Abstract
To gain a better understanding of the role of charred plant materials, which were produced during the burning of vegetation by human activity and wildfires, in the formation of humic and fluvic acids in Japanese volcanic ash soils, the quantitative contribution of charred and buried plant fragments to their acids in whole soils were investigated using three volcanic ash soil samples. Charred fragments were the main components in the fraction of less than specific gravity 1.6 g cm−3 (< 1.6 fraction), which was isolated after HCl–HF treatment of the soil samples. The percentage contribution of organic C content in the < 1.6 fractions to that of the whole soils ranged from 13.9 to 32.0%. All humic acids obtained from the < 1.6 fractions and whole soils were classified into Type A, which is characterized by a high degree of darkening and the presence of a graphite-like structure. However, the color coefficient (ΔlogK) and relative color intensity (RF) values of the humic acids in the < 1.6 fractions were different from those in whole soils. In all soils studied, the amounts of NaOH-extractable humic (OH-HA) and fulvic acids (OH-FA) were much greater than those of Na4P2O7-extractable humic (SPP-HA) and fulvic acids (SPP-FA), respectively, and the amounts of humic acids were substantially greater than those of fulvic acids. The proportion of the quantitative contribution of humic and fulvic acids in the < 1.6 fractions to those in the whole soils ranged from 12.0 to 43.8% for OH-HA, from 3.80 to 9.56% for OH-FA, and from 2.92 to 22.3% for SPP-HA, respectively. The proportion was very small for SPP-FA. It was assumed that in Japanese volcanic ash soils, parts of charred plant materials are subjected to oxidative degradation over a long period of time after burning, and are converted to fulvic acids and, particularly, humic acids.
INTRODUCTION
In Japan, volcanic ash soils (Ando soils or Kurobokudo) developed in deposits of volcanic fallout are widely distributed. These soils often display a thick black or brownish black A horizon with a high humus content that may reach 300 mg per g soil (CitationMinistry of Agriculture and Forestry, Japanese Government 1964). An important feature of humus in the soils is the predominance of black (Type A) humic acids with a high degree of darkening and a graphite-like structure (e.g. CitationKumada 1987). The physicochemical and spectroscopic properties of Type A humic acids have been well documented over the past years (e.g. CitationKumada 1987). The chemical structures of the humic acids have also been investigated (CitationKramer et al. 2004; CitationKumada 1987). Furthermore, we had assumed, based on several findings, that charred plant materials, which were produced during the burning of vegetation by human activity and wildfires, could be an important source of Type A humic acids in Japanese volcanic ash soils (CitationShindo and Honma 2001; CitationShindo et al. 2004a,Citationb). In contrast, the mechanisms of the formation of fulvic acids in volcanic ash soils have received little attention, although the chemistry of fulvic acids has progressed (e.g. CitationWatanabe et al. 1994). The objective of the present study was to gain a better understanding of the role of charred plant materials in the formation of humic and fulvic acids in Japanese volcanic ash soils. We mainly investigated the quantitative contribution of charred and buried plant fragments to humic and fulvic acids in whole soils using three volcanic ash soils with different organic C contents.
MATERIALS AND METHODS
Soil samples
Three volcanic ash soil samples differing in organic C content, horizon and location were used in this study. These samples were collected at depths of 15–63 cm from three different profiles of typical volcanic ash soils in Honshu District, Japan (). Each soil sample was air-dried and passed through a 2-mm mesh sieve. Plant remains, such as stems and roots, in the samples were carefully removed under a stereomicroscope.
