Abstract
Chemical properties of hydrophobic acid (HoA) fractions in water-soluble organic matter in soil and water are concerned with its interactions with mineral soil surfaces and organic pollutants. In 2004 we examined the seasonal and vertical changes in chemical properties of the HoA fraction in a Cambisol profile and compared these properties with those in the HoA fraction of an adjacent stream (aquatic humic substances) in a temperate forested watershed using high performance size exclusion chromatography (HPSEC) and 1H and 13C nuclear magnetic resonance (NMR) spectroscopy. The HoA fractions from Oi, Oe/Oa, A and B horizon soils in summer had lower O-alkyl C proportions than those recorded in samples in other seasons. The proportions of aromatic C in HoA fractions from A and B horizons were highest in summer. These seasonal variations were less significant than variations with soil depth. O-alkyl C proportions in HoA fractions decreased with increasing soil depth from the Oi to the A horizon. The HoA fractions from the B horizon showed a higher alkyl C proportion than samples from other horizons in winter and spring. These changes with soil depth from the Oi to A horizons might result from selective utilization of carbohydrate carbon by microorganisms, whereas those in the B horizon may result from sorption to mineral surfaces. The HoA fractions in the stream were similar in relative molecular weight, distribution of each type of proton and carbon species in HoA fractions from the B horizon, whereas stream HoA fractions collected in summer would be derived from organic horizons. This indicated that vertical changes in the chemical properties of HoA fractions in soil and pathways of water to the stream would largely affect the chemical properties of HoA fractions in the stream.
INTRODUCTION
The hydrophobic acid (HoA) fraction of dissolved organic matter (DOM) in soils, which is defined as the XAD-8 resin adsorbable fraction (CitationLeenheer 1981), corresponds to humic substances and precursors of humic substances in soils and rivers (CitationAnderson et al. 1990; CitationGuggenberger et al. 1994; CitationQualls et al. 1991). It plays an important role in the transport of nutrients and pollutants in soils (CitationFranco et al. 2001; CitationQualls et al. 1991) because of its heterogeneous structure and variety of interactions with other compounds and its soil solid phase. Many studies have suggested that HoA fractions of DOM in soil and water bind organic contaminants and affect the uptake of contaminants by microorganisms and fishes (CitationHaitzer et al. 1999; CitationJohnsen et al. 1989; CitationKukkonen et al. 1990; CitationMcCarthy et al. 1989). These interactions of HoA fractions with contaminants can be substantially influenced by the chemical properties of the HoA fraction (CitationMcCarthy et al. 1989; CitationRaber and Kögel-Knabner 1997).
In addition, sorption of the HoA fractions to mineral surfaces is a function of its chemical properties. CitationKaiser et al. (1997) showed that mineral soils preferentially adsorbed the HoA fraction that had a high proportion of carboxyl groups and aromatic moieties. Therefore, the chemical properties of the HoA fraction may change during its passage through soils, and the pathway of the HoA fraction may influence the chemical properties of the HoA fractions in rivers (aquatic humic substances) and aquatic ecological systems.
Microbial degradation and sorption to mineral surfaces may be responsible for changes in the chemical properties of the HoA fraction. Microbial activity appears to control the production of DOM (CitationChrist and David 1996; CitationMcDowell and Likens 1988) and to change in chemical composition of litter (CitationWilson et al. 1983). Moreover, the biodegradability of organic substances in soil should be related to the molecular characteristics of the soil, that is, microorganisms preferentially utilize carbohydrate components (CitationAlmendros and Dorado 1999; CitationBaldock et al. 1992; CitationKalbitz et al. 2003; CitationYanagi et al. 2003). This selective degradation may lead to seasonal variations in the chemical properties of the HoA fraction.
A number of investigators have observed seasonal and vertical variations in the chemical properties of bulk DOM and have suggested that these variations are derived from changes in the quantitative ratios of DOM fractions (CitationCandler et al. 1988; CitationKaiser et al. 2001), which are defined by CitationLeenheer's (1981) method and show significant differences in chemical properties (CitationGuggenberger et al. 1994). CitationKaiser et al. (2001) assumed that the chemical properties of the HoA fraction changed seasonally. However, these researchers did not clarify the changes in the chemical properties of the HoA fraction.
In our previous work (CitationAsakawa et al. 2006a) we found that the elemental composition, relative molecular weight and 1H nuclear magnetic resonance (NMR) spectrum of the HoA fraction varied with season and soil depth, suggesting an effect of seasonal and spatial changes in microbial activity. In the present study, HoA fractions were extracted from a Dystric Cambisol over four seasons by shaking with water, and the HoA fractions were then analyzed using liquid-state 13C NMR spectroscopy. Moreover, we analyzed the HoA fractions in DOM in a stream using 1H and 13C NMR spectroscopy and high performance size exclusion chromatography (HPSEC), and we compared these results with data from soil HoA fractions to assess the contribution of the soil HoA fraction to the stream.
MATERIALS AND METHODS
Site description and sampling
The study site was a small watershed at Kitadani National Forest, Yamashiro, Kyoto prefecture, Japan (34°47′N, 135°50′E, 188 m elevation). This site is covered by a temperate broad-leaved secondary forest dominated by Japanese oak (Quercus serrata Thunb.) and Aquifoliaceae (Ilex pedunculosa Miq.). The ground vegetation is dominated by Sasa sp. (Pleioblastus chino Makino). The mean annual temperature and annual precipitation in 2004 were 15.5°C and 1449 mm, respectively. Variations in monthly precipitation and mean monthly temperature are shown in . The location of the soil pit was carefully selected to be representative of the watershed, and horizontal and vertical distances from the soil pit to an adjacent stream were 50 m and 20 m, respectively. The soil was Dystric Cambisol (World Reference Base (WRB) classification; CitationFood and Agriculture Organization 1998) and derived from granite material. Chemical and physical properties of the soil horizons are shown in .
