Abstract
Several experiments were conducted using barley (Hordeum vulgare L. cv. Minorimugi) grown hydroponically in a medium containing cadmium (Cd) to clarify the role of phytosiderophores (PS) in chelator-assisted phytoextraction of Cd and the influence of iron (Fe) on Cd uptake. In the first experiment, plants were grown for 7 days in media containing 5 µmol L−1 Cd sulfate (CdSO4), in which the concentration of Fe was 0 or 10 µmol L−1. A marked increase in Cd uptake was obtained in plants grown under the Fe-deficient condition compared with those grown under the Fe-sufficient (10 µmol L−1) condition. In the second experiment, plants were grown for 7 days in media containing 5 µmol L−1 CdSO4, in which the concentration of Fe was 10 or 100 µmol L−1. As a result, the uptake of Cd in plants grown with excess Fe (100 µmol L−1) was significantly lower than the uptake in plants grown under a normal concentration (10 µmol L−1) of Fe. These results indicated that Cd uptake was affected by Fe concentration in the medium and, in particular, a lower concentration of Fe in the media allowed the influx of Cd into plants. Thus, Fe application to the rhizosphere might be effective in reducing Cd concentration in plants. In the third experiment, the ability of solubilizing PS for insoluble Cd mixed with gelatinous Fe was examined. The results showed that PS could solubilize Cd from sulfide of Cd (CdS) gel. In the fourth experiment, roots of Fe-deficient or Fe-sufficient plants were fed with 0 or 10 µmol L−1 mugineic acid (MA), one of the PS, for 4 h in media containing 5 µmol L−1 CdSO4. The uptake of Cd by the plants was not increased with MA. In the fifth experiment, the amount of PS release in Fe-deficient barley suffering from Cd toxicity was measured. Release of PS was partially reduced by 0.05 µmol L−1 CdSO4 and largely reduced by 0.5 or 5 µmol L−1 CdSO4. Based on these results, it was suggested that, when PS were released by roots of grasses to the rhizosphere, PS could mobilize insoluble Cd around the roots. It was also suggested that PS could not convey Cd into the root cells in the form of a PS–Cd complex. Thus, the main role of PS for chelator-assisted phytoextraction of Cd might be the collection of Cd to the rhizosphere, resulting in the enhancement of Cd concentration in the rhizosphere.
INTRODUCTION
Cadmium pollution in soil leads to human disease through the food chain (CitationChaney 1983b; CitationJackson and Alloway 1992). The Itai-itai disease with bone softening and kidney failure is the most well-known human disease ascribed to Cd pollution (CitationFriberg et al. 1985). To secure food safety for human, soils polluted with Cd have to be cleansed.
Phytoremediation, that is, using the capacity of plants to uptake heavy metals, is one way of removing Cd from soil (CitationChaney 1983a). In phytoremediation, the application of chelators, such as ethylene diamine tetraacetic acid (EDTA), aimed at solubilizing the insoluble heavy metals in soil was suggested by CitationCunningham and Ow (1996) and CitationChaney et al. (1997) to enhance the uptake rate of heavy metals by plants. This method was referred to as chelator-assisted phytoextraction by CitationSalt et al. (1998). For this method, plant-born chelators, such as phytosiderophores (PS), may be appropriate. In contrast to synthetic chelators, which can solubilize a variety of nutrients in soils but can pollute the groundwater with heavy metals, PS function only in the rhizosphere and their efflux may not be harmful to the environment.
Phytosiderophores are the chelators for ferric Fe and are released from the roots of grasses suffering from Fe deficiency (CitationTakagi 1972; CitationTakagi 1976; CitationTakagi et al. 1984). It is known that PS can form complexes with some metals (CitationMurakami et al. 1989; CitationSugiura and Tanaka 1981) and the addition of PS to the medium enhances the uptake rate of Fe by plants (CitationAlam et al. 2005; CitationRömheld and Marschner 1986).
