-dimeric 4-mercaptobutyl GSL were the predominant GSLs in leaves (> 1 µmol g−1 DW or > 10% of the total GSL content). In addition, the anti-oxidant activity (n = 3) of intact leaf and root powders, whole DS-GSL eluates and purified DS-4-methoxyglucobrassicin were measured using the XYZ-dish method. DS-4-methoxyglucobrassicin showed the greatest anti-oxidant activity value (3.8 unit g−1 DW), and the value for whole DS-GSL eluates (1.6) was higher than that of intact leaves (1.5) and roots (0.87).
INTRODUCTION
Rocket salad (Eruca sativa Mill.), commonly known as “rucola” in Italy and “kibana-suzushiro” in Japan, is an annual herb belonging to the Brassicaceae family. With its spicy hot flavor, rocket is widely consumed fresh as salad, prepared as a steamed vegetable or used as a spice or food ingredient in European countries. In Asia, particularly in India and Pakistan, the plant serves as an important source of oil and its seed meal is commonly used as animal feed because it is a rich source of protein despite its high glucosinolate (GSL; ) and erucic acid contents (CitationAshraf and Noor 1992; CitationPita Villamil et al. 2002). Most studies have focused on the effects of nitrate and ammonium nutrition on nitrate content in rocket (CitationBianco et al. 1998; CitationSantamaria and Gonnella 2001; CitationSantamaria et al. 1998; CitationSantamaria et al. 2002), as well as on its GSLs and their breakdown products, particularly the isothiocyanates (CitationBennett et al. 2002; CitationCerny et al. 1996; CitationJirovetz et al. 2002; CitationMiyazawa et al. 2002; CitationWeckerle et al. 2001). When crushed, rocket leaves release a strong smell of horseradish combined with sesame seeds. The characteristic pungent or bitter taste and flavor of leafy vegetables, particularly those belonging to the Brassicaceae (i.e. Cruciferae) family may be related to the presence of GSLs and their associated hydrolytic products: isothiocyanates, thiocyanates and nitriles.
Over 120 individual GSLs, a group of sulfur-containing secondary metabolites have been identified from 11 plant families of dicotyledonous angiosperms, particularly those from the Brassicaceae (CitationFahey et al. 2001). Approximately twenty of these GSLs, possessing the same basic structure (), and differing only in their side chains (R), are commonly present in appreciable amounts in any particular plant (CitationOlsen and Sørensen 1981). The GSLs are named according to their R-group and aglycone moieties, whose biosynthetic derivation arises from a few known amino acids. Aliphatic GSLs, bearing an
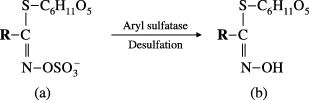
Figure 1 Enzymatic desulfation of glucosinolates. (a) Glucosinolate; (b) desulfated natural glucosinolate.
In the plant, intact GSLs and their breakdown products constitute part of the general defense mechanisms against herbivores and microorganisms (CitationWallsgrove et al. 1999). The specific biological activity of different GSLs varies considerably, thus, it is important to precisely identify and quantify the specific GSLs in any tissue under investigation (CitationKiddle et al. 2001). In recent years, various GSLs have been isolated and identified from the seeds and leaves of rocket salad. (CitationBennett et al. 2002; CitationCerny et al. 1996; CitationIori et al. 1999; CitationJirovetz et al. 2002; CitationKim et al. 2004). However, in our experience, a number of minor GSLs consistently separated using high-pressure liquid chromatography (HPLC) have not yet been identified. Fortunately, two indolyl GSLs were detected from the concentrated extracts of rocket leaves according to their retention times, but not characterized in detail (CitationKim et al. 2004). Moreover, there have been several previous attempts to obtain GSL profiles from different tissues within one plant. Major differences in the relative amount of individual GSLs have been observed between plant parts, indicating that individual GSLs have defined distribution patterns (CitationMacfarlane-Smith and Griffiths 1988; CitationSang et al. 1984).
