Abstract
We studied the effect of crop residues with various C:N ratios on N2O emissions from soil. We set up five experimental plots with four types of crop residues, onion leaf (OL), soybean stem and leaf (SSL), rice straw (RS) and wheat straw (WS), and no residue (NR) on Gray Lowland soil in Mikasa, Hokkaido, Japan. The C:N ratios of these crop residues were 11.6, 14.5, 62.3, and 110, respectively. Based on the results of a questionnaire survey of farmer practices, we determined appropriate application rates: 108, 168, 110, 141 and 0 g C m−2 and 9.3, 11.6, 1.76, 1.28 and 0 g N m−2, respectively. We measured N2O, CO2 and NO fluxes using a closed chamber method. At the same time, we measured soil temperature at a depth of 5 cm, water-filled pore space (WFPS), and the concentrations of soil NH+ 4-N, NO− 3-N and water-soluble organic carbon (WSOC). Significant peaks of N2O and CO2 emissions came from OL and SSL just after application, but there were no emissions from RS, WS or NR. There was a significant relationship between N2O and CO2 emissions in each treatment except WS, and correlations between CO2 flux and temperature in RS, soil NH+ 4-N and N2O flux in SSL and NR, soil NH+ 4-N and CO2 flux in SSL, and WSOC and CO2 flux in WS. The ratio of N2O-N/NO-N increased to approximately 100 in OL and SSL as N2O emissions increased. Cumulative N2O and CO2 emissions increased as the C:N ratio decreased, but not significantly. The ratio of N2O emission to applied N ranged from −0.43% to 0.86%, and was significantly correlated with C:N ratio (y = −0.59 ln [x] + 2.30, r 2 = 0.99, P < 0.01). The ratio of CO2 emissions to applied C ranged from −5.8% to 45% and was also correlated with C:N ratio, but not significantly (r 2 = 0.78, P = 0.11).
Key words:
INTRODUCTION
Nitrous oxide (N2O) is one of the major greenhouse gases and has a 296-fold higher greenhouse effect than carbon dioxide (CO2) over a 100-year time frame, and its concentration in the air is increasing to 0.8 p.p.b. y−1 (CitationIntergovernmental Panel on Climate Change 2001a). The estimated global N2O emission is reported to be 16.2 Tg N y−1, of which 25% is emitted from agricultural soils (CitationMosier et al. 1998). However, estimated values of N2O emissions have a large uncertainty (CitationIntergovernmental Panel on Climate Change 2001a; CitationMosier et al. 1998).
The application of crop residues to soil has been shown to increase N2O emission (CitationAulakh et al. 1991; CitationCochran et al. 1997; CitationHuang et al. 2004; CitationMori et al. 2005; CitationPotthoff et al. 2005). In general, N2O is produced by nitrification and denitrification in soil. Because , NH+ 4, NO− 3 and organic C are used in these processes, applying crop residues to the soil and mineralizing it generally increases N2O production. Application of crop residues to soil activates aerobic decomposition. Following that, the drawdown of oxygen activates denitrification (CitationPotthoff et al. 2005). CitationVigil and Kissel (1991) reported that applying residues with a low C:N ratio encouraged mineralization, but applying residues with a higher C:N ratio advanced N immobilization. CitationAkiyama and Tsuruta (2003) reported a negative logarithmic relationship between N2O emission and the C:N ratio of applied organic matter at the same N application rate in an Andisol field, whereas CitationHuang et al. (2004) reported that the ratio of N2O emission to applied residue N increased
Table 1 Chemical properties of the soil horizons
Table 2 Quantities of nitrogen and carbon applied in crop residues in each treatment
MATERIALS AND METHODS
Site description
We selected fallow land with Gray Lowland (GL) soil in Mikasa, Hokkaido, Japan (43°14.4′ N, 141°50′ E). Onion had been cultivated in the field for more than 10 years, and the field was fallow for 10 months before our experiments. Mean annual temperature and precipitation are 7.4°C and 1,212 mm, respectively, and the soil does not freeze in winter. At a depth of 0–10 cm, the soil pH (H2O) was 5.8 and the cation exchange capacity (CEC) was 25.5 cmolc kg−1. Soil C and N concentrations were 32.1 and 2.8 g kg−1, respectively, and sand, silt and clay comprised 12.1%, 51.2% and 36.7%, respectively, at a depth of 0–10 cm ().
