Abstract
Allophanic Andosols are widely used as a major material in commercial nursery media for fruit vegetables in Japan because of their remarkable physical properties, such as a high water-holding capacity. In the present study, our objectives were: (1) to examine the effect of phosphogypsum (PG) on the chemical properties of Andosols, (2) to investigate the effect of PG on the growth and Ca uptake of melon seedlings. The effect of PG on chemical properties of Andosols was studied using five Andosols with different inorganic and organic colloidal components. The change in soil pH (H2O) was dependent on the soil samples; an increase was observed in the case of Kawatabi 3Bw soil; a sharp decrease in Kawatabi A2 soil; and almost no change or a slight decrease in Kameoka A1, A2 and Bw soils. The water-soluble Ca content was examined as an index of Ca availability in Andosols treated with PG. The increment in water-soluble Ca by PG application was depressed by allophane. The effect of PG application to the nursery media prepared from Andosols on growth and Ca uptake of melon (Cucumis melo L.) was examined in 2002. Three different varieties, Amusu, Earl's and Midorishima, were used in this experiment. The pH value of nursery media was stable at 6.4 ± 0.1 regardless of PG application rate. In contrast, electrical conductivity was clearly increased by PG application, and was reached at 1.2 dS m−1 in 4.0 g L−1 application. The application of PG increased water soluble Ca of nursery media from 1.7 to 5.2 cmolc L−1. Both top and root growth of melon seedlings were enhanced regardless of varieties, dry matter weights were maximized at 4 g L−1 PG application. The Ca uptake of melon seedlings was promoted by PG application in all the varieties. It was suggested that the relative root growth rate of melon seedlings was closely related to the Ca uptake of melon seedlings.
INTRODUCTION
The seedling and transplanting stages are very important for maintaining the fruit quality and yield of melons (Cucumis melo L.) because a melon plant yields only a few fruits at harvest and the vegetative growth stage shifts into the reproductive stage during the process of its growth. It has been shown that cold temperature stress at the transplanting stage decreases the growth of muskmelon, resulting in a possible decrease in yield (CitationKorkmaz and Dufault 2001). CitationOzawa (1990, Citation1998) has stated the importance of root development in prince melon to avoid water stress during hot seasons; the report also stated that in crops in which the seedlings are transplanted, root growth should be accelerated immediately after transplanting. Thus, root development, in particular, is the key for promoting initial growth.
The melon plant is widely known as a crop with high calcium (Ca) content even among crops that have an affinity for Ca (CitationFujimoto and Yamane 1990). Aside from high content, Ca nutrients play an important role in the growth and fruit quality of melons. Calcium deficiency causes rapid fermentation of the fruit under forced culture conditions, an incidence of glassiness and reduces fruit hardness (CitationSerrano et al. 2002). In addition, Ca deficiency reduces root development in melon (CitationFujimoto 2000), indicating that Ca possibly facilitates good rearing of seedlings through root development. The relationship between Ca as a nutrient and melon growth have been studied in field experiments mainly by using limestone (CitationSaito et al. 1993); however, this relationship has not been studied at the seedling stage.
In contrast, Ca availability in nursery media and/or soil materials was not considered, although soil forms are major part of the material for nursery media. Andosols are excellent media for developing root growth and proliferation because of their remarkable physical properties, such as high water-holding capacity (CitationKimble et al. 2000). Virgin Allophanic Andosols are widely used as a major material in commercial nursery media, particularly for fruit and vegetables in the Kanto district, which lies in the central part of Japan, where Allophanic Andosols are widely distributed. CitationSaigusa et al. (1990) clarified that because Ca supply could not be fulfilled by liming, the application of Ca was a prerequisite for the plants that grew in Allophanic Andosols, irrespective of soil pH.