The isothiocyanates are an important group of hydrolytic products of GSLs and are potentially considered to possess anti-carcinogenic properties. In particular, sulforaphane (the isothiocyanate of glucoraphanin) and indole-3-carbinol (the isothiocyanate of glucobrassicin) are believed to confer beneficial effects on human health through a variety of mechanisms (CitationBradfield and Bjeldanes 1984; CitationFahey et al. 2001; CitationGross et al. 2000; CitationMithen et al. 2000; CitationNho and Jeffery 2004). Anti-oxidative activity, particularly free radical scavenging activity, is important because of the putative role of free radicals as cancer-causing agents in both foods and biological systems. Therefore, in a biological system, anti-oxidative properties are frequently used as an index of the activity of a defense mechanism against active oxygen species or free radicals. At the present time, there is an increased interest in non-synthetic anti-oxidants of plant origin, including ascorbic acid, carotenoids, flavonoids, phenolic compounds and vitamins. To date, only the anti-oxidative properties of whole extracts and purified GSLs from brussels sprouts (Brassica oleracea L. var. gemmifera Zenker), broccoli (Brassica oleracea L. var. italica Plenck.), red cabbage, cabbage (Brassica oleracea L.) and cauliflower (Brassica oleracea L. var. botrytis L.) have been systematically studied by measuring their effect on lipid peroxidation using the deoxyribose assay, a radical cation 2,2′-azinobis-3-ethyl-benzothiazoline-6-sulfonate (ABTS), and the bleomycin assay (CitationPlumb et al. 1996). The authors concluded that the total anti-oxidative activities of extracts from cooked and autolysed brussels sprouts were not significantly different because the phenolic compounds and purified GSLs expressed only weak anti-oxidative properties. By contrast, CitationKim et al. (2004) obtained relatively high anti-oxidative activities for two desulfo-glucosinolates (DS-GSLs), DS-4-(β-D-glucopyranosyldisulfanyl)butyl GSL and DS-dimeric 4-mercaptobutyl GSL, purified from rocket leaves using the XYZ-dish chemiluminescence (CL) method (H2O2-acetaldehyde system; CitationYoshiki et al. 1995). Each DS-GSL bore an asymmetrical S–S bond that could serve as an electron donor.
Consequently, the aim of the present study was to identify and characterize GSLs as their desulfated analogs, particularly two indolyl GSLs, present in rocket salad using HPLC and electrospray ionization mass spectrometry (ESI-MS). Different plant tissues, seed, leaf and root, were analyzed to determine their complete GSL profiles and to look for differences between the organs in the relative proportions of individual GSLs. In addition, the anti-oxidative properties of the intact plants and their desulfated analogs were assessed using the XYZ-dish method.
MATERIALS AND METHODS
Plant materials
Rocket (Eruca sativa Mill. cultivar “Odyssey”) seeds were purchased from Sakata Seed Company (Yokohama, Japan). Seeds were kept at 100°C for 30 min in the oven to deactivate endogenous myrosinase and the seeds were defatted using hexane several times and then dried. Seed powder was analyzed for GSL profiles with four replications.
For plant samples, seeds were sown in a plastic pot containing vermiculite on 24 September 2002 and grown in a greenhouse at the National Agricultural Research Center for Hokkaido Region (longitude 141°21′E; latitude 43°04′N). Two weeks later individual seedlings were removed and placed 0.1 m apart in a plastic container (300 mm × 130 mm × 120 mm, 1.8 L). The plantlets were cultivated hydroponically in the greenhouse and nourished with Hoagland-type solutions (CitationHoagland and Arnon 1938) as follows: (macronutrients: KCl 6 mmol L−1, Ca[NO3]2 2 mmol L−1, NH4NO3 5 mmol L−1, NH4H2PO4 1 mmol L−1, MgSO4 1 mmol L−1; micronutrients: MnCl2·4H2O [1.81 g L−1], H3BO3[2.86 g L−1], ZnSO4·7H2O [0.22 g L−1], [NH4]6Mo7O24·4H2O [0.09 g L−1], CuSO4·5H2O [0.08 g L−1], Fe-ethylendiamine–N, N, N1, N1-tetraacetic acid (EDTA) [7.54 g L−1]). The initial pH of the nutrient solution was adjusted to 6.0.
