Abstract
The transformation of added phosphorus (P) to soil and the effect of soil properties on P transformations were investigated for 15 acid upland soils with different physicochemical properties from Indonesia. Based on oxide-related factor scores (aluminum (Al) plus 1/2 iron (Fe) (by ammonium oxalate), crystalline Al and Fe oxides, cation exchange capacity, and clay content) obtained from previous principal component analyses, soils were divided into two groups, namely Group 1 for soils with positive factor scores and Group 2 for those with negative factor scores. The amounts of soil P in different fractions were determined by: (i) resin strip in bicarbonate form in 30 mL distilled water followed by extraction with 0.5 mol L−1 HCl (resin-P inorganic (Pi) that is readily available to plant), (ii) 0.5 mol L−1 NaHCO3 extracting Pi and P organic (Po) (P which is strongly related to P uptake by plants and microbes and bound to mineral surface or precipitated Ca-P and Mg forms), (iii) 0.1 mol L−1 NaOH extracting Pi and Po (P which is more strongly held by chemisorption to Fe and Al components of soil surface) and (iv) 1 mol L−1 HCl extracting Pi (Ca-P of low solubility). The transformation of added P (300 mg P kg−1) into other fractions was studied by the recovery of P fractions after 1, 7, 30, and 90 d incubation. After 90 d incubation, most of the added P was transformed into NaOH-Pi fraction for soils of Group 1, while for soils of Group 2, it was transformed into resin-Pi, NaHCO3-Pi and NaOH-Pi fractions in comparable amounts. The equilibrium of added P transformation was reached in 30 d incubation for soils of Group 1, while for soils of Group 2 it needed a longer time. Oxide-related factor scores were positively correlated with the rate constant (k) of P transformation and the recovery of NaOH-Pi. Additionally, not only the amount of but also the type (kaolinitic) of clay were positively correlated with the k value and P accumulation into NaOH-Pi. Soils developed from andesite and volcanic ash exhibited significantly higher NaOH-Pi than soils developed from granite, volcanic sediments and sedimentary rocks. Soil properties summarized as oxides-related factor, parent material, and clay mineralogy were concluded very important in assessing P transformation and P accumulation in acid upland soils in Indonesia.
INTRODUCTION
Phosphorus (P) sorption experiments in acid upland soils in Indonesia showed that soils varied widely in their capacity to sorb P (CitationHartono et al. 2005). Principal component analyses and stepwise regression showed that oxide-related factor (aluminum [Al] plus 1/2 iron [Fe][by ammonium oxalate], crystalline Al and Fe oxides, cation exchange capacity [CEC] and clay content) were the main components actively contributing to their P sorption maxima and bonding energies in Langmuir simulation. The soils with high scores of oxide-related factor exhibited high P sorption maxima and bonding energies. Desorption experiments showed that by extracting with 0.01 mol L−1 CaCl2, the soils had low desorbability of the sorbed P (CitationHartono et al. 2005). One of the explanations for this result was that it might correspond to irreversible reactions of adsorbed P with soil compounds that lead to a stronger bond through rearrangement of the phosphate ions on the surface (CitationKuo and Lotse 1973).
In general, added fertilizer P transforms into relatively insoluble compounds of Al and Fe in acid soils (CitationGhani and Islam 1946). Long-term transformation of fertilizer added P under cultivation in the field in acid soils (e.g. according to field experiments) is transformed not only into the form of inorganic P of Al and Fe fractions, but also into the form of organic P fractions and these P were retained with a different bond (CitationOberson et al. 2001; CitationSchmidt et al. 1996; CitationVerma et al. 2005; CitationZheng et al. 2002).
Most of the upland soils in Indonesia are acidic in reaction and are deficient in available P. Acid soils account for approximately 57% of the total upland soils and are developed from different types of parent materials (CitationSubagyo et al. 2000). Phosphorus fertilization is a key component of increasing soil productivity in these acid upland soils. The adsorption of additional P decreases as the quantity of P already adsorbed increases (CitationBarrow 1990); thus, repeated additions have cumulative benefits. Accordingly, the best strategy to manage P in these upland soils is to apply P fertilizer in very large amounts in an initial addition or in frequent small applications, depending on the characteristics of the soils to have large and long-term residual values (CitationCassman et al. 1993; CitationKamprath 1967; CitationLinquist et al. 1996).