Recently, the application of phosphogypsum (PG) as an alternative for Ca supply has been re-recognized as a useful fertilization technique. The common name for PG was derived from a byproduct of phosphorus production; however, it contains low phosphates and, in particular, the concentration of water-soluble phosphate was under 0.1 p.p.m. (CitationTakasu and Saigusa 2004). Phosphogypsum increases water-soluble Ca in soils because of its high solubility in water (0.2 g L−1) compared with that of calcium carbonate, and it is effective in supplying Ca to the “Komatsuna” plant (Brassica rapa L.) (CitationTakasu et al. 2004). Therefore, considering the solubility of PG, it is predicted that seedling growth will be promoted by the supply of PG to nursery media, which are mainly composed of Allophanic Andosols, in addition to liming.
The objectives of this study were to evaluate Ca availability in Andosols with PG application and to clarify the effect of PG application to nursery media on Ca uptake and growth of melon seedlings and, in particular, root development.
MATERIALS AND METHODS
Changes in the chemical properties of Andosols treated with phosphogypsum
Five virgin Andosols with different inorganic and organic colloidal components were used in this experiment (). Kawatabi A2 horizon and 3Bw horizon soils in Miyagi prefecture were classified into Aluandic Andosols, and Kameoka A1, A2 and Bw horizon soils in Ibaraki prefecture were classified into Silandic Andosols according to the World Reference Base for Soil Resources (CitationFood and Agriculture Organization, International Soil Reference and Information Center (ISRIC) and International Society of Soil Science (ISSS) 1998). We also
Table 1 Physical and chemical properties of soils selected
Table 2 Elemental composition of phosphogypsum
Experiment on melon seedlings
Experimental design
The experiment was carried out at a greenhouse at the Japan Horticultural Production and Research Institute. A split-plot design was used along with 10 replications. Cucumis melo L., a variety of melon, was grown in the main plot and PG application rate was the sub-plot.
Seedling preparation and fertilization
Three melon varieties, namely cv. Amusu, cv. Earl's and cv. Midorishima, which have been developed from
Table 3 Physical and chemical characteristics of the nursery media
Survey and analysis
The plant growth of melon seedlings was investigated at 24 days after sowing, and four growth indices, leaf number, plant length, dry weight of tops and dry weight of roots, were measured. However, an outlier of an Amusu variety with PG 2 g L−1 was excluded from the date according to the Grubbs–Smirnov test. Chemical analysis of the nursery media was conducted without air-drying. The pH (H2O) and EC of the nursery media were measured using a glass electrode and EC meter, respectively. The ratio of nursery media to distilled water was 1:5 by volume. Exchangeable bases were extracted at pH 7.0 using 1 mol L−1 ammonium acetate solution and then measured using an atomic absorption spectrometer (Z-6100, Hitachi, Tokyo, Japan). Water-soluble bases were extracted in the ratio of 1:5 by volume and then measured in a similar manner to exchangeable bases. Cation exchange capacity (CEC) was determined using the semi-micro Schollenberger method. The tops of the plants were dried at 70°C for 48 h and 10 seedlings were grouped together. The N content was measured using a NC recorder (MT-600 Yanaco, Tokyo, Japan). After wet digestion with nitrate-perchloric acid, P2O5 was analyzed using a spectrophotometer, and Ca, Mg and K contents were determined using an atomic absorption spectrometer (Z-6100, Hitachi).
RESULTS AND DISCUSSION
Changes in the chemical properties of Andosols treated with PG
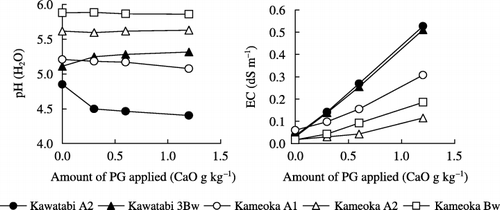
The changes in soil pH and EC by PG application are shown in . The change in soil pH differed among soil samples; Kameoka A2 and Bw horizon exhibited almost the same pH regardless of the PG application rate. Kawatabi A2 horizon and Kameoka A1 horizon soils exhibited a decrease with PG application. In particular, the soil pH of Kawatabi A2 horizon soil sharply decreased when PG was applied at the rate of 0.3 CaO g kg−1, and subsequently it decreased gradually from 4.9 to 4.4 with an increased application rate. In contrast, the soil pH gradually increased in the case of Kawatabi 3Bw horizon soil, and an increase of 0.21 units was observed when PG was applied at the rate of 1.2 CaO g kg−1. Gypsum application has been shown to cause an increase, decrease or negligible change in the soil pH down the profile under both field and laboratory conditions (CitationShainberg et al. 1989).