Plants were watered every 2–3 days in the morning with the nutrient solutions. All samples were harvested on 12 November (49 days after sowing) and separated into leaves and roots. Samples were then lyophilized, ground and stored in a plastic bottle until chemical analysis. Four plants per treatment were analyzed to determine GSL contents.
Chemicals
The HPLC-grade acetonitrile (CH3CN), gallic acid, hydrogen peroxide (H2O2, 35%), acetaldehyde (CH3CHO, approximately 90%) and ODS C18 matrix (Wakosil 40 C18, 30–50 µm particles) for column chromatography were purchased from Wako Pure Chemical Industries (Osaka, Japan). Aryl sulfatase (type H-1, EC 3.1.6.1) was obtained from Sigma-Aldrich Chemical Company (St Louis, MO, USA), and sinigrin (2-propenyl GSL) from Tokyo Kasei Kogyo Company (Tokyo, Japan) as an internal (extraction) standard. DEAE-Sephadex A−25 was supplied by Amersham Biosciences (Uppsala, Sweden).
Desulfo-glucosinolate analysis
Crude GSLs were extracted with 70% (v/v) boiling MeOH from defatted seed meal and ground plants and applied to DEAE-Sephadex A−25. The GSLs were then converted to their DS-GSLs by overnight treatment with aryl sulfatase (). The eluate containing the desulfated products was analyzed using an HPLC-equipped Inersil ODS-2 column (4.6 mm × 250 mm, GL Science, Tokyo, Japan) at 227 nm as previously described (CitationKim et al. 2004). Individual DS-GSLs were identified by comparing their retention times using HPLC and MS spectra. DS-4-methoxyglucobrassicin was purified from the leaves of rocket salad using a 40C18 ODS open column (30 cm × 3.0 cm and 80 cm × 1.0 cm internal diameter) after desulfation (CitationKim et al. 2004).
Mass spectrometry
Analysis of DS-GSLs was carried out on an API-100 instrument (Perkin-Elmer Sciex Instruments, Pomona, CA, USA) equipped with an atmospheric pressure ESI source. The MS operating conditions were as follows: ionspray voltage, 4.8 kV (positive-ion mode); orifice voltage, 40 V; nebulizer gas, air; curtain gas, nitrogen. The purified DS-4-methoxyglucobrassicin directly collected from the HPLC effluent was directly injected in 0.1% AcOH solution into the ESI solvent flow (10 µL min−1) using a loop injection valve. Spectra were scanned in the range m/z 100–1000. The API 100 instrument was used with TUNE software (C-Preliminary Release version) for data acquisition and evaluation (CitationKim et al. 2004).
Anti-oxidative activity
The anti-oxidative properties of plant powders and the purified GSL were detected by CL using the H2O2-acetaldehyde system as previously described in detail (CitationKim et al. 2004). The anti-oxidative activities were quantitatively measured in comparison with CL intensity (peak area for 1 min at 23°C) of gallic acid (100 µL of 1 mmol L−1/1 mL, 100 µmol L−1 = 0.10 unit g−1) as the synthetic anti-oxidant, and the value (unit g−1) was calculated based on 1 unit = CL intensity of a 1 mmol L−1 gallic acid solution (CitationIwai et al. 2000).