The differences in soil properties related to oxide-related factor and clay mineralogy in these upland soils may have different P distributions to the added P. Information on P transformation of added water-soluble P into various P pools and the effect of the soil properties to the P transformation process in these acid upland soils have not been well evaluated. Understanding the transformation of added P, for example, in one cropping season (90 days), in these soils is very important to identify appropriate P management strategies. The objectives of the present study were to examine: (1) the transformation process of added P to soils, (2) the differences in the dominant P fraction among soil types and how these fractions are determined by soil properties.
MATERIALS AND METHODS
Soil samples
Surface horizons from 15 acid upland soils from Sumatra, Java and Kalimantan were used in this study. Soil samples were air-dried and crushed to pass through a 2-mm mesh sieve. Selected chemical and physical properties that can affect P dynamics were analyzed by CitationHartono et al. (2005). Principal component analysis demonstrated that three principal components in Indonesian acid upland soils influenced P sorption, namely oxide-related factor (Al + 1/2 Fe [by ammonium oxalate], crystalline Al and Fe oxides, CEC and clay content) (PC1), acidity plus 1.4 nm mineral-related factor (exchangeable Al and 1.4 nm minerals) (PC2), and organic carbon (C)-related factor (organic C and organically bound Fe) (PC3) (CitationHartono et al. 2005). Parent material of the soil samples and their chemical and physical properties are presented in . In , soil samples were divided into two groups according to the factor scores of PC1 of surface soils. Group 1 denotes soils with factor scores of PC1 more than zero and Group 2 denotes soils with factor scores of PC1 less than zero. The dominant silicate clay minerals in the soils of Group 1 were kaolinite (>90%), except for soils from Gajrug, which were dominated by smectite (95%), and soils from PD1 were kaolinite (71%) and Al-interlayered vermiculite–chlorite intergrades (29%). The dominant silicate clay minerals in the soils of Group 2 were kaolinite for soils from PD2, Kota Bumi and Rimbo Bujang (>95%) and kaolinite and vermiculite for soils from Sebuluh, Kota Bangun, SM-BP and SM 1, and kaolinite and smectite for soils from Teluk Dalam. The percentage of kaolinite in soils from Kota Bangun, SM-BP, SM1 and Teluk Dalam was more than 75%, while in soils from Sebuluh it was approximately 50% (CitationHartono et al. 2005). All of the Java soils belonged to Group 1, whereas the Kalimantan soils belonged to Group 2.
Incubation
Duplicate 0.5-g samples of each soil were treated with 300 mg P kg−1 as a solution of KH2PO4, mixed thoroughly, and incubated for 1, 7, 30 and 90 days at 25°C and 80% of field capacity, which corresponds to 40–65% in gravimetric moisture content (w/w). Controls without the addition of P were also included for each soil.
The P addition rate, 300 mg P kg−1, was chosen based on the lowest P sorption maxima obtained in the previous study. Ninety days was chosen as the end of incubation to evaluate P transformation in one cropping season.
Phosphorus fractionation
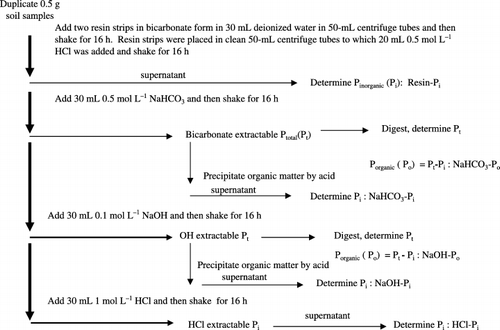
Soil incubation was followed by P fractionation. The protocol of P fractionation according to CitationTiessen and Moir (1993) is summarized in . This P fractionation method was selected because it is considered to have a wide range of P fractions of inorganic and organic P, from the most available fraction to the most unavailable fraction to plants. The modified Hedley method, which is similar but includes CHCl3 treatment before NaHCO3 extraction to lyse microbial cells, was
Table 1 Parent material and some chemical and physical properties of the soils used
Figure 1 Flow chart of the phosphorus (P) fractionation into various inorganic and organic P fractions.
Phosphorus transformation evaluation
The transformation of added P into other fractions was evaluated by the recovery of P fractions after each period of incubation. The values were obtained by subtracting the control from the recovery of P fractions. Statistical analyses (correlation analysis and anova followed by a Tukey's test) were applied using SYSTAT 8.0 (CitationSPSS Inc. 1998).