CitationPavan et al. (1987) reported that PG increased the soil pH in Oxisols and decreased it in Inceptisols; these authors suggested that the number of H+ ions released probably exceeded that of OH− ions released in the Inceptisols with a high Al content, while OH− release could be dominant in Oxisols with a low-Al Oxisols. CitationSaigusa et al. (1992) reported that 8–15 cmolc kg−1 of constant negative charge derived from 2:1–2:1:1 minerals was found in Non-allophanic Andosols, and no positive charge was detected in humic Non-Allophanic Andosols. It was suggested that Kawatabi A2 horizon soil possessed a high constant negative charge and that H+ ions were easily released by the isomorphous substitution of Al3+ ions held by a constant negative charge, resulting in a sharp decrease in the pH. However, it was also reported that thick high-humus, Non-allophanic Andosol did not show an appreciable change (CitationSaigusa et al. 1996). The differences between the previous report and the results of this study were attributed to the different strengths of the buffering capacity of humus.
Tomas and Hargrove (1984) reported that acidity can be neutralized by OH− ions released by the ligand exchange between Al or Fe hydroxide and dissolved SO2− 4 and Cl− ions. Measuring available sulfur content extracted using 0.01 mol L−1 Ca(H2PO4)2, high sulfur content (i.e. 322.6 mg kg−1) was obtained in Kawatabi 3Bw horizon, while 18.3 mg kg−1 was recorded in Kawatabi A2 horizon soil. Thus, it was suggested that in Kawatabi 3Bw horizon, the number of OH− ions released could exceed that of H+ ions released by the ligand exchange of SO2− 4 ions. In contrast, it was
Figure 2 Effect of phosphogypsum (PG) application on water-soluble cations in Andosols. (a) Water-soluble Ca (w-Ca), (b) water-soluble Mg (w-Mg), (c) water-soluble K (w-K) and (d) water-soluble Na (w-Na).
The soil EC clearly increased in proportion to the application rate of PG for all soil samples. This may reflect the relatively high solubility of PG in water (i.e. with 2.1 g L−1). However, the increment in the soil EC differed among soil samples; the soil EC of Non-allophanic Andosols was higher than that of Allophanic Andosols. For this reason, it was thought that the increased amount of the base concentration of the soil solution by the application of PG was different across the soil types tested.
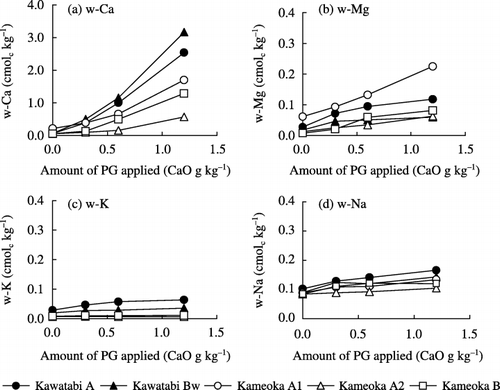
Water-soluble cations were examined as an index of cation availability in Andosols treated with PG (). With an increase in the PG application rate, the water-soluble Ca (w-Ca) content increased in all soil samples, ranging from 0.57 to 3.17 cmolc kg−1 when 1.2 CaO g kg−1 of PG was applied. Furthermore, PG application also increased the contents of other water-soluble cations,
Figure 3 Relationship between allophane content and the apparent increment in water-soluble Ca in Andosols treated with phosphogypsum (PG).