Isolation of charred and buried plant fragments from whole soils
Charred and buried plant fragments in the soils were isolated using a modification of the specific gravity (s.g.) method described by CitationShindo et al. (2004a). The separation medium of s.g. 1.6 g cm−3 was prepared using sodium polytungstate powder (SOMETU-BERLIN) and deionized water (water). An adequate amount of the sieved soil samples (0.5–2 g) was placed in a Teflon beaker, to which 5 mL of 12 mol L−1 HCl and 5 mL of 46–48% (approx. 16.75 mol L−1) HF was added. The beaker was heated at 200°C on a hotplate until the contents became dry. This treatment was repeated again (CitationJapanese Society of Analytical Chemistry 1981). After the HCl–HF treatment, the residues were washed with 10 mL of l mol L−1 HCl and 30 mL of water, successively, by centrifugation (1,700 g for 20 min) to remove minerals. The washed residues were transferred on a membrane filter (0.1 µm, cellulose nitrate, Advantec Toyo, Tokyo, Japan), washed thoroughly with water until chloride ions were no longer detected with AgNO3, and then oven-dried at 90°C for 12 h. Subsequently, the residues on the filter were dislodged with a microspatula and placed in a 50 mL polyethylene centrifuge tube. To the tube, 20 mL of s.g. 1.6 g cm−3 sodium polytungstate solution was added. This tube containing suspension was allowed to stand for 30 min, sonified at 39 kHz and 100 W for
Table 1 Brief description of the soils used†
The yield of the < 1.6 fraction was determined in duplicate, and portions of the yield were used for microscopic observation and determination of organic C. As a large amount of the < 1.6 fraction was required to analyze the humus composition of the fraction, the isolation of the fraction was newly repeated more than 10 times. The combined and crushed samples were used for the analysis.
Microscopic observation of the < 1.6 fraction
The < 1.6 fraction was observed under a stereomicroscope, and part of the <1.6 fraction was mounted on an aluminum stub with a carbon adhesive tape and coated with a thin conductive layer of gold. The morphological characteristics of the charred plant fragments on the stub were observed in detail using a JSM-6100 scanning electron microscope (SEM).
Determination of organic C in the < 1.6 fractions and whole soils
Organic C content was determined using the dichromate-sulfuric acid oxidation method described in CitationKononova (1966). In the present study, an adequate amount of the < 1.6 fractions or the whole soil was digested with the oxidant at approximately 200°C for 30 min in a 100 mL flask with a condenser on a hotplate (CitationShindo et al. 2004a). After digestion, the remaining chromate ions (VI) were titrated with ferrous ammonium sulfate using orthophenanthroline as an indicator for the end point. Organic C content was calculated from the difference of the titration values between the blank and the sample. The analysis was carried out in duplicate.
Analysis of the humus composition of the < 1.6 fractions and whole soils
The analysis of the humus composition was carried out according to the method described by CitationKumada (1967) and conducted in duplicate. Organic matter was extracted successively with 0.1 mol L−1 NaOH and 0.1 mol L−1 Na4P2O7 at 100°C for 30 min. The NaOH and Na4P2O7 extracts were separated into humic and fulvic acids by the addition of concentrated H2SO4. In this study, NaOH-extractable humic (OH-HA) and fulvic acids (OH-FA) and Na4P2O7-extractable humic (SPP-HA) and fulvic acids (SPP-FA) were obtained. The precipitate, humic acid, was washed successively with H2SO4 (1:100) and water and then dissolved in 0.01 mol L−1 NaOH. The absorbance of the humic acid solution at 400 and 600 nm was measured using a JASCO Ubest-50 spectrophotometer. The amounts of C in the humic and fulvic acids were determined using the KMnO4 oxidation method. In this study, 1 mL of 0.02 mol L−1 KMnO4 consumed was calculated as corresponding to 0.48 mg C (CitationIkeya and Watanabe 2003). The type of humic acid was determined using color coefficient (ΔlogK) and relative color intensity (RF) values, where the ΔlogK value is the logarithm of the ratio of the absorbance of humic acid at 400 nm to that at 600 nm and the RF value represents the absorbance of humic acid at 600 nm multiplied by 1,000 and divided by the number of mL of 0.02 mol L−1 KMnO4 consumed by 30 mL of humic acid solution.
RESULTS AND DISCUSSION
In all soils studied, microscopic observation indicated that charred plant fragments, which are black or blackish brown, were the main components in the < 1.6 fractions isolated after direct HCl–HF treatment. The morphological characteristics of the fragments were similar among the soil samples used. As an example, microscopic photographs of the < 1.6 fraction of soil sample No. 1 are given in . The fragments were mainly amorphous, although the shapes of several fragments were similar to those of vascular tissues of woody and herbaceous plants (CitationEsau 1960). These findings agreed with the previous results observed for the fractions of less than s.g. 1.6 g cm−3, which were isolated after HCl–HF treatment of s.g. 1.6–2.0 g cm−3 fractions of 24 volcanic ash soil samples (CitationShindo et al. 2004a).