RESULTS
Original P distribution
Original P distribution is presented in . The values of resin-Pi and NaHCO3-Pi and -Po, which were considered to be biologically available P (labile P) of the two groups, were much lower than those of residual P, NaOH-Pi and -Po. Most P in these soils was accumulated in residual P, which was relatively insoluble. Phosphorus was also significantly accumulated as organic P in the form of labile organic P (NaHCO3-Po) and
Table 2 Original phosphorus (P) distribution and total P of the soil samples
Table 3 Range of the total recovery of the added phosphorus
A lack of resin-Pi and NaHCO3-Pi in the original soils indicated low P availability, and suggested that P fertilizer application in these upland soils was low and infrequent. The low application in these upland soils was also confirmed by the relatively low amount of NaOH-Pi, which ranged from 38 to 98 mg P kg−1 in Group 1 and from 13 to 90 mg P kg−1 in Group 2. For comparison, the amount of NaOH-Pi in acid upland soil from Matalom, Philippines, was 133 mg P kg−1 when 466 kg P ha−1 was applied (CitationDobermann et al. 2002).
Kinetics of P in different fractions during the incubation
The range of total recovery of added P after 1, 7, 30 and 90 days incubation is presented in . The recovery of added P was close to 100% in all periods of incubation, and only a small portion may be fixed in the residual P.
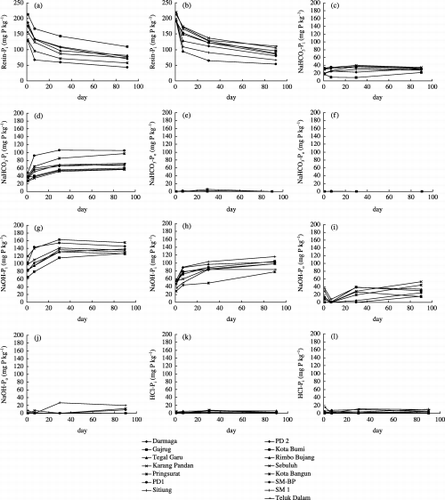
Resin-Pi
Recovery of added P in the form of resin-Pi is presented in for soils with factor scores of PC1 more than zero (Group 1) and for soils with factor scores of PC1 less than zero (Group 2). After 1 day incubation, added P was distributed in resin-Pi ranging from 128 to 213 mg P kg−1 in Group 1 and from 190 to 220 mg P kg−1 in Group 2. After 7 days incubation, the recovery ranged from 67 to 167 mg P kg−1 in Group 1 and from 94 to 174 mg P kg−1 in Group 2. After 30 days incubation, the recovery of resin-Pi ranged from 60 to 144 mg P kg−1 in Group 1 and from 65 to 137 mg P kg−1 in Group 2. After 90 days incubation, the recovery of resin-Pi ranged from 43 to 110 mg P kg−1 in Group 1 and 54 to 111 mg P kg−1 in Group 2.
Soils of Group 1 exhibited lower resin-Pi compared with soils of Group 2 after 1 day incubation. After 7, 30 and 90 days incubation, both groups exhibited similarly low amounts of resin-Pi compared with the initial P addition (300 mg P kg−1). These results suggested that
most resin-Pi was transformed to other fractions. From 1 to 7 days incubation, recovery of resin-Pi decreased significantly, but the difference was not significant from 7 to 30 days or from 30 to 90 days incubation. This suggests that in all the soils studied, after 7 days incubation the added P transformed slowly to other fractions.NaHCO3-Pi
The recovery of added P in the NaHCO3-Pi fraction is presented in for soils of Group 1 and for soils of Group 2. After 1 day incubation, added P was distributed in NaHCO3-Pi from 17 to 33 mg P kg−1 in Group 1 and from 26 to 48 mg P kg−1 in Group 2. After 7 days incubation, the recovery ranged from 10 to 36 mg P kg−1 in Group 1 and from 34 to 92 mg P kg−1 in Group 2. After 30 days incubation, the recovery ranged from 9 to 34 mg P kg−1 in Group 1 and from 51 to 106 mg P kg−1 in Group 2. After 90 days incubation, the recovery ranged from 22 to 35 mg P kg−1 in Group 1 and from 56 to 106 mg P kg−1 in Group 2.
The distribution of the added P to this fraction was higher in Group 2. There was not any significant increase in Group 1 during the incubation period, suggesting that most added P was not transformed into this fraction after 30 and 90 days incubation. In contrast, soils of Group 2 showed a significant increase in this fraction after 30 and 90 days incubation, suggesting that significant amounts of added P were transformed into this fraction.