RESULTS AND DISCUSSION
Separation and identification of DS-GSLs using liquid chromatograph mass spectrometry
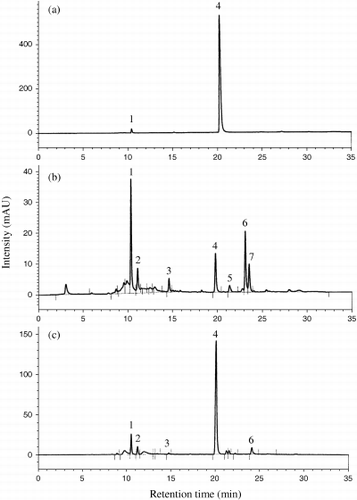
The HPLC chromatograms of DS-GSLs separated from defatted seeds, leaves and roots of rocket salad are shown in . The DS-GSLs were identified based on their retention times compared with those in our database. For confirmation, mass spectra of the individual DS-GSLs were obtained using positive-ion ESI-MS analysis. The HPLC profiles were very similar to the HPLC chromatograms, except for indolyl GSLs, reported by CitationBennett et al. (2002), who used the same solvent mixture and the same DS-GSL extraction method. Their identities, including chemical structures, trivial (semisystematic) names and molecular weights are listed in . Peak numbers followed the elution order of HPLC profiles are shown in , and their retention times from the HPLC chromatograms shown in . The DS-GSLs were identified from their protonated molecular ions [M+H]+ in the mass spectra of DS-GSLs. Peaks 1 and 3 were identified using ESI-MS as DS-glucoraphanin and DS-glucoerucin and had molecular weights of 357 (m/z 358, [M+H]+) and 341 (m/z 342, [M+H]+), respectively (data not shown). DS-sinigrin (SIN, peak 2) is commonly used as an external or internal standard. Interestingly, two indolyl GSLs in the previous study (CitationKim et al. 2004) were detected from the concentrated extracts of rocket leaves according to their retention times compared to our database (i.e. HPLC chromatograms of broccoli, Chinese cabbage, cauliflower, rapeseed and vegetable turnip rape). In the present study, the two indolyl GSLs (peaks 4 and 6) were characterized in detail using ESI-MS and identified as DS-glucobrassicin and DS-4-methoxyglucobrassicin (, respectively). The protonated molecular ion [M+H]+ and a group-specific
Table 1 Structures of the desulfo-glucosinolates isolated from rocket salad
Figure 2 High-pressure liquid chromatography (HPLC) profiles of desulfo-glucosinolates (DS-GSL) isolated from rocket salad (a) seeds, (b) leaves and (c) roots. Peak numbers refer to the GSLs listed in Table 1. 1, DS-glucoraphanin; 2, DS-sinigrin, used as an internal standard; 3, DS-4-(β-D-glucopyranosyldisulfanyl)butyl GSL; 4, DS-glucoerucin; 5, DS-glucobrassicin; 6, DS-dimeric 4-mercaptobutyl GSL; 7, DS-4-methoxyglucobrassicin.
The identified DS-GSLs consisted of two aliphatic DS-GSLs (DS-glucoraphanin and DS-glucoerucin), two
Figure 3 Molecular ion region of electrospray ionization mass spectrometry of two indolyl desulfo-glucosinolates isolated from rocket salad. (a) Spectrum of DS-glucobrassicin [m/z 369, (M+H)+]; (b) spectrum of DS-4-methoxyglucobrassicin [m/z 399, (M+H)+]. Spectra were recorded between m/z 100 and 1000, but are shown between m/z 100 and 550.
