Abstract
The Japanese woody plant Chengiopanax sciadophylloides is well known for its extraordinary accumulation of manganese (Mn), and is used as a model for studying Mn uptake and utilization by plants. To clarify the role of manganese dioxide (MnO2) solubilization for Mn acquisition and further Mn hyperaccumulation in this plant, we examined the lowering of pH in the rhizosphere and Mn accumulation of this plant using regenerated plants. Plants regenerated from C. sciadophylloides calli lowered the pH of the culture broth continuously and simultaneously solubilized MnO2 added to the medium. The Mn content of the plant increased up to 1,300 mg kg−1 within 4 weeks of culture. Release of protein or specific organic acid from the roots was not observed. The medium used for plant culture maintained MnO2 solubilization ability after removal of the plant; however, this ability was lost by adjustment to the same medium pH of pre-culture conditions. In addition, pH lowering and MnO2 solubilization were suppressed by adding 1 mmol L−1 of the plasma H+-ATPase inhibitor Na3VO4 to the medium, and completely inhibited when 5 mmol L−1 of Na3VO4 was added. These results suggested that H+ leaking from plasma H+-ATPase plays an important role in MnO2 solubilization in the rhizosphere of C. sciadophylloides and in Mn accumulation in this plant.
INTRODUCTION
Among the six transition metal elements required as essential nutrients for higher plants, manganese (Mn) is an important element that is needed for many processes, such as photosynthesis, amino acid synthesis and superoxide dimustase activity. A relatively large amount of Mn as well as Fe is required for photosynthesis, and its deficiency causes serious damage, such as chlorosis in plants. However, Mn deficiency is observed in arable land worldwide even though there is an adequate amount of Mn in soil (CitationWelch 1995). This is because only a reduced form of Mn (Mn2+) is available to plants and oxidized forms such as MnO2 are not (CitationRenzel 1997). Hence, studies examining MnO2 solubilization and the following acquirement of Mn2+ by plants are speculated to be salutary for crop production. However, the MnO2 solubilization mechanism by the plant itself has not been investigated vigorously, and many points related to Mn acquirement by plants are unclear.
Metal hyperaccumulators are plants that accumulate some specific metal at an extraordinary high level, and are good models for investigations of metal uptake, tolerance, transport and utilization in plants. Among 400 species of metal hyperaccumulator plants, 11 plants were introduced as Mn hyperaccumulators by CitationBrooks (1998), and recently Austromyrtus bidwillii and Phytolacca acinosa were also reported to be Mn hyperaccumulators (CitationBidwell et al. 2002; CitationXue et al. 2004). Some of these plants have been investigated for their storage patterns of Mn and participation of organic acids in Mn hyperaccumulation. In A. bidwillii, the Mn concentration of the leaf reached 19,200 mg kg−1 dry weight, and it was revealed that the leaf contained a sufficient amount of organic acid (oxalic, malic, malonic, succinic). However, mechanisms of MnO2 solubilization and uptake in these hyperaccumulator plants have scarcely been investigated, and we have very little information about their Mn acquisition abilities.
Chengiopanax sciadophylloides (formally Acanthopanax sciadophylloides) is the sole Japanese Mn-hyperaccumulator woody plant reported by CitationTakada et al. (1994, Citation1997). In this plant, Mn is present mainly in the cell wall and vacuole of the leaf, and forms a complex with oxalic acid in the vacuole (CitationMemon and Yatazawa 1984). In general, plants have a poor ability to solubilize MnO2, and usually Mn-hyperaccumulator plants are found in highly Mn-contaminated soil areas. However, Mn hyperaccumulation of C. sciadophylloides is observed in the normal soil environments of Japanese forest areas (CitationTakada et al. 1994). This distinct character of C. sciadophylloides suggests that this plant has a special ability to change MnO2 to ionized Mn2+, which plants can easily take up, and the elucidation of the mechanism of MnO2 solubilization by this plant may give us a good countermeasure to Mn deficiency in plants. To clarify this mechanism, we investigated MnO2 solubilization in the rhizosphere of C. sciadophylloides plants regenerated from calli and the participation of H+-ATPase in this mechanism.