Regarding the activity on PS for Cd solubilization and the enhancement of Cd uptake by plants in soil, experiments by CitationAwad and Römheld (2000) and CitationRömheld and Awad (2000) showed that Cd uptake was enhanced by released PS in Fe-deficient wheat (Triticum aestivum L. cv. Ares or cv. Piko) transplanted to calcareous soil containing supplemental Cd. Their results meant that PS might be useful for chelator-assisted phytoextraction. In hydroponic experiments, however, CitationShenker et al. (2001) and CitationHill et al. (2002) denied the activity of PS in Cd uptake. CitationShenker et al. (2001) indicated that PS did not enhance the uptake of Cd by wheat (Triticum aestivum L. cv. AgCs) and barley (Hordeum vulgare L. cv. CM 72). CitationHill et al. (2002) indicated that Cd uptake in maize was decreased by the addition of root washings of Fe-deficient maize to the medium. The causation of the differences in the role of PS for Cd uptake between the soil experiment and the hydroponic experiment was unclear. We needed to conduct experiments using another approach to clarify the efficiency of PS for chelator-assisted phytoextraction.
The physiological effect of Fe on Cd uptake should be considered separately from the effect of PS in our experiments. As it was likely that Cd uptake was affected by the Fe nutritional status in some plants. For example, in dicots, CitationLombi et al. (2002) showed that Cd uptake was significantly enhanced by Fe deficiency in the Ganges ecotype of Thlaspi caerulescens. In grasses, CitationNakanishi et al. (2006) suggested a possibility that Fe deficiency enhanced Cd uptake in rice, although CitationShenker et al. (2001) suggested that the uptake rate of Cd was not affected by the Fe nutritional status in wheat and barley. The physiological effect of Fe on Cd uptake is also unclear, even in grasses, and we need to clarify whether Cd uptake is affected by Fe or not.
There is controversy about the role of PS and the influence of Fe in Cd uptake in plants. Therefore, the objective of this experiment was to solve these questions about the role of PS for chelator-assisted phytoextraction of Cd and the physiological effect of Fe on Cd uptake. In this experiment, barley was used as a model plant of grasses because of its highest capacity for PS release (CitationKawai et al. 1987; CitationMarschner et al. 1989).
MATERIALS AND METHODS
Preparation of barley seedlings
Barley (Hordeum vulgare L. cv. Minorimugi) seeds were sterilized with 2% chlorinated lime solution for 60 min and rinsed with tap water for 60 min. After germination between wet towels at 20°C for 24 h, the seeds were placed on sterilized plastic net floated on a 1 mmol L−1 CaCl2 solution in a plastic bucket covered with aluminum foil, which was removed 2 days later, and kept in a phytotron at a 17°C day (14 h) and 10°C night (10 h). Six days later, the medium was replaced with 1/5-strength modified Hoagland and Arnon No.2 medium (pH 5.5). The full-strength modified Hoagland and Arnon No.2 medium consisted of 6 mmol L−1 KNO3, 4 mmol L−1 Ca(NO3)2, 1 mmol L−1 NH4H2PO4, 2 mmol L−1 MgSO4, 20 µmol L−1 Fe-EDTA, 3 µmol L−1 H3BO3, 0.5 µmol L−1 MnSO4, 0.2 µmol L−1 CuSO4, 0.4 µmol L−1 ZnSO4 and 50 nmol L−1 H2MoO4 (CitationKawai et al. 1993). Barley seedlings were transplanted to a continuously aerated 1/2-strength medium (pH 5.5) in 10-L buckets when the length of the second leaf of the seedlings reached 20% of that of the first leaf, and grown for 2 days. Then, the prepared barley seedlings were used in the following experiments. In all experiments the pH of the media was adjusted to 5.5 with 0.5 mol L−1 HCl or NaOH.
Treatment of plants grown under the different status of Fe with Cd
Seedlings of barley were transplanted to 1/2-strength media containing 5 µmol L−1 CdSO4, in which the Fe concentration was 0 or 10 µmol L−1 and harvested after 7 days. In 1/2-strength medium without Fe, 0.5 mmol L−1 NH4H2PO4 was replaced by 0.5 mmol L−1 NaH2PO4 (CitationTakagi 1993). In a separate experiment, seedlings of barley were transplanted to 1/2-strength media containing 5 µmol L−1 CdSO4, in which the Fe concentration was 10 (normal condition) or 100 (excess condition) µmol L−1 and harvested after 7 days. Cadmium at 5 µmol L−1 was used in these experiments when Cd was added to the media because our preliminary experiment indicated that this Cd concentration induced Cd-toxicity symptoms and distinct Cd uptake by the plants.