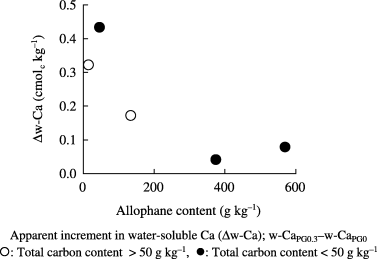
Table 4 Changes in the chemical properties of the nursery media with phosphogypsum (PG) application
Thus, it was suggested that the apparent increment of water-soluble Ca was more affected by allophane content than humus content, although the humus content also affected the Ca availability in Andosols.
Experiment on melon seedlings
Changes in the chemical properties of nursery media
The chemical properties of the nursery media after PG application are shown in . The application of PG seldom changed the pH (H2O) of the nursery media; the average pH (H2O) value was 6.4 ± 0.1. This result was similar to a change in the pH (H2O) of Allophanic Andosols in experiment 1. In contrast, EC showed a clear increase in proportion to the PG application rate; it reached 1.2 dS m−1 with a PG application at 4 g L−1 and 1.6 dS m−1 at 8 g L−1.
The application of PG increased the levels of exchangeable Ca, which reached 22.6 cmolc L−1 when 8.0 g L−1 of PG was applied. Exchangeable Ca to Mg equivalent ratios (Ca/Mg ratio) were found to be 5.8, 6.5, 7.5 and 8.9 for the PG application rates of 0, 2, 4 and 8 g L−1, respectively. CitationHasegawa (1987) reported that excess Ca absorption by melon plants caused Mg deficiency, which is referred to as leaf dying, because of the Ca and Mg imbalance in the soil. Although the Ca/Mg ratio in soils increased after PG application, symptoms of Mg deficiency were not observed in this experiment.
With an increase in the PG application rate, the water-soluble Ca content of the media increased, the content ranged from 1.7 to 5.2 cmolc L−1. Furthermore, PG application also increased the contents of other water-soluble cations in the order Mg > K > Na, similar to experiment 1. The water-soluble Ca/Mg ratio was lower (i.e. 3.9, 4.7, 5.6 and 6.5) than the exchangeable Ca/Mg ratio, regardless of the application rate of PG.
Growth of seedlings
When seedlings were directly sown in the media, no difference in germination rate was observed among the experimental plots. The investigation data is shown in . No significant difference in leaf number was observed among these plots. Plant length tended to be promoted by PG application, except when PG was applied at 2.0 g L−1 to the plot with Earl's variety. However, this difference was not statistically significant in any of the experimental plots. The maximum dry weight of top was observed in all varieties when PG was applied
Table 5 Effect of phosphogypsum (PG) application on the growth of melon seedlings at 24 days after sowing
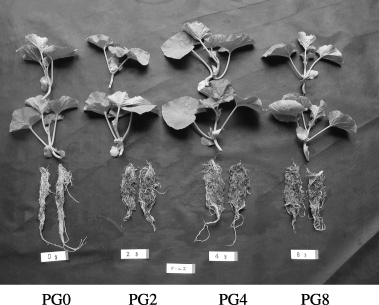
Similar to the top growth, the greatest root weight was observed in all varieties with PG application at 4 g L−1 (). The Amusu, Earl's and Midorishima varieties showed approximately 35%, 56% and 34% greater weights than that of the control, respectively. Significant differences against the control at 5% level were observed when PG was applied at 4 g L−1 in Amusu and Midorishima varieties and at 4 g L−1 and 8 g L−1 in Earl's variety. Thus, an increase in nutrient uptake area because of root growth may result in an increase in top weight. The top : root ratio (T/R ratio) decreased in all varieties with PG application at 4 g L−1; the value ranged from 4.0 to 4.9. This result indicates that PG application affects the root growth rather than the top growth of melon seedlings.