MATERIALS AND METHODS
Culture conditions of calli and regenerated plants
Calli and regenerated plants were cultured in sterile conditions at 25°C, 16-h day/8-h night, 25 µmol s−1 m−2 of photon flux density in a growth chamber.
The callus of C. sciadophylloides used in the present study was a kind gift from D. Atsushi Taniguchi (Forest Tree Breeding Center, Hitachi, Japan). The callus was cultured on 10 mg L−1 gellan gum medium containing full-strength Murashige and Skoog (MS) salt (CitationMurashige and Skoog 1962), 1 mg L−1 2,4-dichorophenoxy acetic acid (2,4-D) and 20 g L−1 sucrose, and serially passed and cultured until the regeneration of plants.
Calli were transplanted to the same medium but with 0.01 mg L−1 2,4-D to induce the formation of asexual embryo. The asexual embryos were transplanted to regeneration medium (1/4 MS salt, no 2,4-D and sucrose) to produce regenerated plants.
Examination of MnO2-solubilization ability
Ten milliliters of 1/4 MS medium without Mn2+, sucrose and 2,4-D (1/4 MS–Mn) was prepared in a 50 mL plastic centrifuge tube and supplied with 316.5 mg L−1 powdered MnO2 (Wako Chemical, Osaka, Japan) equivalent to 200 mg L−1 Mn. Regenerated plant samples cultured on regeneration medium for 1 month were transplanted to the tube and continued cultivation for a given period (6 or 7 days and 1 month). The above-ground parts of the plant samples (n = 4) were collected at weekly intervals, and dried at 77°C for 2 days, following two washes with ultrapure-water. After decomposition of the dried sample in a mixture of nitric acid–perchloric acid, Mn in the above-ground parts was quantitatively analyzed using an atomic absorption spectrometer (AAS, Type AA6500S, Shimadzu, Kyoto, Japan).
The pH and Mn content of the filtrated culture medium were also measured with a glass electrode and an AAS, respectively. The same experiments without regenerated plants (–plant) were also carried out as control tests.
Effect of pH adjustment of the culture medium on Mn solubilization
Regenerated plants were cultured in 1/4 MS(–Mn) for 1 week and then removed from the medium. A portion of the samples was adjusted to pH 5.3 by adding 1 mol L−1 NaOH. After adding powdered MnO2 equivalent to 200 mg L−1 Mn, the treated medium was incubated at 25°C with shaking, (150 rounds per minute) for 24 h. Solubilized Mn in the medium was measured with an AAS after filtration through 0.22 µm mesh.
Inhibition of H+-ATPase activity in the rhizosphere
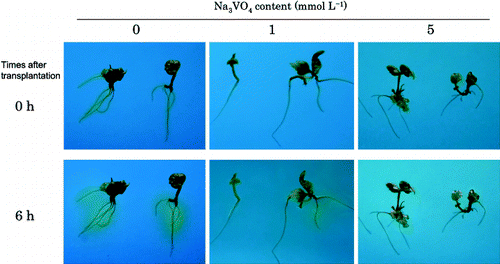
The experiments were carried out using the methods reported by CitationYan et al. (2002). Regenerated plants were transferred to pH indication medium (5 g L−1 gellan gum, 0.06 mg L−1 bromocresol green, 1 mmol L−1 CaSO4 and 2.5 mmol L−1 K2SO4, pH5.8) containing 0, 1 or 5 mmol L−1 of a plasma membrane H+-ATPase inhibitor Na3VO4. Plants were incubated for 6 h in the light at 25°C, and rhizosphere pH change indicated by the color of bromocresol green was observed every 2 h after transplanting.