NaHCO3-Po
The recovery of added P in the NaHCO3-Po fraction is presented in for soils of Group 1 and for soils of Group 2. The two groups showed that the added P was not distributed into this fraction.
NaOH-Pi
The recovery of added P in the NaOH-Pi fraction is presented in for soils of Group 1 and for soils of Group 2. After 1 day incubation, NaOH-Pi ranged from 65 to 120 mg P kg−1 in Group 1 and from 28 to 57 mg P kg−1 in Group 2. After 7 days incubation, the recovery ranged from 79 to 144 mg P kg−1 in Group 1 and from 44 to 89 mg P kg−1 in Group 2. After 30 days incubation, the recovery ranged from 115 to 163 mg P kg−1 in Group 1 and from 49 to 103 mg P kg−1 in Group 2. After 90 days incubation, the recovery ranged from 126 to 155 mg P kg−1 in Group 1 and from 77 to 116 mg P kg−1 in Group 2.
The soils of Group 1 exhibited a larger NaOH-Pi fraction than those of Group 2 in each period of incubation. After 30 and 90 days incubation, in the former soils, approximately 40 to 50% of added P was transformed into this fraction. The equilibrium of P transformation was reached at 30 days in soils of Group 1 because an increase in this fraction was not observed after 30 days incubation. For soils of Group 2, the amount of NaOH-Pi was comparable with that of NaHCO3-Pi and the former fraction tended to increase during incubation.
NaOH-Po
The recovery of added P in the NaOH-Po fraction is presented in for soils of Group 1 and for soils of Group 2. shows that there is no regular pattern concerning the distribution of added P to this fraction. Most soils of Group 1 and some soils of Group 2 exhibited some amounts of the NaOH-Po fraction after 30 and 90 days incubation, and the former group exhibited higher amounts of the NaOH-Po fraction.
HCl-Pi
The recovery of added P in HCl-Pi is presented in for soils of Group 1 and for soils of Group 2. The two groups of soils showed that the added P was not distributed in this fraction. This is because the soils were acid and contained negligible amounts of free CaCO3 to form Ca-P, which is extracted by 1 mol L−1 HCl.
DISCUSSION
Decreasing trend in resin-Pi during the incubation in relation to soil properties
Soils of Group 1 with high clay content exhibited lower resin-Pi in the early stage of incubation (1 day), although this difference disappeared later, and also exhibited lower NaHCO3-Pi fraction than soils of Group 2, with lower clay content, in each period of incubation. This supports the results of CitationKamprath and Watson (1980), in which finer-textured soils normally have larger P-buffering capacities and lower extractable P contents than coarser-textured soils. The difference in P transformation process between soils of Group 1 and Group 2 suggested that the form and distribution of added P were related to the soil characteristics summarized as oxide-related factor (PC1), that is, Al + 1/2 Fe (by ammonium oxalate), crystalline Al and Fe oxides, CEC and clay content.
With respect to the soil from Gajrug of Group 1, it exhibited exceptionally high resin-Pi among soils of Group 1 and was similar to some of the soils from Group 2 () in all periods of incubation, although the clay contents of this soil were relatively high (65%) (). The soil from Gajrug had the highest factor scores of PC2 as well PC1 (CitationHartono et al. 2005), which means that this soil contained high amounts of exchangeable Al and the silicate clay mineral of this soil was dominated by 1.4 nm mineral, namely smectite. In addition, it also had a significant amount of Al and Fe oxides. This suggests that exchangeable Al in smectite was not reactive with H2PO− 4. Exchangeable Al and smectite positively affected labile resin-Pi. Exchangeable Al and 1.4 nm minerals were reported to decrease P sorption maximum (CitationHartono et al. 2005). Smectite was also reported to have a lower P adsorbing surface than kaolinite (CitationSollins 1991), suggesting that clay mineralogy affects the P transformation process as shown in the case of the Gajrug soil.
Table 4 First-order resin-Pi in equilibrium (c) and the kinetic rate constant for phosphorus (P) transformation (k)
The trend of decreasing recovery of resin-Pi as a function of incubation time is more or less uniform for all soils and is well described by the first-order kinetic equation Pt = c + Pde−kt , where Pt is the amount of resin-Pi at time t, c is the amount of resin-Pi in equilibrium, Pd is the amount of decayed-resin-Pi, k is the rate constant and t is the reaction time in days. The resin-Pi in equilibrium (c) and the first-order kinetic rate constant (k) for P transformation is presented in . The values of c and k varied among soils. Most soils of Group 1 had higher k values than the soils of Group 2. Unlike the k values, c values show no consistent pattern with regard to soil group.