Experimental plots
We set up five experimental plots with four types of crop residue, onion leaf (OL), soybean stem and leaf (SSL), rice straw (RS) and wheat straw (WS), and no residue (NR). Each residue was dried completely at 70°C and cut into 5-cm pieces. The C:N ratios were 11.6, 14.5, 62.3 and 110, respectively. From the results of a questionnaire survey of farmer practices, we estimated the residue N application rate to each field. Then, the appropriate application rates, which did not exceed the maximum application rate, were determined. The residue C or N application rates were 108, 168, 110, 141 and 0 g C m−2 and 9.3, 11.6, 1.76, 1.28 and 0 g N m−2 ().
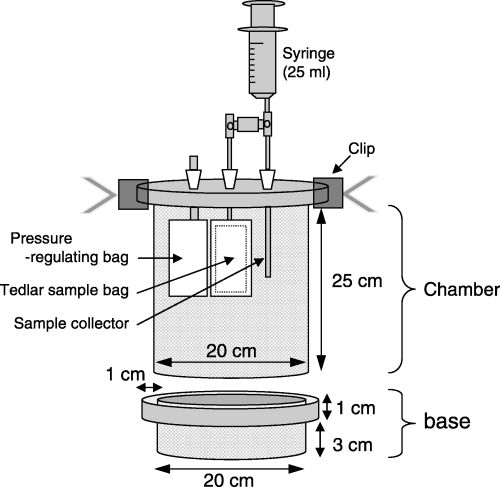
We set up the experimental plots on 1 September 2005. We dug out all soil to a depth of 5 cm from each plot (1 m × 1 m) and mixed it thoroughly. We divided the soil into five equal parts by weight, and mixed one residue into each part (leaving the NR unamended). We placed root-resistant, water-permeable sheets (Toyobo BKS9812) at the bottom of each hole to avoid mixing the treated soil and subsoil, and then laid each mixed soil onto the sheet. The saturated hydraulic conductivity of the sheet (4.8 × 10−6 m s−1) was higher than that of the soil in the same field (CitationHu et al. 2002). Finally, we set up three bases in each plot for the gas sampling chambers (). Each base measured 20 cm in diameter and was made of stainless steel, and the upper part had a slight depression (1 cm deep, 1 cm wide). The lower part (3 cm deep) remained in the soil throughout the experimental period ().
Measuring N2O, CO2 and NO fluxes and estimating N2O and CO2 emissions
N2O, CO2 and NO fluxes from each plot were measured using a closed chamber method (CitationKusa et al. 2002). We sampled three times in the first week (2, 5 and 8 September), two times in the next week and a half (13 and 18 September), and then once per week until 30 October (six times). The gas sampling chamber was 20 cm wide and 25 cm high, and was made of white-painted stainless steel (). The cover, made of white acryl, was fitted with a sample collector, a pressure-regulating bag, and a Tedlar sample bag (0.5 L). Gas samples were withdrawn from the sample collector by syringe into the Tedlar bag. The chamber was set on the base's depression, which was filled with water to prevent aeration during measurement. We took gas samples before
closing the chamber (0 min) for analysis of N2O, CO2 and NO. After the chamber was closed, we took one gas sample for CO2 after 6 min, and another for N2O and NO after 15 min (CitationKusa et al. 2002; CitationNakano et al. 2004). All samples were taken in triplicate (one per base).In the laboratory, the gas fluxes were calculated using a linear-recurrence method (CitationKusa et al. 2002). CO2 was analyzed using an infrared CO2 analyzer (Model ZFP5YA3I, Fuji Electric, Tokyo, Japan) within 12 h. The detection limit of CO2 flux was ±3.7 mg C m−2 h−1. N2O was analyzed using electron-capture detection gas chromatography (Model GC-14B, Shimadzu, Kyoto, Japan) within 1 month. The detection limit of N2O flux was ±7.7 µg N m−2 h−1. NO was analyzed using a chemiluminescence nitrogen oxide analyzer (Model 265P, Kimoto Electric, Osaka, Japan) within 12 h. The detection limit of NO flux was ±0.99 µg N m−2 h−1.