Solubilization of the Cd gel with mugineic acid
For the preparation of Cd gel (CdS) suspension, 5 mmol L−1 CdSO4 (Kanto Chemical Co., Tokyo, Japan) and 10 mmol L−1 Na2S (Kanto Chemical Co.) were mixed. Similarly, 5 mmol L−1 Fe2(SO4)3 (Kanto Chemical Co.) and 10 mmol L−1 Na2S were also mixed for the preparation of the Fe gel. Two weeks later, the following solutions were made where 2 mL of Cd gel and 2 mL of Fe gel suspension were mixed with or without mugineic acid (MA), one of the PS. The final volume of the solutions was made to 10 mL by diluting with pure water, where the concentration of MA was 30 µmol L−1. It has been shown that this concentration of MA could efficiently solubilize Fe and enhance the Fe concentration in the xylem of barley when fed to the roots in water culture (CitationKawai et al. 2001). The solutions were incubated in an oven at 55°C for 120 min and shaken every 40 min. The solutions were filtered through a filter paper, Advantec No. 5C (Toyo Roshi Kaisha, Tokyo, Japan). Mugineic acid used in this experiment was collected from root washings of barley and purified according to the method of CitationTakagi et al. (1984). The compound was desalted using cation exchange resin (Dowex 50W, Dow Chemical Co., Midland, MI, USA) and crystallized from water–methanol–ethanol solution. The purity of authentic MA was determined using nuclear magnetic resonance (NMR).
Short-term feeding experiment with Cd and mugineic acid
Seedlings of barley were grown for 5 days in 1/2-strength Fe-deficient medium without Cd. One hour after the onset of daytime 5 days after transplanting, the seedlings were transferred to 1/2-strength Fe-deficient medium containing 5 µmol L−1 CdSO4 without (0 µmol L−1) or with (10 µmol L−1) MA, and harvested after 4 h. Similarly, seedlings of barley were grown for 5 days in 1/2-strength Fe-sufficient medium without Cd, transferred to 1/2-strength Fe-sufficient medium containing 5 µmol L−1 CdSO4 without (0 µmol L−1) or with (10 µmol L−1) MA in the morning and harvested after 4 h. These two experiments were conducted separately.
Measurement of elements
All of the filtrated solutions of the gel solutions were analyzed for Cd and Fe using atomic absorption spectroscopy (Atomic Absorption Spectrophotometer 170–30, Hitachi, Tokyo, Japan).
In the case of the plant samples, harvested plants were dried at 70°C for over 24 h after rinsing the roots with deionized water. The plants were separated into shoots and roots, weighed, digested with nitric–perchloric acid mixture (5:1) (CitationPiper and Piper 1950), and analyzed for Cd and Fe using atomic absorption spectroscopy.
Measurement of phytosiderophores released from barley treated with different Cd concentrations
Seedlings of barley were transplanted to 1/2-strength media without Fe, which contained 0, 0.05, 0.5 or 5 µmol L−1 CdSO4. After growth for 1 week, the roots of plants were soaked in beakers filled with 500 mL of deionized water (four plants per beaker) for 3 h after the onset of daytime for the collection of PS.
After the removal of the roots of plants from the beaker with root washings, approximately 10 mg of thymol (Kanto Chemical Co.) was added to the root washings to defend PS from microbial degradation. Solutions of the root washings were introduced individually into the columns of cation exchange resin (Amberlite IR-120B, Organo Corp., Tokyo, Japan) and the resin was washed with a sufficient amount of deionized water. Subsequently, PS adsorbed to the resin was eluted with 140 mL of 1 mol L−1 NH4OH and concentrated under vacuum. The amounts of PS were measured using the Fe solubilizing assay of CitationTakagi (1976).
Statistical analysis
All experiments except the measurement of PS release were conducted in quadruplicate and were repeated three times. The measurement of PS release was conducted in triplicate and was repeated twice. All data were subjected to an anova (CitationSAS Institute 1988) using the computer “sas” in Iwate University, Japan. Differences between means were evaluated using the Ryan–Einot–Gabriel–Welsch multiple range test (P < 0.05).