The k values () suggested that the soils of Group 1 were faster than the soils of Group 2 in the added P transformation process. Again, soil from Gajrug exhibited the lowest k value among the Group 1 soils and the highest c value in these soils, suggesting the effect of exchangeable Al and 1.4 nm mineral (smectite).
The correlation between factor scores of PC1, PC2, PC3 and recovery of P fractions after 90 days incubation and c and k values is presented in . The factor score of PC1 is positively significantly correlated with the k value (P < 0.05). This suggests that the transformation process in soils of Group 1 was faster than in soils in Group 2 because the P adsorbing surfaces in soils of Group 1 were much larger than those in Group 2 (CitationGriffin & Jurinak 1974; CitationKuo & Lotse 1973), which is reflected by higher oxide-related factor scores.
Table 5 Simple correlation coefficient (Pearson r) between factor scores of PC1, PC2, PC3 and the recovery of P fractions after 90 days of incubation and resin-Pi in equilibrium (c) and rate constant (k)
In contrast, PC2 was positively significantly correlated with the c value and resin-Pi (P < 0.01), suggesting that exchangeable Al and 1.4 nm minerals increased resin-Pi. This result confirmed that soil acidity, which is associated with exchangeable Al, and the content of 1.4 nm minerals, of which smectite and vermiculite are common, should be included in models used to estimate P sorption maxima in Indonesian acid upland soils.
Transformation of P into different fractions
The two groups of soils showed a different direction in added P transformation after 7, 30 and 90 days incubation when their resin-Pi were similar. and a correlation analysis () suggested that soils in Group 1 accumulated most of the remaining added P as NaOH-Pi fraction and only a small amount as NaHCO3-Pi fraction. In contrast, the soils of Group 2 accumulated P not only as NaOH-Pi fraction, but also as NaHCO3-Pi in significant amounts. With respect to NaHCO3-Pi, there were two possibilities regarding where this P fraction was derived from: (1) from the surface of minerals (CitationMattingly 1975; CitationTiessen and Moir 1993), (2) from the precipitated Ca-P and Mg forms (CitationOlsen and Sommers 1982) because of reactions with dissolved Ca and Mg. The possibility of precipitated Ca-P and Mg-P forms was calculated by using the amount of added P (300 mg P kg−1), water content during incubation, soil pH (1:1.5) and the exchangeable Ca and Mg of the soils after zero (0) days incubation. From the calculation of both cations to precipitate with H2PO− 4, only Ca-P precipitation could possibly occur in both Group soils (CitationLindsay 1979).
However, because there was not any increase in NaHCO3-Pi observed during the period of incubation in Group 1 soils, it is concluded that the abundant oxides surface retards the transformation of the added P into NaHCO3-Pi extractable forms. Thus, for soils of Group 1, P accumulation in NaOH-Pi results from the high P adsorbing surfaces provided by soil properties related to oxide-related factor and kaolinite. The majority of the remaining added P was chemisorbed by Al and Fe oxides and the broken edges of Al layers of kaolinite. The broken edges of Al layers of kaolinite are variable charge surfaces containing – Al(OH) groups with P sorption properties similar to those of Al and Fe oxides surfaces (CitationParfitt 1978), although chemisorption by kaolinite was less than that by Al and Fe oxides (CitationSollins 1991). The appearance of NaHCO3-Pi fraction in each stage of incubation showed that at a given amount of P chemisorbed by Al and Fe oxides (NaOH-Pi) also made equilibrium with the P to sorbed on the surfaces of minerals (NaHCO3-Pi) besides with resin-Pi.
In soil from Gajrug (Group 1), in which the predominant silicate clay mineral was smectite, although the P transformation process was relatively slow and resin-Pi was high compared with other Group 1 soils, the remaining added P was not accumulated in NaHCO3-Pi () but rather in NaOH-Pi (). This was because of a high contribution of oxide-related factor components (i.e. Al + 1/2 Fe [by ammonium oxalate], crystalline Al and Fe oxides, CEC and clay content) ().