N2O and CO2 emissions were calculated using the following equation:
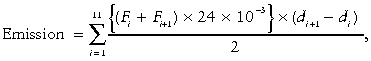 ,
where F
i
is the N2O or CO2 flux at the ith sampling (N2O: µg N m−2 h−1; CO2: mg C m−2 h−1) and d
i
denotes the day of the ith sampling.
,
where F
i
is the N2O or CO2 flux at the ith sampling (N2O: µg N m−2 h−1; CO2: mg C m−2 h−1) and d
i
denotes the day of the ith sampling.
Calculation of the emission fractions of N2O (EFN2O) and CO2 (EFCO2 )
We assumed that in each treatment the N2O and CO2 emissions originated from the N or C in the crop residues and soil organic matter. Therefore, we calculated the emission fractions of N2O (EFN2O) and CO2 (EFCO2 ) as:
Other measurements and soil sampling
Soil temperature at a depth of 5 cm was measured during each gas flux measurement. Soil samples were taken from 0–5 cm at three points in each plot. After measurement of water content, each sample was extracted with distilled water for measurement of the NO− 3-N and water-soluble organic carbon (WSOC) concentrations and with 2 mol L−1 KCl for measurement of the NH+ 4-N concentration. NO− 3-N and WSOC concentrations were analyzed using ion chromatography (QIC analyzer, Dionex Japan, Osaka, Japan) and a Total organic carbon (TOC) analyzer (Model TOC-5000A, Shimadzu, Kyoto, Japan), respectively. The NH+ 4-N concentration was analyzed using colorimetry with indophenol-blue (Uvmini-1240, Shimadzu, Kyoto, Japan). Three replicate undisturbed soil samples (0–5 cm) were also taken with a steel corer (100 mL) on 1 September and 30 October and analyzed for dry bulk density. The dry bulk density increased by 30% during that interval owing to soil consolidation after setting the treatments. We assumed that the change was linear, and calculated water-filled pore space (WFPS) from the interpolated dry bulk density and water content. Daily precipitation data were obtained from the local Iwamizawa weather station (43°12.6′ N, 141°47.3′ E).
RESULTS
N2O and CO2 fluxes
shows N2O and CO2 fluxes over the study period. N2O fluxes in OL (455 µg N m−2 h−1) and SSL (567 µg N m−2 h−1) reached their maximum on the third day (4 September) and then gradually decreased. However, in RS, WS and NR, N2O fluxes were close to zero throughout the study period. CO2 fluxes in OL (506 mg C m−2 h−1) and SSL (413 mg C m−2 h−1) also reached a maximum on the third day and then gradually decreased.
There were positive relationships between N2O and CO2 fluxes in all plots except for WS (). There were strong correlations in OL (n = 33, r 2 = 0.81, P < 0.001) and SSL (n = 33, r 2 = 0.89, P < 0.001).
Soil temperature, WFPS, soil NH+ 4-N and NO− 3-N concentrations, WSOC and NO flux
shows the variation in soil temperature, WFPS, soil NH+ 4-N, NO− 3-N, WSOC and NO flux, and shows the relationship of N2O and CO2 fluxes with soil temperature, WFPS and soil NH+ 4-N, NO− 3-N
Figure 2 Variations in (a) N2O flux, (b) CO2 flux, (c) soil temperature, (d) water-filled pore space (WFPS), (e) soil -N, (f) -N, (g) water-soluble organic carbon (WSOC) and (h) NO flux from plots treated with onion leaf (OL), soybean stem and leaf (SSL), rice straw (RS), wheat straw (WS) or no residue (NR). SD, standard deviation.
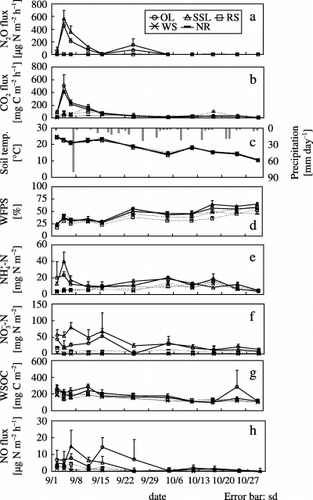
Figure 3 Relationships between N2O and CO2 fluxes from plots treated with (a) onion leaf (OL) and soybean stem and leaf (SSL) and (b) rice straw (RS), wheat straw (WS) or no residue (NR).