Nutrient uptake of melon seedlings
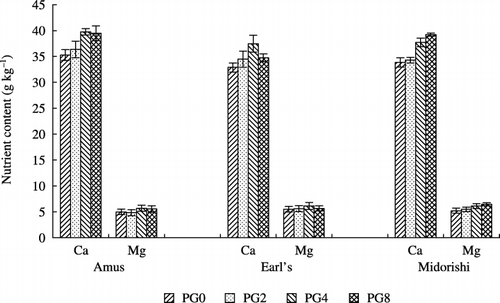
The major nutrient contents in the tops of melon seedlings were determined at 24 days after sowing (). The average Ca contents of the three melon varieties, Amusu, Earl's and Midorishima, were 3.62%, 3.34% and 3.57%, respectively. CitationFujimoto and Yamane (1990) reported that the Ca content in the melon leaves of Amusu variety was 5.86% at 2 weeks after transplantation; the value was 5.89%, 7.77% and 11.4% in the upper, middle and lower parts of the leaves, respectively, at the harvest stage, during which a single stem bore two fruits. The Ca content level at which symptoms appeared in the melon leaves of Amusu variety was examined in a hydroponics culture; it was clarified that the Ca content in leaves 16–18 decreased to a level of 9.0 g kg−1 after the flowering stage (CitationFujimoto 2000). Although the Ca
content of melon seedlings was clearly increased by PG application in this experiment, the Ca content in the seedlings was relatively lower than those of previous field studies. Significant differences against the control at 5% level were observed when PG was applied at 4 g L−1 in the Amusu and Earl's varieties and at 4 g L−1 and 8 g L−1 in the Midorishima variety. Calcium is well known to be an essential key constituent for root hair formation (CitationHofer 1996). It was observed that the roots of the melon seedlings held a large amount of soil and peat moss, which is a material of nursery media by PG application, in particular in Earl's. Thus, it can be inferred that thinner roots, such as root hairs, developed with the application of PG.It is well known that Ca is antagonistic to Mg uptake. However, in this experiment, Mg content also increased after PG application, irrespective of the varieties. This is because of both enhancement of the nutrient absorption site and an increase in water-soluble Mg by the exchange between Ca and Mg.
The relationship between the amount of Ca uptake and the dry weight of the roots is shown in Fig. 6. A different trend was observed among the tested varieties. Root dry matter proportionally increased with the amount of Ca uptake in both the Amusu and Earl's varieties. In contrast, the maximum rate of dry root matter production was not in agreement with the highest amount of the Ca uptake in the Midorishima variety. This implies that another factor, such as EC, in the nursery media was also related to the root growth of the melon seedlings.
Conclusion
The application effect of PG on Andosols with different colloidal components was evaluated using the measurements of soil pH, EC and water-soluble Ca. The results revealed that the changes in the pH of Allopanic Andosols were not appreciable regardless of the allophone content, whereas the soil pH of Non-allopanic Andosols changed markedly. Furthermore, the increment in water-soluble Ca by PG application was inversely proportional to the allophane content in Andosols.
As it was clarified that PG application could supply water-soluble Ca without significantly changing the pH in Allophanic Andosols, the effect of PG application on the chemical properties of nursery media mainly prepared from Allophanic Andosols and the Ca uptake of melon seedlings were examined. The results revealed that PG application to the nursery media enhanced rooting and plant growth in melon seedlings. Therefore, it is proposed that PG application to nursery media might be a promising method of supplying Ca to melon seedlings: the optimum PG application rate for melon seedlings is 4 g L−1 from the view point of maximum root dry matter.
ACKNOWLEDGMENTS
We are grateful to Mr Kunihiko Tsukudo for his overall encouragement and Mr Shin Hiranuma for his grateful support. We thank all the staff in the Agricultural Materials Research Laboratory, CO-OP Chemical Co. Ltd. for their helpful cooperation, especially Ms Tomoko Takahashi for her technical assistance in the laboratory work.
REFERENCES
- Korkmaz , A and Dufault , RJ . 2001 . Developmental consequences of cold temperature stress at transplanting on seedling and field growth and yield. Muskmelon . JAmSocHorticSci , 126 : 410 – 413 .