The NaOH-Pi fraction was considered to be P that was chemisorbed on amorphous Al and Fe minerals (CitationDobermann et al. 2002; CitationTiessen et al. 1984; CitationZheng et al. 2003). In the soils examined in the present study, factor scores of PC1 were strongly correlated with the NaOH-Pi fraction (), suggesting that P in these upland soils was chemisorbed on both amorphous and crystalline Al and Fe oxide minerals.
Significant P accumulation in the NaHCO3-Pi in the soils of Group 2 was considered to be enhanced primarily by low P fixing surfaces that contribute to the increase in NaOH-Pi. It was P adsorbed on the surface of minerals and/or precipitated Ca-P forms.
Organic P in the form of NaHCO3-Po was not detected in all periods of incubation and only small amounts of NaOH-Po were detected, particularly after 30 and 90 days incubation in both groups of soils. In this experiment, only inorganic P fertilizer (KH2PO4) was applied. The insignificant effect of added inorganic P fertilizer on NaHCO3-Po and NaOH-Po contents was previously observed by other investigators in their short-term experiments (CitationBeauchemin and Simard 2000; CitationHedley et al. 1982; CitationIvarsson 1990; CitationZheng et al. 2003). However, the small occurrence of NaOH-Po in some soils of Group 1 and Group 2, for example, in the 30 and 90 day incubations, was supposed to result from the incorporation of added P into microbial biomass. The Po from dead microorganisms was chemisorbed by Al and Fe oxides and/or the broken edge of Al layers of kaolinite and its amount was higher in soils with high clay and Al and Fe oxide contents (soils of Group 1) as reported by CitationO’Halloran (1993) and CitationBeauchemin and Simard (2000). There was not any added P distributed in NaHCO3-Po in both groups, suggesting that NaHCO3-Po acted as a transitory pool rather than as a sink in P transformation.
This experiment was conducted under the condition that neither additional input of substrates for microbial activities nor influence of plant growth was expected. It is likely that P transformation in the organic fractions will be more dynamic if plants were included in the system because of P uptake, root exudates and more variable microbial activity. However, as it has been reported that added inorganic P fertilizer had little impact on organic P fractions in short-term applications when plant growth was included (CitationBeauchemin and Simard 2000; CitationHedley et al. 1982; CitationIvarsson 1990; CitationZheng et al. 2003); thus, the results of this experiment are still predictable of field conditions, particularly in one cropping season (90 days).
Effect of parent material on NaHCO3-Pi and NaOH-Pi fractions
The effect of parent material on NaHCO3-Pi and NaOH-Pi fractions is presented in . Soils from sedimentary rocks exhibited significantly (Tukey's test, P < 0.05) higher NaHCO3-Pi than soils developed from andesite, volcanic ash, volcanic sediment and volcanic ash–sedimentary rock mixture, but not from soils developed from granite.
Table 6 Effect of parent material on NaHCO3-Pi and NaOH-Pi fractions after 90 days of incubation
In contrast, soils developed from andesite and volcanic ash exhibited significantly (Tukey's test, P < 0.01) higher NaOH-Pi than soils developed from granite, volcanic sediments, volcanic ash–sedimentary rock mixture and sedimentary rocks. Standard deviations of NaHCO3-Pi and NaOH-Pi were relatively low on all parent materials.
Differences in parent materials could be used to assess the transformation and accumulation of P applied in these upland soils. Soils that were developed from volcanic ash and andesite accumulated the most applied P into NaOH-Pi because of relatively high soil properties related to oxide-related factor. In contrast, in soils from sedimentary rocks, granite and volcanic sediments because of relatively low soil properties related to oxide-related factor, applied P was accumulated not only into NaOH-Pi fraction but also into NaHCO3-Pi fraction with significant amounts. Most of the volcanic soils were situated in Java and, in part, in Sumatra, while most Kalimantan soils were developed from sedimentary rocks. These results can be used to determine P transformation and accumulation when fertilizer P is applied in acid upland soils in Java, Sumatra and Kalimantan according to their parent materials.
This study suggested that the application of P fertilizer in very large amounts in these upland soils could be done in the kaolinitic soils with high oxide-related factor component and developed from andesite and volcanic ash parent materials. This is because most of the added P was accumulated in the NaOH-Pi fraction. This fraction acted as a sink for added P and long-term residual values could be expected (CitationBeck and Shanchez 1994; CitationGuo et al. 2000; CitationZheng et al. 2003).