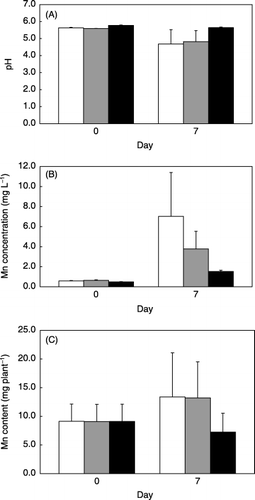
Ten milliliters of 1/4 MS(–Mn) supplemented with 316.5 mg L−1 MnO2 and 0, 1 or 5 mmol L−1 Na3VO4 was prepared in a 50 mL plastic centrifuge tube and regenerated plant was transplanted into it. After cultivation for 1 week, the pH of the culture medium and the Mn content of the above-ground part of the regenerated plant were investigated.
RESULTS
Plants regenerated from C. sciadophylloides callus
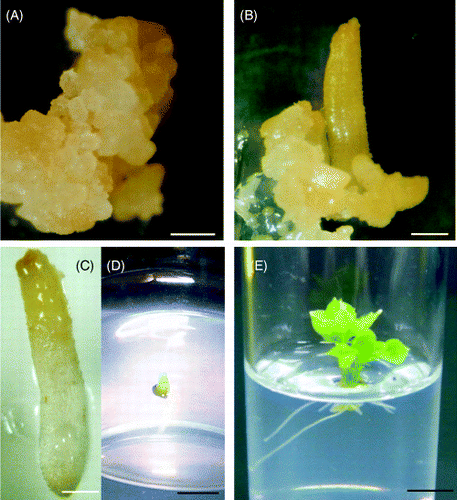
In the series of experiments reported here, we used plants regenerated from calli because the seeds of this plant seldom germinate successfully. Asexual embryos were induced from C. sciadophylloides calli () by lowering the 2.4-D content of the medium to 0.01 mg kg−1 (), and part (half) of the embryos
Figure 1 Plants regenerated from Chengiopanax sciadophylloides calli. Calli of C. sciadophylloides (A) were induced to asexual embryo formation (B) by 2,4-D content lowering. Asexual embryos, green and white in color (C), were transplanted to the regeneration medium with the green part of the asexual embryos above the medium (D). Leaves and roots were produced from the green and white part of the asexual embryo, respectively (E). Size bars in A–E indicate 1 mm, 1 mm, 1 mm, 3 mm and 5 mm, respectively.
MnO2 solubilization ability of regenerated plants
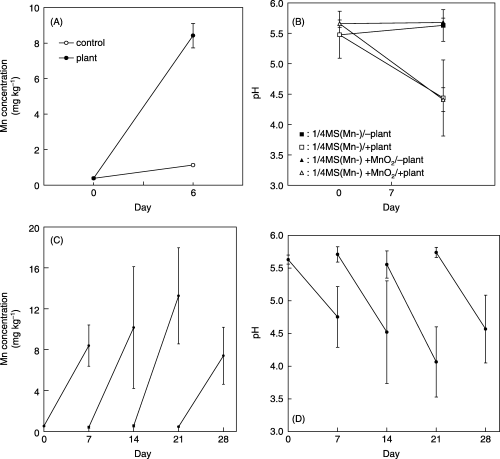
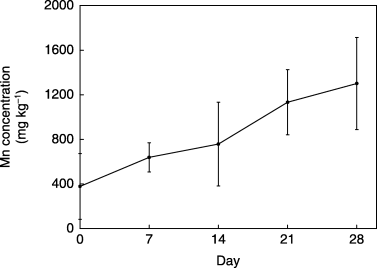
Powdered MnO2 was hardly dissolved in 1/4 MS medium, but its solubility increased markedly by culturing the regenerated plant in the medium. Mn2+ content of the medium increased from 0.39 to 8.42 mg L−1 during the 6 day cultivation, in contrast to the slight increase to 1.14 mg L−1 in the control medium (). Furthermore, the pH of the medium (–plant) did not change for 1 week (5.66–5.68), but lowered to 4.41 when the regenerated plant was cultivated, and this lowering was also observed in the same condition without adding MnO2 to the medium (). When the cultivation of the regenerated plants continued for 4 weeks with weekly medium exchange, the Mn content of the medium was markedly increased every week () with continuous pH lowering (). These data strongly suggest that regenerated plants of C. sciadophylloides could solubilize MnO2 accompanied with pH lowering, and this solubilization system occurred constitutively and independently of MnO2 existence. With an increase in Mn solubilization in the culture medium, the Mn content of the above-ground part of the regenerated plants increased up to 1,300 mg kg−1 in 4 weeks ().