RESULTS AND DISCUSSION
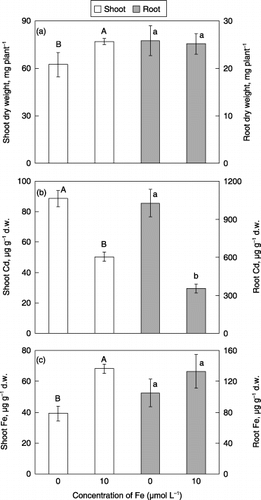
In the first experiment, the effect of lower Fe concentration of the medium on Cd uptake by barley was investigated. When barley was exposed to Cd, necrotic spots on the leaves were observed visually both in plants grown in Fe-deficient and Fe-sufficient media. In particular, more spots and chlorotic symptoms were observed visually in Fe-deficient plants. When treated with Cd, Fe-deficient plants had significantly lower shoot dry weight than Fe-sufficient plants, but the root dry weight of Fe-deficient plants was not significantly different from that of Fe-suffcient plants (). Cadmium concentrations were significantly higher both in the shoots and the roots of Fe-deficient plants compared with those of Fe-sufficient plants (). Iron concentrations were significantly lower in the shoots of Fe-deficient plants ().
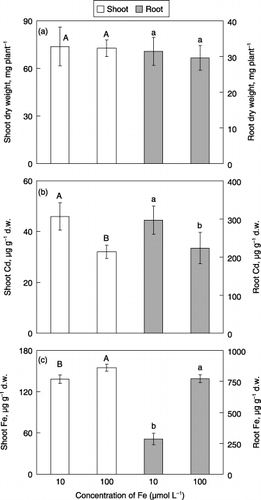
In the second experiment, the effect of excess Fe (100 µmol L−1) in the medium on Cd uptake by barley was investigated. Necrotic spots on the leaves were observed visually in both plants and these plants were visually almost similar. Dry weights both in shoots and roots were similar in both plants (). Cadmium concentrations were significantly lower and Fe concentrations were significantly higher both in the shoots and the roots of plants grown with excess Fe ().
The results of these experiments revealed that the uptake of Cd in barley grown hydroponically was affected by Fe concentration in the medium and Cd concentrations in shoots and roots were markedly
Figure 1 Effect of Fe deficiency on (a) dry weights, (b) Cd concentrations and (c) Fe concentrations in the shoots and roots of barley plants grown in media with 5 µmol L−1 CdSO4. Each value is the mean ± standard error (n = 4) and different letters at the top of each bar indicate significant differences (P < 0.05) according to the Ryan–Einot–Gabriel–Welsch multiple range test. d.w., dry weight.
A question arose from these results, that is, whether or not PS were related to the increase in the uptake rate of Cd in Fe-deficient plants. There was a possibility that Cd uptake under Fe-deficient conditions might be enhanced by PS carrying Cd from the rhizosphere into roots, because PS are released from the roots to the rhizosphere in grasses suffering from Fe deficiency (CitationTakagi 1976).
Figure 2 Effect of excess Fe on (a) dry weights, (b) Cd concentrations and (c) Fe concentrations in the shoots and roots of barley plants grown in media with 5 µmol L−1 CdSO4. Each value is the mean ± standard error (n = 4) and different letters at the top of each bar indicate significant differences (P < 0.05) according to the Ryan–Einot–Gabriel–Welsch multiple range test. d.w., dry weight.
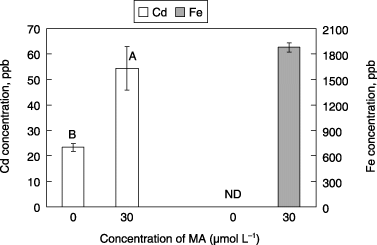
In the third experiment, the ability of MA solubilizing Cd was examined. In our experiment, CdS was used as the insoluble form of Cd because it is well known that Cd2+ is precipitated forming CdS with S2– reduced from SO2− 4 in anaerobic conditions in soil (CitationPostgate 1979), and it decreases the solubility of Cd, resulting in a decrease in the uptake of Cd by plants (Citationvan den Berg et al. 1998). When MA was added to the mixture of the gel of Cd and Fe, the concentrations of both Cd and Fe in
Figure 3 Solubilized amount shown as the concentration of Cd and Fe in the filtrated solutions by mugineic acid (MA) from mixtures of Cd and Fe gel. Cadmium gel and Fe gel were formed by precipitation in the presence of Na2S. Each value is the mean ± standard error (n = 4) and different letters at the top of each bar indicate significant differences (P < 0.05) according to the Ryan–Einot–Gabriel–Welsch multiple range test. ND, not detected.