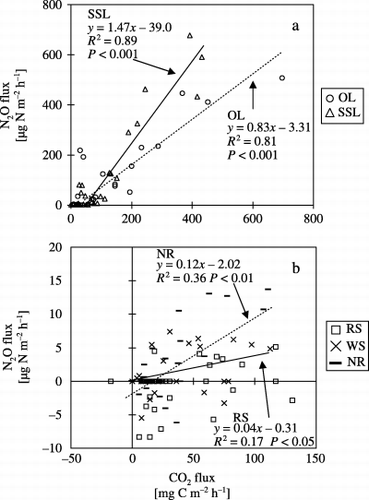
Figure 4 Relationships between N2O (a–e) and CO2 (f–j) fluxes and soil temperature at 5 cm (a,f), water-filled pore space (WFPS) (b,g), soil -N (c,h), -N (d,i) and water-soluble organic carbon (WSOC) (e,j) in plots treated with onion leaf (OL), soybean stem and leaf (SSL), rice straw (RS), wheat straw (WS) or no residue (NR).
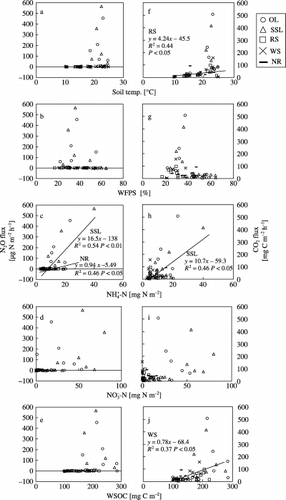
Table 3 Measured N2O and CO2 emissions, and estimated EFN2O and EFCO2 in five treatments
N2O and CO2 emissions and EFN2O and EFCO2
shows the N2O and CO2 emissions and the estimated EFN2O and EFCO2 in each plot. N2O and CO2 emissions from each plot ranged from −1.44 to 89.1 mg N m−2 per period and from 42.7 to 98.0 g C m−2 per period. Both emissions in OL and SSL were larger than those in the other plots. There was a significant positive correlation between N2O emission and crop residue N (y = 8.75x − 6.41, n = 5, r 2 = 0.96, P < 0.01). Moreover, N2O and CO2 emissions increased as the C:N ratio decreased, but not significantly (N2O: y = −0.99x + 92.2, n = 4, r 2 = 0.83, P = 0.09; CO2: y = −22.8 ln [x] + 152, n = 4, r 2 = 0.84, P = 0.09). There were no relationships between CO2 emission and C application rate (data not shown).
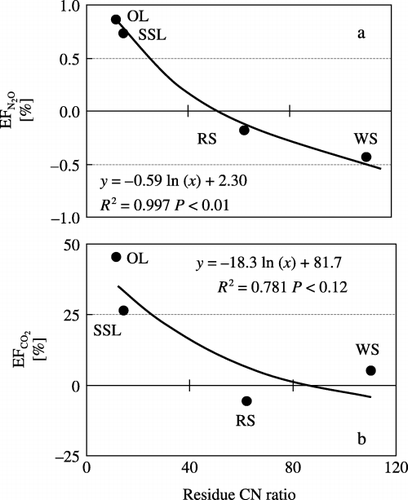
Emission fractions were −0.43% to 0.86% for N2O and −5.77% to 39.9% for CO2. EFN2O and EFCO2 were higher in OL and SSL than in the other plots. There was a negative logarithmic relationship between EFN2O and
residue C:N ratio (, EFN2O: y = −0.59 ln [x] + 2.30, n = 4, r 2 = 0.99, P < 0.01), and between EFCO2 and residue C:N ratio ( EFCO2 : y = −18.3 ln [x] + 81.7, n = 4, r 2 = 0.78, P = 0.11).DISCUSSION
N2O flux
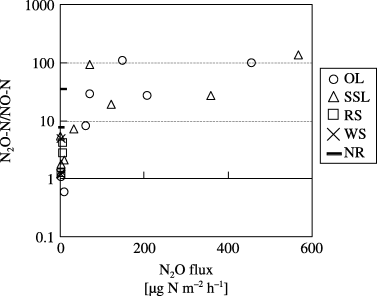
N2O fluxes increased greatly soon after the application of onion leaf and soybean residues, which had low C:N ratios. Similarly, CO2 fluxes and soil NH+ 4-N concentrations increased after the application of those residues, then soil NO− 3-N concentrations increased. The lack of change in N2O and CO2 fluxes in the NR suggests that onion leaf and soybean residues decomposed rapidly and nitrification took place. CitationAulakh et al. (1991) and CitationHuang et al. (2004) reported that N2O and CO2 fluxes increased more after the application of residues with low C:N ratios compared with high C:N ratios. CitationVigil and Kissel (1991) reported that crop residue C:N ratio had a significant negative relationship to the rate of mineralization of residues. CitationEiland et al. (2001) reported that residues with a high C:N ratio posed a nutrient limitation on decomposers during the early stage of decomposition. We assume that residues with a lower C:N ratio were more easily mineralized and the probability of nitrification and denitrification was high, so N2O was easily produced. CitationBouwman (1990) summarized the reports written by CitationAnderson and Levine (1986) and CitationLipschultz et al. (1981), and reported that N2O-N/NO-N was < 1 during nitrification and > 100 during denitrification. Because N2O-N/NO-N increased with increasing N2O flux (), we suggest that N2O was mainly produced by denitrification in the OL and SSL (CitationBouwman 1990; CitationKusa et al. 2002, Citation2006). The production of N2O during denitrification could have been enhanced by the supply of N and C from the decomposing onion leaf and soybean residues, which had low C:N ratios.