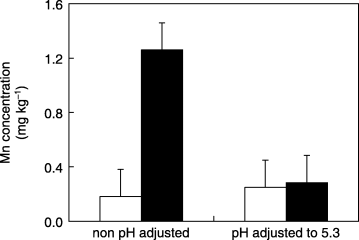
The medium used for plant culture retained MnO2 solubilization ability even after removing the plants from the medium, but once the medium pH was adjusted to pH 5.3, the same pH of the –plant medium, the MnO2 solubilization ability disappeared (). In addition, MnO2 solubilization was not affected by heat treatment (data not shown). These results indicate that MnO2
solubilization was not caused by the activity of unknown enzyme(s) released from C. sciadophylloides roots, but may be caused by simple pH lowering.Role of plasma membrane H+-ATPase in MnO2 solubilization
To clarify the participation of plasma membrane H+-ATPase in pH lowering and MnO2 solubilization in the
Figure 4 Effects of pH adjustment of the culture medium on MnO2 solubilization. Portions of culture medium with no plants (□) and after removal of regenerated plants (▪) were adjusted to pH 5.3, and the activity of MnO2 solubilization was investigated by measuring the Mn concentration increase over 1 week. Data are expressed as the mean ± standard deviation of four replicates.
DISCUSSION
Regenerated plants of C. sciadophylloides lowered the pH and solubilized MnO2 in the rhizosphere in the medium continuously even in the presence of MnO2. Although several investigators have dealt with pH lowering in the rhizosphere induced by deficiencies of Fe or P (CitationDell’Orto et al. 2000; CitationYan et al. 2002), none showed constant pH lowering in the rhizosphere under adequate nutrient conditions or the participation of pH lowering in the Mn acquisition of plants. We speculate that this is not attributable to the lack of available Mn because the Mn2+ concentration of the culture medium just after the addition of MnO2 (7.1 µmol L−1) was almost the same as that for popular hydroponics. In addition, we could demonstrate that this pH lowering resulted from H+ releasing by the activity of plasma membrane H+-ATPase, and suppression of the action directly resulted in the inhibition of Mn solubilization and accumulation in C. sciadophylloides. Aside from investigating the role of H+-ATPase, we searched for other Mn solubilization factors that might be released from C. sciadophylloides in the rhizosphere. The existence of several types of organic acids such as oxalate, malate, malonate and succinate, which are present in the leaves of C. sciadophylloides (CitationMemon et al. 1984) and in the Australian Mn hyperaccumulator A. bidwillii (CitationBidwell et al. 2002), in the medium was checked, but no candidate peak was detected using High performance liquid chromatography (HPLC) analysis (data not shown). We also looked for the presence of an enzyme or peptide that has the ability to solubilize MnO2. However, the MnO2 solubilization ability of the culture medium was not affected by heat treatment, and neither protein nor peptide releasing were detected by silver staining on sodium dodecylsulfate-polyacrylamide gel electrophoresis (SDS-PAGE) gel (data not shown). Judging from these results and additional conformation from the pH control test in , we concluded that the Mn solubilization of C. sciadophylloides resulted from the consequent pH lowering by the plasma membrane.