The heavy application of P fertilizer in soils developed from sedimentary rocks with low oxide-related factor components and smectite as the predominant clay mineral is not recommended because of a relatively slow P transformation process and significant accumulation in labile P fractions (resin-Pi and NaHCO3-Pi). Although P will be more available for plants, there will be the risk of P loss and the transfer from soils to waterbodies. Frequent and small amounts of P application are recommended.
Conclusions
Parent materials, oxide-related factor contributed by oxalate-soluble Al and Fe, crystalline Al and Fe oxides, CEC and clay content were very important in assessing P transformation and P accumulation in acid upland soils in Indonesia. As for clay, the type of silicate clay minerals should be considered because P in smectic soil, which is associated with exchangeable Al, was more extractable and slower with respect to the P transformation process compared with kaolinitic soils. The P transformation process in these upland soils was very fast, especially for kaolinitic soils with high amounts of oxide-related factor components. The equilibrium was reached in 30 days and most of the P added was accumulated into the NaOH-Pi fraction. The low P adsorbing surfaces enhanced the P transformed into NaHCO3-Pi in soils with low amounts of oxide-related factor. Soils developed from andesite and volcanic ash accumulated the added P into NaOH-Pi fraction significantly more than soils developed from granite, volcanic sediments and sedimentary rocks. In contrast, soils from sedimentary rocks, granite and volcanic sediments, because of relatively low oxide-related factor components, accumulated the added P not only into NaOH-Pi fraction but also into NaHCO3-Pi fraction in significant amounts. The latter fraction was bonded on the surface of minerals or precipitated Ca-P forms for soils with high amounts of calcium. This finding is of significance for further reviews in programming P fertilization in Indonesian acid upland soils.
ACKNOWLEDGMENTS
We thank the farmers in Java, Sumatra and East Kalimantan for their assistance in the collection of the soil samples. We also thank Mr Tetsuhiro Watanabe for his assistance in the field, Dr Hitoshi Shinjo for his assistance in the statistical analysis and Mr M. Turner for his assistance in the preparation of this manuscript.
REFERENCES
- HartonoA FunakawaS KosakiT2005Phosphorus sorption–desorption characteristics of selected acid upland soils in Indonesia Soil SciPlant Nutr 51 787799
- Kuo , S and Lotse , EG . 1973 . Kinetics of phosphate adsorption and desorption by hematite and gibbsite . Soil Sci , 116 : 400 – 406 .
- Oberson , A , Friesen , DK , Rao , IM , Bühler , S and Frossard , E . 2001 . Phosphorus transformation in an Oxisol under contrasting land-use systems: the role of soil microbial biomass . Plant Soil , 237 : 197 – 210 .
- Schmidt , JP , Buol , SW and Kamprath , EJ . 1996 . Soil phosphorus dynamics during seventeen years of continuous cultivation: fractionation analyses . Soil SciSocAmJ , 60 : 1168 – 1172 .
- Verma , S , Subehia , SK and Sharma , SP . 2005 . Phosphorus fractions in an acid soil continuously fertilized with mineral and organic fertilizers . BiolFertilSoils , 41 : 295 – 300 .
- Zheng , Z , Simard , RR , Lafond , J and Parent , LE . 2002 . Pathways of soil phosphorus transformation after 8 years of cultivation under contrasting cropping practices . Soil SciSocAmJ , 66 : 999 – 1007 .
- Subagyo , H , Suharto , N and Siswanto , AB . 2000 . “ Agricultural lands in Indonesia ” . In Indonesian Land Resources and Its Management , Edited by: Abdurachman , A , Amien , LI , Agus , F and Djaenuddin , D . 21 – 65 . Bogor : Center for Soil and Agroclimate Research . (in Indonesian)
- “ BarrowNJ1990 Relating chemical processes to management system. InPhosphorus Requirements for Sustainable Agriculture in Asia and Oceania ” . 199 – 209 . Manila, , Philippines : International Rice Research Institute .
- Cassman , KG , Singleton , PW and Linquist , BA . 1993 . Input/output analyses of the cumulative soybean response to phosphorus on an Ultisol . Field Crop Res , 34 : 23 – 36 .
- Kamprath , EJ . 1967 . Residual effect of large applications of phosphorus on high phosphorus fixing soils . AgronJ , 59 : 25 – 27 .
- Linquist , BA , Singleton , PW , Cassman , KG and Keane , K . 1996 . Residual phosphorus and long-term management strategies for an Ultisol . Plant Soil , 184 : 47 – 55 .