![Figure 3 Molecular ion region of electrospray ionization mass spectrometry of two indolyl desulfo-glucosinolates isolated from rocket salad. (a) Spectrum of DS-glucobrassicin [m/z 369, (M+H)+]; (b) spectrum of DS-4-methoxyglucobrassicin [m/z 399, (M+H)+]. Spectra were recorded between m/z 100 and 1000, but are shown between m/z 100 and 550.](/cms/asset/825fe0a6-1ad2-466c-8d54-dc6468e4d6e1/tssp_a_10382391_o_f0003g.gif)
Table 2 Variation in proportion (expressed as % of total glucosinolate [GSL]) of individual desulfo-GSLs (DS-GSLs) identified in the rocket salad cultivar “Odyssey”
Proportions of GSLs among different plant organs
The proportions of the individual DS-GSLs to total GSL content in the seeds, leaves and roots are shown in . The highest total GSL content was observed in the seeds (125 µmol g−1 dry weight [DW]), and this value was approximately six-fold greater than that recorded in the roots (22 µmol g−1 DW) and 12-fold that of the leaves (11 µmol g−1 DW). Given its very low total GSL content, rocket salad is suitable for consumption as a leafy salad vegetable. The European Community fixed the total GSL level of defatted rapeseed meal at below 35 µmol g−1 DW of seed and the recommendation for rapeseed was below 20 µmol g−1 DW from 1991 because of undesirable effects on humans and livestock owing to pungency and goitrogenic activity (CitationMilford and Evans 1991). Predicting the total or individual GSL content in edible parts of rocket, compared to their contents as determined by breeders, would reduce the time necessary for whole plant analysis. Moreover, total GSL contents vary widely in the different organs of any given plant (CitationKim et al. 2001, Citation2003; CitationSang et al. 1984). Therefore, it is difficult to determine which plant organs contain the majority of the total whole plant GSL content (CitationBarillari et al. 2005). As the roles of GSLs in plants become clearer, it may be necessary to monitor the content or the relative proportion of individual GSLs rather than total GSL.
Two DS-GSLs, DS-glucoraphanin and DS-glucoerucin, were found to be the predominant GSLs in both seeds and roots, while three DS-GSLs (DS-glucoraphanin, DS-glucoerucin and DS-dimeric 4-mercaptobutyl GSL) were the main components in the leaves (> 1 µmol g−1 DW or > 10% of the total GSL content). Thus, our study is in agreement with the finding that seeds generally present simpler HPLC profiles together with higher GSL levels compared to leaves (CitationKim et al. 2001; CitationSang et al. 1984). Interestingly, DS-dimeric 4-mercaptobutyl GSL, which presented the greatest proportion (52%) of total DS-GSLs in the leaves, was not found in the seeds and only detected in roots in trace amounts (4%). The two indolyl DS-GSLs were found in the leaves as trace amounts. DS-4-(β-D-glucopyranosyldisulfanyl)butyl GSL accounted for 7% of DS-GSLs in the leaves, but only trace amounts (< 1%) in the roots.
Anti-oxidative activity of the new GSL
Anti-oxidants are believed to play a very important role in the human body's defense system against reactive oxygen species such as superoxide radicals, singlet oxygen, H2O2 and hydroxyl radicals. The anti-oxidant activities of intact leaf/root powder, whole DS-GSL eluates and purified DS-4-methoxymglucobrassicin were measured using the XYZ-dish method (). The activity of intact leaf powder (1.5 unit g−1) was greater than that of intact root powder (0.87 unit g−1) even though the total GSL content (11 µmol g−1 DW) of the leaves was half that of the roots (22 µmol g−1 DW) (). This indicates that not only GSLs, but other phytochemicals such as flavonoids and polyphenols, present anti-oxidant activities (CitationPlumb et al. 1996).
DS-4-methoxyglucobrassicin showed the greatest activity among the samples examined (3.8 unit g−1), two-fold
Table 3 Anti-oxidative activity in rocket salad
ACKNOWLEDGMENT
The authors wish to thank the Japanese Society for the Promotion of Science (JSPS) for financial assistance.
REFERENCES
- Ashraf , M and Noor , R . 1992 . Growth and pattern of ion uptake in Eruca sativaMill. under salt stress . Angew Bot , 67 : 17 – 21 .