To date, several investigators have reported the effect of Mn-reducing rhizosphere bacteria on Mn uptake by plants (CitationMarschner et al. 1991, Citation2003; CitationTrimble and Ehrlich 1968), but the present study may be the first and sole report showing that the plant can lower the pH of its rhizosphere by itself in normal culture condition. This suggests that proton release in the rhizosphere by the plant itself also plays a role in Mn acquirement, and also suggests that this mechanism may have, at least partially, a role in Mn hyperaccumulation in this plant. This finding is speculated to reinforce the tolerance of this plant to Mn deficiency, but further investigation on the role of pH lowering in Mn acquirement in other plants is necessary to use this finding for crop cultivation in low Mn-available soil areas.
Similar to the case of the relationship between a ZIP family transporter TcZNT1 and Cd hyperaccumulation in Thlaspi caerulescens (CitationPence et al. 2000), it is easy to speculate that active Mn2+ transport into the plant may also play an important role in the Mn hyperaccumulation of C. sciadophylloides. To date, some homologues of ZIP, NRAMP and CAX family transporters has been reported for their Mn2+ transport abilities (CitationHirschi et al. 2000; CitationKorshunova et al. 1999; CitationThomine et al. 2003) and it has been confirmed by our group that C. sciadophylloides has homolog genes of ZIP and NRAMP transporters (CsZIP1: AB242563, CsNramp1: AB242564). Investigations on their Mn2+ transport ability and participation in Mn-specific hyperaccumulation are now in progress. Moreover, proton release by plasma membrane H+-ATPase produced an electrochemical potential gradient that is utilized for nutrient uptake (CitationPalmgren 2001). Not only MnO2 solubilization, but also continuous proton release from C. sciadophylloides roots may work as powerhouses of Mn2+ uptake.
In the present study, we proposed one candidate mechanism for the Mn acquirement of C. sciadophylloides, but whether this mechanism works for Mn hyperaccumulation and Mn-specific acquisition remains to be determined. In addition, we need to examine further the effect of Na3VO4 to ensure the role of the plasma membrane H+-ATPase in MnO2 solubilization. Na3VO4 may also have an effect on other proteins (CitationPalmgren and Harper 1999). Further investigation of these issues is ongoing.
ACKNOWLEDGMENTS
This work was supported in part by Grant-in-Aid for Young Researchers (B) to T. Mizuno (No. 181878004500) and Grant-in-Aid for Scientific Research on Priority Areas “Nutrient uptake and transport in plants—Identification of molecules responsible for transport and their regulation mechanisms” to H. Obata and T. Mizuno (No. 181805600900) from the Ministry of Education, Culture, Sports, Science and Technology of Japan. This work was also supported in part by Grant-in-Aid for Scientific Research (C) to H. Obata (No. 181551007300) from the Japan Society for the Promotion of Science.
REFERENCES
- Welch , RM . 1995 . Micronutrient nutrition of plants . CritRevPlant Sci , 14 : 49 – 82 .
- Renzel , Z . 1997 . Root exudation and microflora populations in rhizosphere of crop genotypes differing in tolerance to micronutrient deficiency . Plant Soil , 196 : 255 – 260 .
- Brooks , RR . 1998 . “ General Introduction ” . In Plants That Hyperaccumulate MetalsTheir Role in Phytoremediation, Microbiology, Archaeology, Mineral Exploration and Phytomining , Edited by: Brooks , RR . 1 – 14 . New York : CAB International .
- Bidwell , SD , Woodrow , IE , Batianoff , GN and Sommer-Knudsen , J . 2002 . Hyperaccumulation of manganese in the rainforest tree Austromyrtus bidwillii(Myrtaceae) from Queensland, Australia . FuncPlant Bio , 29 : 899 – 905 .
- Xue , SG , Chen , YX , Reeves , RD , Baker , AJM , Lin , Q and Fernando , DR . 2004 . Manganese uptake and accumulation by the hyperaccumulator plant Phytolacca acinosaRoxb. (Phytolaccaceae) . EnvironPollut , 131 : 393 – 399 .