- Ozawa , K . 1990 . Effect of root expands on growth and yield of princemelon in the Ogasawara islands . BullTokyo AgricExpStn , 22 : 1 – 9 . (in Japanese with English summary)
- Ozawa , K . 1998 . Regulation of plant water and fertilizer absorption due to roots grown in sub-soil . BullTohoku NatlAgricExpStn , 93 : 51 – 100 . (in Japanese with English summary)
- Fujimoto , J and Yamane , T . 1990 . Nutrient absorption and translocation of melon plant bearing two fruits on single stem . JpnJSoil SciPlant Nutr , 61 : 298 – 301 . (in Japanese)
- Serrano , M , Amoros , A , Pretel , MT , Martinez-Madrid , MC , Madrid , R and Romajaro , F . 2002 . Effect of calcium deficiency on melon (Cucumis melo L.) texture and glassiness incidence during ripening . Food SciTechInt , 8 : 147 – 154 .
- Fujimoto , J . 2000 . Diagnosis of calcium deficiency in melon (Cucumis meloL.) . JJpnSocAgrTechManage , 7 : 3 – 7 . (in Japanese)
- Saito , T , Takahashi , B , Inoue , H and Watanabe , K . 1993 . Effect of Ando sub-soil used as bed soil and calcium fertilizer utilization on growth and fruit quality of muskmelon . BullCollAgrVetMed, Nihon Univ , 50 : 11 – 21 . (in Japanese with English summary)
- Kimble , JM , Ping , CL , Sumner , ME and Wilding , LP . 2000 . “ Section E. Pedology, 6. Classification of soils, 3. Andisols ” . In Handbook of Soil Science , Edited by: Sumner , ME . 222 Boca Raton : CRC Press .
- Saigusa , M , Nishiya , M , Matsuyama , N , Shoji , S and Abe , T . 1990 . Liming and calcium nutrition in acid Andisols . BullExpFarm, Tohoku Univ , 6 : 33 – 38 . (in Japanese)
- TakasuE SaigusaM2004 Effect of phosphogypsum application on chemical properties of soil and soil solution in Alluvial soils in Japan . Proc. of the 6th International Symposium on Plant–Soil Interactions at low pH . pp. 310 – 311 .
- 2004 . TakasuE HiranumaS YamadaH ShimadaN YoshidaY SaigusaM2004 Effect of phosphogypsum application on the chemical property of the soil and Ca uptake of Komatsuna plant . Proc. of the Annual Meeting of the Japanese Society of Soil Science and Plant Nutrition . Sep. 14–16 2004 , Fukuoka, Japan. Vol. 141 , (in Japanese)
- Food and Agriculture Organization, International Soil Reference and Information Center (ISRIC) . “ International Society of Soil Science (ISSS) 1998 World Reference Base for Soil Resources ” . World Soil Resources Reports 84 Food and Agriculture OrganizationRome .
- Japanese Society of Soil Science and Plant Nutrition . 1990a . “ Cation exchange capacity (Schollenberger method) ” . In Soil Standard Analysis and Measurement Methods , Edited by: Soil Standard Analysis and Measurement Methods Editorial Committee . 208 – 211 . Tokyo : Hakuyusha . (in Japanese)
- Japanese Society of Soil Science and Plant Nutrition and Soil Standard Analysis and Measurement Methods Editorial Committee. 1990b . “ Exchangeable cations ” . In Soil Standard Analysis and Measurement Methods , 215 – 219 . Tokyo : Hakuyusha . (in Japanese)
- Japanese Society of Soil Science and Plant Nutrition . 1990c . “ Water soluble cations ” . In Soil Standard Analysis and Measurement Methods , Edited by: Soil Standard Analysis and Measurement Methods Editorial Committee. 204 – 208 . Tokyo : Hakuyusha . (in Japanese)
- Japanese Society of Soil Science and Plant Nutrition and Soil Standard Analysis and Measurement Methods Editorial Committee. 1990d . “ Acid oxalate and pyrophosphate extractable Fe, Al, Si ” . In Soil Standard Analysis and Measurement Methods , 288 – 297 . Tokyo : Hakuyusha . (in Japanese)
- Saigusa , M , Shoji , S , Ito , T and Honna , T . 1992 . Revaluation of exchange acidity y1in Andisol . JpnJSoil SciPlant Nutr , 63 : 216 – 218 . (in Japanese)
- Barrow , NJ . 1967 . Studies on extraction and on availability to plants of adsorbed plus soluble sulfate . Soil Sci , 104 : 242 – 249 .