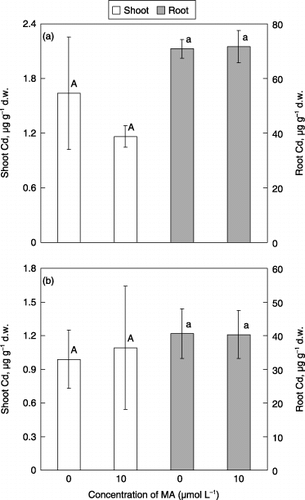
The fourth experiment examined whether or not MA could enhance the uptake of Cd in barley. Cadmium and MA were fed to both Fe-deficient and Fe-sufficient barley grown without Cd, because expression of PS-metal transporter might possibly be different between these plants. In addition, the plants were fed with Cd and MA for 4 h in hydroponic conditions because the feeding experiment with PS was generally conducted using short-term experiments to avoid microbial degradation of PS (CitationAlam et al. 2004). As a result, Cd was detected in the shoots and the roots by 4 h feeding with Cd, and there was no significant difference in the Cd concentration of the shoots and the roots of barley between the plants supplied with or without MA in both Fe-deficient and Fe-sufficient plants (). Cadmium uptake of the plants fed with Cd for 4 h in the condition of Fe deficiency () seemed to be higher than that of Fe sufficiency (), regardless of the supply of MA; however, these experiments were conducted separately and the uptake amount of Cd between these plants was difficult to compare. These results indicated that MA did not enhance the uptake rate of Cd, regardless of possible expression of PS transporter and did not convey Cd into the root cells in the form of a PS–Cd complex. It should be noted that this
Figure 4 Effect of feeding mugineic acid (MA) on Cd concentrations of the shoots and roots. (a) Plants grown in the medium without Fe were transferred to Fe-deficient medium containing 5 µmol L−1 CdSO4 without (0 µmol L−1) or with (10 µmol L−1) MA and kept for 4 h, and (b) plants grown in the medium with Fe were transferred to Fe-sufficient medium containing 5 µmol L−1 CdSO4 without (0 µmol L−1) or with (10 µmol L−1) MA and kept for 4 h. Each value is the mean ± standard error (n = 4) and different letters at the top of each bar indicate significant differences (P < 0.05) according to the Ryan–Einot–Gabriel–Welsch multiple range test.
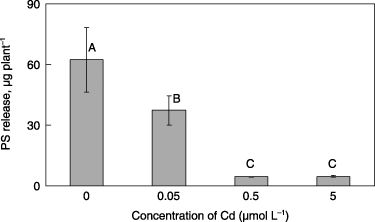
Furthermore, the fifth experiment showed that PS was released from the roots of Fe-deficient plants grown without Cd, but the amount of released PS decreased according to the increase in Cd concentration in the media and was drastically repressed at 0.5 and 5 µmol L−1 Cd ().
These results clearly exhibited that the enhancement of Cd uptake in Fe-deficient barley in our first experiment was not related to the effect of PS. In our experiment,
Figure 5 Amount of phytosiderophores (PS) released from the roots of barley plants grown in Fe-deficient media with 0, 0.05, 0.5 or 5 µmol L−1 CdSO4. Each value is the mean ± standard error (n = 3) and different letters at the top of each bar indicate significant differences (P < 0.05) according to the Ryan–Einot–Gabriel–Welsch multiple range test.
We suggest that Fe application should be tried to decrease the Cd concentration in grains as the edible part of plants grown in fields or in wetlands where removal of Cd by phytoremediation is difficult. It is possible that Fe application enhances the amount of Fe in the soil solution, resulting in the repression of the Cd uptake by plants. Therefore, the application of several types of Fe fertilizer may be considered for alleviation of Cd toxicity in practice.