N2O emission and EFN2O
We found positive relationships between N2O emission and crop residue N and between N2O and CO2 fluxes, but a negative logarithmic relationship between CO2 emission and residue C:N ratio. Therefore, N2O emission could have been affected by the decomposition of residues. N2O emissions have been shown to increase with increasing fertilizer N application rate (CitationGroenigen et al. 2004; CitationSmith et al. 1998). In the present study, onion leaf and soybean residues, with more N than the other residues, could have been easily decomposed on account of their low C:N ratios. For that reason, more N was supplied to the soil and N2O producers.
EFN2O increased as the C:N ratio of residues decreased. CitationMillar and Baggs (2005) applied six crop residues with the same amount of N and found significant negative correlations between N2O emission and the residue's soluble C:N ratio. CitationAkiyama and Tsuruta (2003) also reported a negative logarithmic relationship between N2O emission and the C:N ratio of applied organic matter in an Andisol field. This relationship between EFN2O and residue C:N ratio might arise because a lower C:N ratio makes the residue more easily decomposed (CitationEiland et al. 2001; Vigil & Kissel 1991). However, CitationHuang et al. (2004) reported that the EFN2O of residues was positively correlated with the residue C:N ratio, and suggested that most of the N2O was produced by denitrification. The soil C concentration CitationHuang et al. (2004) used was 21 g kg−1, and sand and clay contents were 2.1% and 51%, respectively (they did not show WFPS). Therefore, the soil of their study was more clayey and had a lower C concentration than the soil used in our study. CitationGroffman and Tiedje (1989) reported that clay loam soil compared with loam or sandy soils showed a high denitrification rate. Applying a C source, which is easily available, may increase the reduction of N2O to N2 (CitationWeier et al. 1993). Thus, we believe that in their study, N and C supplied from residues with a low C:N ratio could have allowed active reduction of N2O to N2 and, thus, decreased the emission of N2O from residues with a low C:N ratio. In our study, most of the N2O was produced by denitrification in OL and SSL. Therefore, applying residues with a low C:N ratio to the soil may enhance the process of denitrification. However, the type of N (N2O or N2) emitted from the soil might be affected by soil moisture content or soil type (CitationCheng et al. 2004; CitationScholefield et al. 1997; CitationWeier et al. 1993).