- Pita Villamil , JM , Perez-Garcia , F and Martinez-Laborde , JB . 2002 . Time of seed collection and germination in rocket, Eruca vesicaria(L.) Cav. (Brassicaceae) . GenetResourCrop Evol , 45 : 47 – 51 .
- Bianco , VV , Santamaria , P and Elia , A . 1998 . Nutritional value and nitrate content in edible wild species used in southern Italy . Acta Hortic , 467 : 71 – 87 .
- Sanatamaria , P and Gonnella , M . 2001 . Ways of reducing rocket salad nitrate content . Acta Hort , 548 : 529 – 536 .
- Satamaria , P , Elia , A , Papa , G and Serio , F . 1998 . Nitrate and ammonium nutrition in chicory and rocket salad plants . JPlant Nutr , 21 : 1779 – 1789 .
- Satamaria , P , Elia , A , Parente , A and Serio , F . 2002 . Effect of solution nitrogen concentration on yield, leaf element content, and water and nitrogen use efficiency of three hydroponically-grown rocket salad genotypes . JPlant Nutr , 25 : 245 – 258 .
- Bennett , RN , Mellon , FA , Botting , NP , Eagles , J , Rosa , EAS and Williamson , G . 2002 . Identification of the major glucosinolate (4-mercaptobutyl glucosinolate) in leaves of Eruca sativaL. (rocket salad) . Phytochemistry , 61 : 25 – 30 .
- Cerny , MS , Taube , E and Battaglia , R . 1996 . Identification of bis (4-isothiocyanatobutyl) disulfide and its precursor from rocket salad (Eruca sativa) . JAgricFood Chem , 44 : 3835 – 3839 .
- Jirovetz , L , Smith , D and Buchbauer , G . 2002 . Aroma compound analysis of Eruca sativa(Brassicaceae) SPME headspace leaf samples using GC, GC-MS, and olfactometry . JAgricFood Chem , 50 : 4643 – 4646 .
- Miyazawa , M , Maehara , T and Kurose , K . 2002 . Composition of the essential oil from the leaves of Eruca sativa . Flavour FragrJ , 17 : 187 – 190 .
- Weckerle , B , Michel , K , Balázs , B , Schreier , P and Tóth , G . 2001 . Quercetin 3, 3′, 4′-tri-O-β-D-glucopyranosides from leaves of Eruca sativa(Mill.) . Phytochemistry , 57 : 547 – 551 .
- Fahey , JW , Zalcmann , AT and Talalay , P . 2001 . The chemical diversity and distribution of glucosinolates and isothiocyanates among plants . Phytochemistry , 56 : 5 – 51 .
- Olsen , O and Sørensen , H . 1981 . Recent advances in the analysis of glucosinolates . JAmOil ChemSoc , 58 : 857 – 865 .
- Wallsgrove , RM , Doughty , KJ and Bennett , RN . 1999 . “ Glucosinolates ” . In Plant Amino Acids: Biochemistry and Biotechnology , Edited by: Singh , BK . 523 – 562 . New York : Marcel Dekker .
- Kiddle , G , Bennett , RN , Botting , NP , Davidson , NE , Robertson , AAB and Wallsgrove , RM . 2001 . High-performance liquid chromatographic separation of natural and synthetic desulphoglucosinolates and their chemical validation by UV, NMR and chemical ionisation-MS methods . PhytochemAnal , 12 : 226 – 242 .
- Iori , R , Bernardi , R , Gueyrard , D , Rollin , P and Palmieri , S . 1999 . Formation of glucoraphanin by chemoselective oxidation of natural glucoerucin: A chemoenzymatic route to sulforaphane . BioorgMedChemLett , 9 : 1047 – 1048 .
- Kim , S-J , Jin , S and Ishii , G . 2004 . Isolation and structure elucidation of 4-(β-d-glucopyranosyldisulfanyl) butyl glucosinolate from leaves of Rocket Salad (Eruca sativaL.) and its antioxidative activity . BiosciBiotecBiochem , 68 : 2444 – 2450 .