CitationShenker et al. (2001) reported that the uptake of Cd by barley and wheat showing Fe-deficient symptoms were similar to those without Fe-deficient symptoms, which differed from our results in which Cd uptake was increased under Fe-deficient conditions. Cadmium uptake in CitationShenker et al.'s (2001) experiment might be affected by high concentrations of chelators, because their medium was “theoretical” Fe-deficient medium containing high concentrations of chelators and 10 µmol L−1 Fe. Our Fe-deficient medium had little amount of chelators and Fe. It was inferred that these differences might cause the difference between the results of our experiment and CitationShenker et al. (2001). It is known that PS can deprive Fe from synthetic chelator (CitationAlam et al. 2005). In our opinion, apoplastic Fe on the root surface may repress the uptake of Cd in “theoretical” Fe-deficient medium because it is known that apoplastic Fe can be formed when Fe chelated by artificial chelator was fed to the root (CitationAlam et al. 2005). The formation of apoplastic Fe in hydroponic culture may induce the difference between our results and CitationShenker et al. (2001). Recently, the same research group published a different result. CitationAdhikari et al. (2006) reported that Cd uptake was increased in rice grown in “theoretical” Fe-deficient medium, and the released amount of PS per gram root dry weight was increased in plants grown under higher concentrations of Cd. CitationAdhikari et al. (2006) also wrote that PS might have some effects in increasing the uptake of Cd by plants, although evidence for indicating the effect of PS on Cd uptake was not shown. However, it appears that the released amount of PS per plant did not increase in their results. Rice may have higher activity to maintain PS release when suffering Cd toxicity. In addition, the released amount of PS in rice is much lower than that of barley (CitationKawai et al. 1994; CitationTakagi 1976). In the case of rice, it is possible that the enhancement of Cd uptake resulted from the expression of Fe2+ transporter (CitationEide et al. 1996; CitationNakanishi et al. 2006; CitationRogers et al. 2000).
The function of PS in soil needs to be considered. CitationAwad and Römheld (2000) and CitationRömheld and Awad (2000) found a higher Cd uptake by Fe-deficient wheat compared with Fe-sufficient wheat, which were transplanted to soil. They suggested that the enhancement of the Cd uptake by the plants resulted from the function of PS in solubilizing Cd in the rhizosphere; however, our results showed that PS did not convey Cd into the root cells in the form of a PS–Cd complex. Our understanding about the role of PS for Cd uptake from soil is as follows: (1) PS can extract and convey Cd in soil along with water flow to the root surface, (2) PS in the PS–Cd complex collected in the vicinity of root must be decomposed within 1 or 2 days by microorganisms (CitationTakagi et al. 1988), (3) Cd2+ remaining in soil around the root surface may be absorbed by the roots without diffusion, which differs from the water culture, resulting in higher Cd concentrations in plant tissues. Based on this, we suggest that PS may collect Cd to the rhizosphere resulting in the enhancement of Cd concentration in the rhizosphere.
A problem with our results was that PS release of barley was largely repressed under high Cd concentrations (0.5 and 5 µmol L−1) (); however, PS release may not be repressed in grasses grown after transplanting into Cd-contaminated soil because the Cd concentration of “soil solution” may not always be so high in nature. For example, CitationKashem and Singh (2002) reported that the Cd concentration of soil solution of Cd-contaminated soil, whose Cd content in soil was 2.5 mg kg−1, with oat cultivation was between 0.002–0.007 µmol L−1. Our results showed that PS release was observed even in the range between 0 and 0.05 µmol L−1 Cd in the media (). In addition, it is known that PS release from grasses occurs even in Fe-sufficient conditions (CitationTakagi et al. 1984; CitationYoshida et al. 2004). It is considered that PS may be continuously released, although the released amount of PS may decrease when the stress of Fe deficiency is retrieved in plants. It may be possible that the harvest of transplanted Fe-deficient grasses in the short-term (e.g. 1–2 weeks) may have the high performance of the extraction of Cd, using the function of PS for solubilizing insoluble Cd in soil.
Therefore, it may be worthwhile to trial the transplantation of precultured Fe-deficient grasses with a higher ability for PS production for phytoremediation of soil with light Cd pollution. Further investigation using Cd contaminated soil is required.
In conclusion, first, a physiological role of Fe in Cd uptake was suggested for the repression of the Cd influx into plants. To clarify the mechanisms of Fe in the repression of Cd uptake, we need to investigate the competitive relationship between Fe and Cd uptake on isolated plasma membranes. Second, PS could not convey Cd directly into the root cells in the form of a PS–Cd complex. It was considered that the main role of PS for chelator-assisted phytoextraction of Cd might be the collection of Cd to the rhizosphere resulting in the enhancement of Cd concentration in the rhizosphere.
ACKNOWLEDGMENTS
The authors thank Dr A. Nagasawa of Saitama University for analyzing mugineic acid using nuclear magnetic resonance. The authors thank the Grant-in-Aid for Science Research (18580058) of the Ministry of Education, Science, Culture and Sports, Government of Japan, to support this research. The authors thank the Sasakawa Scientific Research Grant from the Japan Science Society to support this research. The authors thank Dr M. A. Kashem for his valuable suggestions.