In our study, the EFN2O values in RS and WS were negative. Moreover, EFN2O became negative at a residue C:N ratio greater than 49.7. Soil NH+ 4-N and NO− 3-N concentrations in RS and WS were lower than those in NR. Therefore, immobilization of NH+ 4-N and NO− 3-N might have occurred in RS and WS. The application of residues with a high C:N ratio to the soil often immobilizes mineral N in soil (CitationYonebayashi 1997). CitationVigil and Kissel (1991) reported that the border of residue C:N ratio between mineralization and immobilization was approximately 40. However, our C:N ratios of rice and wheat straw were above 40 (62.3 and 110, respectively). In the regression model in , EFN2O became negative at C:N ratio > 49.7, indicating that the application of residues with high C:N ratio to the soil causes the immobilization of soil mineral N and makes EFN2O negative.
Further study of the effects of soil texture and C and N concentrations in soil on the relationship between EFN2O and residue C:N ratios is needed. However, it might be possible to estimate the EFN2O of certain residues by using the relationship between EFN2O and the residue C:N ratio.
Conclusion
N2O emissions from crop residues applied to soil were highest from onion leaf and soybean stem and leaf. N2O emission was higher in plots treated with residues with a low C:N ratio, possibly because residues with a low C:N ratio are more easily decomposed. There was a negative logarithmic relationship between EFN2O and residue C:N ratio (y = −0.59 ln [x] + 2.30, n = 4, r 2 = 0.99, P < 0.01), suggesting the possibility of estimating of residues from the residue C:N ratio.
ACKNOWLEDGMENTS
This work was financially supported by the Global Environmental Research Program of the Ministry of the Environment of Japan (No. S-2). The authors thank Akane Kagemoto and Yuu Hirose for help with collecting data, and Takeshi Morimoto for permission to use their onion fields.
REFERENCES
- Intergovernmental Panel on Climate Change2001aClimate Change 2001: Synthesis Report WG I – Technical Summary Available from URL: http://www.ipcc.ch/pub/syreng.htm (http://www.ipcc.ch/pub/syreng.htm)
- Mosier , A , Kroeze , C , Nevison , C , Oenema , O , Seitzinger , S and Cleemput , O . 1998 . Closing the global N2O budget: nitrous oxide emissions through the agricultural nitrogen cycle . NutrCyclAgroecosyst , 52 : 225 – 248 .
- Aulakh , MS , Doran , JW , Walters , DT , Mosier , AR and Francis , DD . 1991 . Crop residue type and placement effects on denitrification and mineralization . Soil SciSocAmJ , 58 : 1020 – 1025 .
- Cochran , VL , Sparrow , EB , Schlentner , SF and Knight , CW . 1997 . Long-term tillage and crop residue management in subarctic: fluxes of methane and nitrous oxide . CanJSoilSci , 77 : 565 – 570 .
- Huang , Y , Zou , J , Zheng , X , Wang , Y and Xu , X . 2004 . Nitrous oxide emissions as influenced by amendment of plant residues with different C:N ratio . Soil BiolBiochem , 36 : 973 – 981 .
- Mori , A , Hojito , M , Kondo , H , Matsunami , H and Scholefield , D . 2005 . Effects of plant species on CH4and N2O fluxes from a volcanic grassland soil in Nasu, Japan . Soil SciPlant Nutr , 51 : 19 – 27 .
- Potthoff , M , Dyckmans , J , Flessa , H , Muhs , A , Beese , F and Joergensen , RG . 2005 . Dynamics of maize (Zea maysL.) leaf straw mineralization as affected by the presence of soil and the availability of nitrogen . Soil BiolBiochem , 37 : 1259 – 1266 .
- Vigil , MF and Kissel , DE . 1991 . Equations for estimating the amount of nitrogen mineralized from crop residues . Soil SciSocAmJ , 55 : 757 – 761 .
- Akiyama , H and Tsuruta , H . 2003 . Effect of organic matter application on N2O, NO, and NO2fluxes from an Andisol field . Global BiogeochemCycles , 17 ( 4 ) : 1100
- Hu , R , Hatano , R , Kusa , K and Sawamoto , T . 2002 . Effect of nitrogen fertilization on methane flux in a structured clay soil cultivated with onion in central Hokkaido, Japan . Soil SciPlant Nutr , 48 : 797 – 804 .