As shown in , the percentage contribution of organic C content in the < 1.6 fraction to that of the whole soil was 25.3, 32.0 and 13.9% for soil samples 1, 2 and 3, respectively, indicating that charred and buried plant fragments are one of the important C constituents in the soils studied. In European Chernozemic soils (CitationSchmidt et al. 1999) and US agriculture soils (CitationSkjemstad et al. 2002), charcoal C contributed up to 45% and 35% of the soil total organic C, respectively. These findings suggest that charred plants should not be overlooked as one of the constituents of soil organic matter.
In this study, all the humic acids obtained from the < 1.6 fractions () belonged to Type A. According to CitationKumada (1987), all the humic acids obtained from fresh and decayed plant residues and composts belonged to Type Rp, with a low degree of darkening. When the powder specimens of fresh Susuki (Miscanthus sinensis) and Sugi (Cryptomeria japonica) plants (CitationShindo et al. 2003) were treated with HCl–HF as in the case of the soil samples, all the OH-HA obtained from their residues belonged to Type Rp, indicating that Type A humic acids in the < 1.6 fractions were not artificially produced during the HCl–HF treatment in this study.
Figure 1 (a) Stereomicroscopic and (b) scanning electron microscopic images of the < 1.6 fraction isolated from soil sample No. 1.
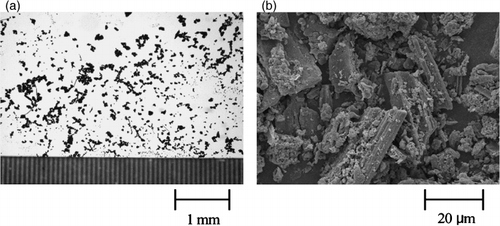
Table 2 Total organic C content of whole soil, and the yield and organic C content of the < 1.6 fraction
Table 3 Quantitative and qualitative characteristics of NaOH-extractable and Na4P2O7-extractable humic and fulvic acids in the < 1.6 fractions isolated from soils (F) and in the whole soils (W)
Similar to the < 1.6 fractions (), all the humic acids obtained from the whole soils () belonged to Type A. However, the following differences were found on the optical properties of humic acids between the < 1.6 fraction and the whole soil: (1) in the OH-HA, although there were no remarkable differences on the ΔlogK values between the < 1.6 fractions (0.521–0.537) and the whole soils (0.520–0.539), the RF values of the < 1.6 fractions (142–159) were higher than those of the whole soils (122–150), (2) in the SPP-HA, the ΔlogK values of the < 1.6 fractions (0.544–0.608) were higher than those of the whole soils (0.469–0.523), while the RF values of the former (101–126) tended to be lower than those of the latter (111–151). These results indicate that Type A humic acids in the soils studied are composed of humic acids with different ΔlogK and RF values.
In the whole soils () and, particularly, the < 1.6 fractions (), the amounts of the OH-HA and OH-FA were much greater than those of the SPP-HA and SPP-FA, respectively. Furthermore, the amounts of the OH-HA and SPP-HA were considerably greater than those of the OH-FA and SPP-FA, respectively. From these data on the humus composition of the < 1.6 fractions and whole soils and the yields of < 1.6 fractions in whole soils (), the degrees of the quantitative contribution of humic and fulvic acids in the < 1.6 fractions to those in the whole soils were estimated (). The percentage contribution of the amounts of the OH-HA and OH-FA in the < 1.6 fraction to those in the whole soils ranged from 12.0 to 43.8% and from 3.80 to 9.56%, respectively. In contrast, the percentage contribution of the amounts of the SPP-HA in the < 1.6 fraction to those in the whole soils ranged from 2.92 to 22.3%. The contribution of the amounts of the SPP-FA in the < 1.6 fraction was not estimated because the amounts of the fulvic acids were very small ().
In this study, the 14C ages of the charred and buried plant fragments were not determined. According to
Table 4 Quantitative contribution (%) of humic and fulvic acids in the < 1.6 fraction to those in whole soil
ACKNOWLEDGMENTS
We thank the members of the Cooperative Research Projects on Ando soils (volcanic ash soils), especially Dr T. Honna and Dr T. Yamamoto, Faculty of Agriculture, Tottori University, for supplying the soil samples, and we also thank Dr H. Honma, Forensic Science Laboratory, Yamaguchi Prefectural Police Headquarters, for his assistance.