REFERENCES
- Chaney , RL . 1983b . “ Potential effects of waste constituents on the food chain ” . In Land Treatment of Hazardous Wastes , Edited by: Parr , JE , Marsh , PB and Kla , JM . 152 – 240 . Park Ridge : Noyes Data Corp. .
- Jackson , AP and Alloway , BJ . 1992 . “ The transfer of Cd from agricultural soils to the human food chain ” . In Biochemistry of Trace Metals , Edited by: Adriano , DC . 109 – 152 . New York : Lewis Publishers .
- Friberg , L , Elinder , CG , Kjellström , T and Nordberg , GF . 1985 . Cadmium and HealthA Toxicological and Epidemiological Appraisal , Volume I and II , Boca Raton : CRC Press .
- Chaney , RL . 1983a . “ Plant uptake of inorganic waste ” . In Land Treatment of Hazardous Wastes , Edited by: Parr , JE , Marsh , PB and Kla , JM . 50 – 67 . Park Ridge : Noyes Data Corp. .
- Cunningham , SD and Ow , DW . 1996 . Promises and prospects of phytoremediation . Plant Physiol , 110 : 715 – 719 .
- Chaney , RL , Malik , M Li , YM . 1997 . Phytoremediation of soil metals . CurrOpinBiotechnol , 8 : 279 – 284 .
- Salt , DE , Smith , RD and Raskin , I . 1998 . Phytoremediation . AnnuRevPlant PhysiolPlant MolBiol , 49 : 643 – 668 .
- Takagi , S . 1972 . “ The absorption mechanism of heavy-metals by the plants, especially on the secretion of iron-solubilizing substances and iron absorption from the plant roots ” . In Studies on Soil Science and Plant Nutrition in Modern Agriculture , Edited by: Japanese Society of Soil Science and Plant Nutrition . 66 – 72 . Tokyo : Yokendo Press . (in Japanese)
- Takagi , S . 1976 . Naturally occurring iron-chelating compounds in oat- and rice-root washings. I. Activity measurement and preliminary characterization . Soil SciPlant Nutr , 22 : 423 – 433 .
- Takagi , S , Nomoto , K and Takemoto , T . 1984 . Physiological aspect of mugineic acid, a possible phytosiderophore of graminaceous plants . JPlant Nutr , 7 : 469 – 477 .
- Murakami , T , Ise , K , Hayakawa , M , Kamei , S and Takagi , S . 1989 . Stabilities of metal complexes of mugineic acids and their specific affinities for iron(III) . ChemLett , 1989 : 2137 – 2140 .
- Sugiura , Y and Tanaka , H . 1981 . Structure, properties, and transport mechanism of iron(III) complex of mugineic acid, a possible phytosiderophore . J. Am. Chem. Soc. , 103 : 6979 – 6982 .
- Alam , S , Kamei , S and Kawai , S . 2005 . Effectiveness of phytosiderophore in absorption and translocation of 59Iron in the presence of plant-borne, synthetic, and microbial chelators . JPlant Nutr , 28 : 1709 – 1722 .
- Römheld , V and Marschner , H . 1986 . Evidence for a specific uptake system for iron phytosiderophores in roots of grasses . Plant Physiol , 80 : 175 – 180 .
- Awad , F and Römheld , V . 2000 . Mobilization of heavy metals from contaminated calcareous soils by plant born, microbial and synthetic chelates and their uptake by wheat plants . JPlant Nutr , 23 : 1847 – 1855 .
- Römheld , V and Awad , F . 2000 . Significance of root exudates in acquisition of heavy-metals from a contaminated calcareous soil by graminaceous species . JPlant Nutr , 23 : 1857 – 1866 .
- Shenker , M , Fan , TW-M and Crowley , DE . 2001 . Phytosiderophores influence on cadmium mobilization and uptake by wheat and barley plants . JEnvironQual , 30 : 2091 – 2098 .
- Hill , KA , Lion , LW and Ahner , BA . 2002 . Reduced Cd accumulation in Zea mays: A protective role for phytosiderophores? . EnvironSciTechnol , 36 : 5363 – 5368 .
- Lombi , E , Tearall , KL , Howarth , JR , Zhao , F-J , Hawkesford , MJ and McGrath , SP . 2002 . Influence of iron status on cadmium and zinc uptake by different ecotypes of the hyperaccumulator Thlaspi caerulescens . Plant Physiol , 128 : 1359 – 1367 .