- Kusa , K , Sawamoto , T and Hatano , R . 2002 . Nitrous oxide emissions for 6 years from a gray lowland soil cultivated with onions in Hokkaido, Japan . NutrCyclAgroecosyst , 63 : 239 – 247 .
- Nakano , T , Sawamoto , T , Morishita , T , Inoue , G and Hatano , R . 2004 . A comparison of regression methods for estimating soil–atmosphere diffusion gas fluxes by a closed-chamber technique . Soil BiolBiochem , 36 : 107 – 113 .
- Eiland , F , Klamer , M , Lind , AM , Leth , M and Baath , E . 2001 . Influence of initial C/N ratio on chemical and microbial composition during long term composting of straw . MicrobEcol , 41 : 272 – 280 .
- Bouwman , AF . 1990 . “ Chapter 4–6 Nitric oxide and nitrogen dioxide ” . In Soils and the Greenhouse Gas Effect , Edited by: Bouwman , AF . 120 – 124 . Great Britain : John Wiley & Sons .
- Anderson , IC and Levine , J . 1986 . Relative rates of nitric oxide and nitrous oxide production by nitrifiers, denitrifiers, and nitrate respirers . ApplEnvironMicrobiol , 51 : 938 – 945 .
- Lipschultz , F , Zafiriou , OC , Wofsy , SC , McElroy , MB , Valois , FW and Watson , SW . 1981 . Production of NO and N2O by soil nitrifying bacteria . Nature , 294 : 641 – 643 .
- Kusa , K , Hu , R , Sawamoto , T and Hatano , R . 2006 . Three years of nitrous oxide and nitric oxide emissions from silandic andosols cultivated with maize in Hokkaido, Japan . Soil SciPlant Nutr , 52 : 103 – 113 .
- Groenigen , JW , Kasper , GJ , Velthof , GL , Dasselaar , A and Kuikman , PJ . 2004 . Nitrous oxide emissions from silage maize fields under different mineral nitrogen fertilizer and slurry applications . Plant Soil , 263 : 101 – 111 .
- Smith , KA , Mctaggart , IP , Dobbie , KE and Conen , F . 1998 . Emissions of N2O from Scottish agricultural soils, as a function of fertilizer N . NutrCyclAgroecosyst , 52 : 123 – 130 .
- Millar , N and Baggs , EM . 2005 . Relationships between N2O emissions and water-soluble C and N contents of agroforestry residues after their addition to soil . Soil BiolBiochem , 37 : 605 – 608 .
- Groffman , PM and Tiedje , JM . 1989 . Denitrification in north temperate forest soils: relationships between denitrification and environmental factors at the landscape scale . Soil BiolBiochem , 21 : 621 – 626 .
- Weier , KL , Doran , JW , Power , JF and Walters , DT . 1993 . Denitrification and the dinitrogen/nitrous oxide ratio as affected by soil water, available carbon, and nitrate . Soil SciSocAmJ , 57 : 66 – 72 .
- Cheng , W , Tsuruta , H , Chen , G , Akiyama , H and Yagi , K . 2004 . N2O and N2production potential in various Chinese agricultural soils by denitrification . Soil SciPlant Nutr , 50 : 909 – 915 .
- Scholefield , D , Hawkins , JMB and Jackson , SM . 1997 . Use of a flowing helium atmosphere incubation technique to measure the effects of denitrification controls applied to intact cores of a clay soil . Soil BiolBiochem , 29 : 1337 – 1344 .
- Yonebayashi , K . 1997 . “ Organic matter in soil ” . In Saishin Dojyogaku , Edited by: Kyuma , K . 43 – 52 . Tokyo : Asakura-syoten . (in Japanese)
- Intergovernmental Panel on Climate Change2001bGood Practice Guidance and Uncertainty Management in National Greenhouse Gas Inventories Available from NGGIP in IPCC website URL: http://www.ipcc-nggip.iges.or.jp/public/gp/english/ (http://www.ipcc-nggip.iges.or.jp/public/gp/english/)