REFERENCES
- Ministry of Agriculture and Forestry, Japanese Government . 1964 . Volcanic Ash Soils in Japan , Tokyo : Sakurai Kosaido .
- Kumada , K . 1987 . Chemistry of Soil Organic Matter , Tokyo : Japan Scientific Societies Press and Elsevier .
- Kramer , RW , Kujawinski , EB and Hatcher , PG . 2004 . Identification of black carbon derived structures in a volcanic ash soil humic acid by Fourier transform ion cyclotron resonance mass spectrometry . EnvironSciTechnol , 38 : 3387 – 3395 .
- Shindo , H and Honma , H . 2001 . “ Significance of burning vegetation in the formation of black humic acids in Japanese volcanic ash soils ” . In Humic Substances: Structures, Models and Functions , Edited by: Ghabbour , EA and Davies , G . 297 – 306 . Cambridge : Royal Society of Chemistry .
- Shindo , H , Honna , T , Yamamoto , S and Honma , H . 2004a . Contribution of charred plant fragments to soil organic carbon in Japanese volcanic ash soils containing black humic acids . OrgGeochem , 35 : 235 – 241 .
- Shindo , H , Ushijima , N , Hiradate , S , Fujitake , N and Honma , H . 2004b . Production and several properties of humic acids during decomposition process of charred plant materials in the presence of H2O2 . Humic Subst. Res. , 1 : 29 – 37 .
- Watanabe , A , Itoh , K , Arai , S and Kuwatsuka , S . 1994 . Comparison of the composition of humic and fulvic acids prepared by the IHSS method and NAGOYA method . Soil SciPlant Nutr , 40 : 601 – 608 .
- Japanese Society of Analytical Chemistry . 1981 . Handbook of Analytical Chemistry , Tokyo : Maruzen . (in Japanese)
- Kononova , MM . 1966 . Soil Organic Matter , 2nd English edn , Oxford : Pergamon Press .
- Kumada , K , Sato , O , Ohsumi , Y and Ohta , S . 1967 . Humus composition of mountain soils in central Japan with special reference to the distribution of P type humic acid . Soil SciPlant Nutr , 13 : 151 – 158 .
- Ikeya , K and Watanabe , A . 2003 . Direct expression of an index for the degree of humification of humic acids using organic carbon concentration . Soil SciPlant Nutr , 49 : 47 – 53 .
- Esau , K . 1960 . Anatomy of Seed Plants , 2nd edn , New York : John Wiley & Sons .
- Schmidt , MWI , Skjemstad , JO , Gehrt , E and Kögel-knabner , I . 1999 . Charred organic carbon in German chernozemic soils . EurJSoil Sci , 50 : 351 – 365 .
- Skjemstad , JO , Reicosky , DC , Wilts , AR and McGowan , JA . 2002 . Charcoal carbon in U.S. agricultural soils . Soil SciSocAmJ , 66 : 1249 – 1255 .
- Shindo , H , Ushijima , N and Amano , Y . 2003 . “ Comparison of elementary and humus composition of woody plants before and after burning ” . In Soil SciPlant Nutr Vol. 49 , 685 – 693 .
- Yamanoi , T . 1996 . Geological investigation on the origin of the black soil distributed in Japan . JourGeolSocJapan , 102 : 526 – 544 . (in Japanese with English summary)
- Shindo , H , Yoshida , M , Yamamoto , A , Honma , H and Hiradate , S . 2005 . δ13C values of organic constituents and possible source of humic substances in Japanese volcanic ash soils . Soil Sci , 170 : 175 – 182 .
- Shindo , H and Honma , H . 1998 . Comparison of humus composition of charred susuki (eulalia, Miscanthus sinensis) plants before and after HNO3treatment . Soil SciPlant Nutr , 44 : 675 – 678 .
- Wada , K . 1986 . “ Part II Date base ” . In Ando Soils in Japan , Edited by: Wada , K . 115 – 276 . Fukuoka : Kyushu University Press .