- Nakanishi , H , Ogawa , I , Ishimaru , Y , Mori , S and Nishizawa , NK . 2006 . Iron deficiency enhances cadmium uptake and translocation mediated by the Fe2+transporters OsIRT1 and OsIRT2 in rice . Soil SciPlant Nutr , 52 : 464 – 469 .
- Kawai , S , Sato , Y , Takagi , S and Nomoto , K . 1987 . Separation and determination of mugineic acid and its analogues by high-performance liquid chromatography . JChromatogr , 391 : 325 – 327 .
- Marschner , H , Treeby , M and Römheld , V . 1989 . Role of root-induced changes in the rhizosphere for iron acquisition in higher plants . ZPflanzenern Bodenk , 152 : 197 – 204 .
- Kawai , S , Itoh , K and Takagi , S . 1993 . Incorporation of 15N and 14C of methionine into mugineic acid family of phytosiderophores in iron-deficient barley roots . PhysiolPlant , 88 : 668 – 674 .
- Takagi , S . 1993 . “ Production of phytosiderophores ” . In Iron Chelation in Plants and Soil Microorganisms , Edited by: Barton , LL and Hemming , BC . 111 – 131 . New York : Academic Press .
- Kawai , S , Kamei , S Matsuda , Y . 2001 . Concentrations of iron and phytosiderophores in xylem sap of iron-deficient barley plants . Soil SciPlant Nutr , 47 : 265 – 272 .
- Piper , CS and Piper , CS . 1950 . Soil and Plant Analysis , Adelaide : Hassell Press .
- SAS Institute . 1988 . SAS/STAT User's Guide No1 ANOVA , Cary : Statistical Analysis System Institute . Version 6
- Postgate , JR . 1979 . The Sulphate-Reducing Bacteria , London : Cambridge University Press .
- van den Berg , GA , Loch , JPG and Winkels , HJ . 1998 . Effect of fluctuating hydrological conditions on the mobility of heavy metals in soils of a freshwater estuary in the Netherlands . Water Air Soil Pollut , 102 : 377 – 388 .
- Alam , S , Akiha , F , Kamei , S and Kawai , S . 2004 . Diurnal variations in absorption and translocation of a ferrated phytosiderophore in barley as affected by iron deficiency . Soil SciPlant Nutr , 50 : 457 – 461 .
- Eide , D , Broderius , M , Fett , J and Guerinot , ML . 1996 . A novel iron-regulated metal transporter from plants identified by functional expression in yeast . ProcNatl AcadSciUSA , 93 : 5624 – 5628 .
- Rogers , EE , Eide , DJ and Guerinot , ML . 2000 . Altered selectivity in an Arabidopsismetal transporter . ProcNatl AcadSciUSA , 97 : 12356 – 12360 .
- Zaharieva , T and Römheld , V . 2000 . Specific Fe2+uptake system in strategy I plants inducible under Fe deficiency . JPlant Nutr , 23 : 1733 – 1744 .
- Curie , C , Panaviene , Z , Loulergue , C , Dellaporta , LS , Briat , J-F and Walker , LE . 2001 . Maize yellow stripe1encodes a membrane protein directly involved in Fe(III) uptake . Nature , 409 : 346 – 349 .
- Adhikari , T , Tel-Or , E , Libal , Y and Shenker , M . 2006 . Effect of cadmium and iron on rice (Oryza sativaL.) plant in chelator-buffered nutrient solution . JPlant Nutr , 29 : 1919 – 1940 .
- Kawai , S , Sasaki , O , Hayasaka , Y and Takagi , S . 1994 . Biosynthesis of avenic acid A in oat cv. Onward: studies with 14C or 15N labeled compounds . Plant Soil , 165 : 285 – 289 .
- Takagi , S , Kamei , S and Yu , M-H . 1988 . Efficiency of iron extraction from soil by mugineic acid family phytosiderophores . JPlant Nutr , 11 : 643 – 651 .
- Kashem , MA and Singh , BR . 2002 . The effect of fertilizer additions on the solubility and plant-availability of Cd, Ni, and Zn in soil . NutrCyclAgroecosys , 62 : 287 – 296 .
- Yoshida , T , Kawai , S and Takagi , S . 2004 . Detection of the regions of phytosiderophore release from barley roots . Soil SciPlant Nutr , 50 : 1111 – 1114 .