- Macfarlane-Smith , WH and Griffiths , DWA . 1988 . A time-course study of glucosinolates in the ontogeny of forage rape (Brassica napusL.) . JSciFood Agric , 43 : 121 – 134 .
- Sang , JP , Minchinton , IR , Johnstone , PK and Truscott , RJW . 1984 . Glucosinolate profiles in the seed, root and leaf tissue of cabbage, mustard, rapeseed, radish and swede . CanJPlant Sci , 64 : 77 – 93 .
- Bradfield , CA and Bjeldanes , LF . 1984 . Effect of dietary indole-3-carbinol on intestinal and hepatic monooxygenase, glutathione S-transferase and epoxide hydrolase activities in the rat . Food ChemToxicol , 22 : 977 – 982 .
- Gross , HB , Dalebout , T , Grubb , CD and Abel , S . 2000 . Functional detection of chemopreventive glucosinolates in Arabidopsis thaliana . Plant Sci , 159 : 265 – 272 .
- Mithen , RF , Dekker , M , Verkerk , R , Rabot , S and Johnson Ian , T . 2000 . The nutritional significance, biosynthesis and bioavailability of glucosinolates in human foods: Review) . JSciFood Agric , 80 : 967 – 984 .
- Nho , CW and Jeffery , E . 2004 . Crambene, a bioactive nitrile derived from glucosinlate hydrolysis, acts via the antioxidant response element to upregulate quinine reductase alone or synergistically with indole-3-carbinol . ToxicolApplPharmacol , 198 : 40 – 48 .
- Plumb , GW , Lambert , N Chambers , SJ . 1996 . Are whole extracts and purified glucosinolates from cruciferous vegetables antioxidatives? . Free RadRes , 25 : 75 – 86 .
- Yoshiki , Y , Okubo , K , Onuma , M and Igarashi , K . 1995 . Chemiluminescence of benzoic and cinnamic acids, and flavonoids in the presence of aldehyde and hydrogen peroxide or hydroxyl radical by Fenton reaction . Phytochemistry , 39 : 225 – 229 .
- Hoagland , DR and Arnon , DI . 1938 . The Water Culture Method for Growing Plants Without Soil , Berkley : California Agric Expt Sta . Circ 347
- Iwai , K , Abe , K and Matsue , H . 2000 . Establishment of XYZ-dish method for a new antioxidative activity assay using photon detection . Nippon Shokuhin Kagaku Kogaku Kaishi , 47 : 181 – 190 . (in Japanese)
- Barillari , J , Canistro , D Paolini , M . 2005 . Direct antioxidant activity of purified glucoerucin, the dietary secondary metabolite contained in rocket (Eruca sativaMill.) seeds and sprouts . JAgricFood Chem , 53 : 2475 – 2482 .
- International Organization for Standardization . 1992 . Rapeseed-determination of gluco-sinolates content , Vol. 9167–1 , 1 – 9 . ISO .
- Milford , GFJ and Evans , EJ . 1991 . Factors causing variation in GS in oilseed rape . Outlook Agric , 20 : 31 – 37 .
- Kim , S-J , Ishida , M , Matsuo , T , Watanabe , M and Watanabe , Y . 2001 . Separation and identification of glucosinolates of vegetable turnip rape by LC/APCI-MS and comparison of their contents in ten cultivars of vegetable turnip rape (Brassica rapaL.) . Soil SciPlant Nutr , 47 : 167 – 177 .
- Kim , S-J , Kawaguchi , S and Watanabe , Y . 2003 . Glucosinolate in vegetative tissues and seeds of twelve cultivars of vegetable turnip rape (Brassica rapaL.) . Soil SciPlant Nutr , 49 : 337 – 346 .
- Yoshiki , Y , Onda , N and Okubo , K . 2004 . Relationship between photon emission and chemopreventive potential of tea . Food Chem. , 87 : 269 – 274 .