- Hue , NV and Adams , F . 1979 . Indirect determination of micrograms of sulfate by barium absorption spectroscopy . CommunSoil SciPlant Anal , 10 : 841 – 851 .
- Kubo , S , Shimada , N and Okamoto , N . 1992 . Effects of rate and forms of applied nitrogen in nursery soils on seedling growth of different vegetable species . JJpnSocHortSci , 60 : 859 – 867 . (in Japanese with English summary)
- Shainberg , I , Sumner , ME , Miller , WP , Farina , MPW , Pavan , MA and Fey , MV . 1989 . Use of Gypsum on Soils. Advances in Soil Science Vol. 9 , 1 – 111 .
- Pavan , MA , Binghum , FT and Peryea , FJ . 1987 . Influence of calcium and magnesium salts on acid soil chemistry and calcium nutrition of apple . Soil SciSocAmJ , 51 : 1526 – 1530 .
- Saigusa , M , Toma , M and Nanzyo , M . 1996 . Alleviation of subsoil acidity in Non-allophanic Andosols by phosphogypsum application in topsoil . Soil SciPlant Nutr , 42 : 221 – 227 .
- Alva , AK , Gascho , GJ and Yang , G. 1991 . Soil solution and extractable calcium in gypsum-amended coastal plain soils used for peanut culture . CommunSoil SciPlant Anal , 22 : 99 – 116 .
- Imai , H and Okajima , H . 1979 . Studies on the nutrient retention power of soils. 1. The effect of AEC and CEC on the chemical composition of the soil solution . JpnJSoil SciPlant Nutr , 50 : 33 – 39 . (in Japanese)
- Imai , H and Okajima , H . 1980a . Studies on the nutrient retention power of soils. 2. The effect of anion adsorption and gypsum formation on ammonium retention by soils . JpnJSoil SciPlant Nutr , 51 : 95 – 101 . (in Japanese)
- Imai , H and Okajima , H . 1980b . Studies on the nutrient retention power of soils. 3. Nitrate adsorption . JpnJSoil SciPlant Nutr , 51 : 102 – 106 . (in Japanese)
- Wada , S . 1984 . Mechanism of apparent salt absorption in ando soils . Soil SciPlant Nutr , 30 : 77 – 83 .
- Qafoku , NP and Sumner , ME . 2002 . Adsorption and desorption of indifferent ions in variable charge subsoils: The possible effect of particle interactions on the counter-ion charge density . Soil SciSocAmJ , 66 : 1231 – 1239 .
- Hofer , RM . 1996 . “ Root Hairs ” . In Plant Roots, The Hidden Half , 2nd edn , Edited by: Waisel , Y. , Eshel , A. and Kafkafi , U. 111 – 126 . New York : Marcel Dekker .
- Hasegawa , T . 1987 . Necrotic leaves of melon in Nakaumi Polder . Kinki Chugoku Agri. Research , 74 : 8 – 12 .
- Saigusa , M , Matsuyama , N and Abe , T . 1992 . Electric charge characteristics of Andisols and its problem on soil management . JpnJSoil SciPlant Nutr , 63 : 196 – 201 . (in Japanese with English summary)
- Matsuyama , N , Saigusa , M and Abe , T . 1994 . Distributions of Allophanic Andosols and Non-allophanic andosols in Kanto and Chubu districts . JpnJSoil SciPlant Nutr , 65 : 304 – 312 . (in Japanese with English summary)