- TakadaJ TakamatsuT SatakeK SaseH1994 Data on elemental concentration in land plants by neutron activation analysis (No. 1) National Institute for Environmental StudiesTsukuba (in Japanese)
- Takada , J , Nishimura , K , Akaboshi , M , Matsubara , T , Katayama , Y and Koyama , M . 1997 . Element content in a number of plant leaves and accumulation of some elements in typical plant species: A case of Okayama Prefecture . JRadioanalNuclearChem , 217 : 65 – 70 .
- Memon , AR and Yatazawa , M . 1984 . Nature of manganese complexes in the manganese accumulator plant Acanthopanax sciadophylloides . JPlant Nutr , 7 : 961 – 974 .
- Murashige , T and Skoog , F . 1962 . A revised medium for rapid growth and bioassays with tobacco tissue culture . PhysiolPlant , 15 : 473 – 497 .
- Yan , F , Zhu , Y , Muller , C , Zorb , C and Schubert , S . 2002 . Adaptation of H+-pumping and plasma membrane H+-ATPase activity in proteoid roots of white lupin under phosphate deficiency . Plant Physiol , 129 : 50 – 63 .
- De Michelis , MI and Spanswick , RM . 1986 . H+-pumping driven by the vanadate-sensitive ATPase in membrane vesicles from corn roots . Plant Physiol , 81 : 542 – 547 .
- O’Neill , SD and Spanswick , RM . 1984 . The effects of vanadate on the plasma membrane ATPase of red beet and corn . Plant Physiol , 75 : 586 – 591 .
- Dell’Orto , M , Santi , S Nisi , PD . 2000 . Development of Fe-deficiency responses in cucumber (Cucumis sativus L.) roots: involvement of plasma membrane H+-ATPase activity . JExpBot , 51 : 695 – 701 .
- Marschner , P , Ascher , JS and Graham , RD . 1991 . Effect of manganese-reducing rhizosphere bacteria on the growth of Gaeumannomyces graminisvar. triticiand on manganese uptake by wheat (Triticum aestivumL.) . BiolFertlSoils , 12 : 33 – 38 .
- Marschner , P , Fu , Q and Rengel , Z . 2003 . Manganese availability and microbial populations in the rhizosphere of wheat genotypes differing in tolerance to Mn deficiency . JPlant NutrSoil Sci , 166 : 712 – 718 .
- Trimble , RB and Ehrlich , HL . 1968 . Bacteriology of manganese nodules III. Reduction of MnO2by two strains of nodule bacteria . ApplMicrobiol , 16 : 695 – 702 .
- Pence , NS , Larsen , PB Ebbs , SD . 2000 . The molecular physiology of heavy metal transport in the Zn/Cd hyperaccumulator Thlaspi caerulescens . ProcNatl AcadSci, USA , 97 : 4956 – 4960 .
- Hirschi , KD , Victor , D , Korenkov , VD , Wilganowski , NL and Wagner , GJ . 2000 . Expression of Arabidopsis CAX2in tobacco. Altered metal accumulation and increased manganese tolerance . Plant Physiol , 124 : 125 – 134 .
- Korshunova , YO , Eide , D , Clark , WG , Guerinot , ML and Pakrasi , HB . 1999 . The IRT1 protein from Arabidopsis thalianais a metal transporter with a broad substrate range . Plant MolBiol , 40 : 37 – 44 .
- Thomine , S , Lelievre , F , Debarbieux , E , Schroeder , JI and Barbier-Brygoo , H . 2003 . ATNRAMP3, a multispecific vacuolar metal transporter involved in plant responses to iron deficiency . Plant J , 34 : 685 – 695 .
- Palmgren , MG . 2001 . Plant plasma membrane H+-ATPases: powerhouses for nutrient uptake . AnnuRevPlantPhysiolPlantMolBiol , 52 : 817 – 845 .
- Palmgren , M and Harper , J . 1999 . Pumping with plant P-type ATPases . JExpBot , 50 : 883 – 893 .