- Tiessen , H and Moir , JO . 1993 . “ Characterization of available P by sequential extraction ” . In Soil Sampling and Methods of Analysis , Edited by: Carter , MR . 75 – 86 . Boca Raton, Florida : Canadian Society of Soil Science, Lewis Publishers .
- Murphy , J and Riley , JP . 1962 . A modified single solution method for the determination of phosphate in natural waters . AnalChimActa , 27 : 31 – 36 .
- Mattingly , GEG . 1975 . “ Labile phosphorus in soils ” . In Soil Sci Vol. 119 , 369 – 375 .
- Olsen , SR and Sommers , LE . 1982 . “ Phosphorus ” . In Methods of Soil Analyses , 2nd edn , Part 2 Agron. Monogr. 9 Edited by: Page , AL , Miller , RH and Keeney , DR . 403 – 430 . Madison : ASA and SSSA .
- Dobermann , A , Geroge , T and Thevs , N . 2002 . Phosphorus fertilizer effects on soil phosphorus pools in acid upland soils . Soil SciSocAmJ , 66 : 652 – 660 .
- SPSS Inc.1998 SYSTAT 80 StatisticsChicago
- Kamprath , EJ and Watson , ME . 1980 . “ Conventional soil and tissue test for assessing the phosphorus status of soils ” . In The Role of Phosphorus in Agriculture , Edited by: Khasawneh , FE , Sample , EC and Kamprath , EC EJ . 433 – 469 . Madison : ASA, CSSA, and SSSA .
- Sollins , P . Effects of soil microstructure on phosphorus sorption in soils of the humid tropics . Phosphorus Cycles in Terrestrial and Aquatic Ecosystems Regional workshop 3: South and Central America, SCOPE/UNEP Proceedings . Edited by: Tiessen , H , Hernandez , D Lopez and Salcedo , IH . pp. 169 – 175 . Saskatoon : University of Saskatchewan .
- Griffin , RA and Jurinak , JJ . 1974 . “ Kinetics of phosphate interaction with calcite ” . In Soil SciSocAmProc Vol. 38 , 75 – 79 .
- Lindsay , WL . 1979 . Chemical Equilibria in Soils , New York : John Wiley & Sons .
- Parfitt , RL . 1978 . Anion adsorption by soils and soil materials . AdvAgron , 30 : 1 – 50 .
- Tiessen , H , Stewart , JWB and Cole , CV . 1984 . Pathways of phosphorus transformations in soils of differing pedogenesis . Soil SciSocAmJ , 48 : 853 – 858 .
- Zheng , Z , Parent , LE and MacLeod , JA . 2003 . Influence of soil texture on fertilizer and soil phosphorus transformations in Gleysolic soils . CanJSoil Sci , 83 : 395 – 403 .
- Beauchemin , S and Simard , RR . 2000 . “ Phosphorus status of intensively cropped soils of the St. Lawrence Lowlands ” . In Soil SciSocAmJ Vol. 64 , 659 – 670 .
- Hedley , MJ , Stewart , JWB and Chauhan , BS . 1982 . “ Changes in inorganic and organic soil phosphorus fractions induced by cultivation practices and laboratory incubations ” . In Soil SciSocAmJ Vol. 46 , 970 – 976 .
- Ivarsson , K . 1990 . The long-term soil fertility experiments in Southern Sweden. IV. Changes in inorganic and organic soil phosphorus fractions after a pot trial . Acta AgricScand , 40 : 205 – 215 .
- O’Halloran , IP . 1993 . Effect of tillage and fertilization on the inorganic and organic phosphorus . CanJSoil Sci , 73 : 359 – 369 .
- Beck , MA and Sanchez , PA . 1994 . Soil phosphorus fraction dynamic during 18 years of cultivation on a Typic Paleudult . Soil SciSocAmJ , 58 : 1424 – 1431 .
- Guo , F , Yost , RS , Hue , NV , Evensen , CI and Silva , JA . 2000 . Changes in phosphorus fractions in soils under intensive plant growth . Soil SciSocAmJ , 64 : 1681 – 1689 .
- Ghani , MO and Islam , MA . 1946 . Phosphate fixation in acid soils and its mechanism . Soil Sci , 62 : 293 – 306 .
- Soil Survey Staff . 1998 . Keys to Soil Taxonomy , 8th edn , Washington : USDA Natural Resources Conservation Service .