Soil (Oi, Oe/Oa, A and B horizons) and water samples were taken in winter (late February), spring (late May), summer (early August) and autumn (late October) 2004. Soil sampling was carried out two or more days after rainfall from the same soil pit. Soil samples were placed in a cooler with ice for transport back to the laboratory and stored in field-moist conditions at 1°C. Water samples were collected from the stream that flows near the soil pit.
Isolation procedure of the hydrophobic acid fraction from the soil
A full description of the procedures of extraction and purification of the HoA fraction of water-extractable
Figure 1 Monthly precipitation and mean monthly air temperature at the nearest observatory of the Japan Meteorological Agency (Kyotanabe; 34°48.6′N, 135°46.3′E) from November 2003 to November 2004.
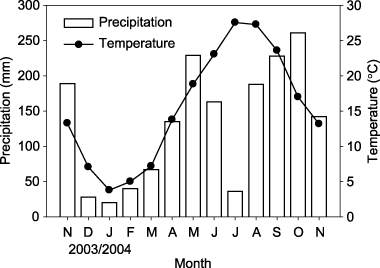
Table 1 Some chemical and physical properties of the studied soil horizons
Water-extractable organic matter (WEOM) was extracted from the Oi, Oe/Oa and A horizon soils within 3 days after sampling. The B horizon sample was extracted within 1 week after sampling. The Oi, Oe/Oa, A and B horizon samples were extracted with ultra-pure water at a soil-to-solution ratio of 1:80, 1:20, 1:5 and 1:5, respectively. The soil-to-solution ratios of organic horizon samples were selected to be as low as possible to be sufficient to soak and shake the samples. The suspensions from the organic horizon and mineral horizon samples were shaken at 1°C for 12 h and 24 h, respectively. The extract was filtrated through a 0.45-µm cellulose acetate membrane filter. These operational conditions were based on the report of CitationAsakawa et al. (2006b).
This WEOM solution was fractionated into operationally defined HoA and non-HoA fractions using the method of CitationThurman and Malcolm (1981) as modified by CitationAsakawa et al. (2006a). The extract was acidified to pH 2 with 6 mol L−1 HCl and passed through a column filled with Supelite DAX-8 resin (Supelco, Bellefonte, PA, USA). The HoA fraction was eluted from the column with 0.1 mol L−1 NaOH. Brown precipitates that formed on the resin bed were dissolved using 0.1 mol L−1 NaOH; thus, the HoA fraction includes the acid-precipitated fraction. The eluate was acidified to pH 1 with 6 mol L−1 HCl immediately and concentrated hydrofluoric acid (HF) was added to make up a final concentration of 0.3 mol L−1. After being shaken overnight at room temperature, this solution was again passed through a column of DAX-8 resin. The HoA fraction was desorbed using 0.1 mol L−1 NaOH, protonated with a strongly acidic cation exchange resin (AG MP-50; Bio-Rad Laboratories, Hercules, CA, USA) and freeze-dried.
Isolation procedure of the hydrophobic acid fraction from the stream
The isolation procedure of the HoA fraction in stream DOM was similar to that of the soil HoA fraction described above. Fresh stream water was pumped through a series of three in-line cartridge filters of decreasing pore size: 5, 0.8 and 0.45 µm (Advantec, Tokyo, Japan). The filtrate was acidified to pH 2 with 6 mol L−1 HCl, which was pumped using peristaltic pumps. The acidified sample was passed through a glass column of DAX-8 resin, and the effluent was neutralized with NaOH for disposal. Aliquots of the filtered water were collected in rinsed 1-L glass bottles for laboratory experiments. In summer, it rained during the collection of the stream HoA fraction, so we aborted the collection of that sample. One day after rainfall, the DAX-8 column was changed and the collection of the sample was resumed to compare the chemical properties of a stream HoA fraction collected before and after rain. The columns and filtered water were returned to the laboratory in a cooler with ice.
The adsorbed organic matter was back eluted from the columns using 0.1 mol L−1 NaOH and immediately acidified with 6 mol L−1 HCl. The humic substance was purified using HF treatment, reconcentrated on the DAX-8 resin, and passed through the AG MP-50 resin as described above. The proportion of the carbon in the HoA fraction of DOM was calculated using DAX-8 resin adsorption analysis in the laboratory.
Liquid state 1H and 13C NMR spectroscopy
The NMR spectra were obtained on a Bruker Avance 500 spectrometer (Bruker GmbH, Karlsruhe, Germany) using sample tubes 5 mm in diameter. Approximately 30–50 mg of the sample was dissolved in 0.4 mL of 0.5 mol L−1 NaOD in D2O. Chemical shifts were referenced to sodium 3-trimethylsilylpropionate-2,2,3,3 D4 (TSP; Euriso-top, Saint Aubin, France).
To obtain quantitative conditions for the integration of the 13C NMR spectra, 13C signals were proton-decoupled using the inverse-gated decoupling technique as follows: spectrometer frequency, 125.76 MHz; pulse width, 45°; acquisition time, 0.839 s. A total repetition time of 2.5 s was used to permit complete relaxation of all the spins. To improve the signal-to-noise ratio, a line broadening of 50 Hz was used. Scans numbering 10,000 to 20,000 were accumulated. Resonance areas were calculated using electronic integration. The assignments of the spectra were made according to the reports of CitationPreston and Blackwell (1985), CitationSchnitzer and Preston (1986), CitationWilson (1987), CitationThorn et al. (1989) and CitationRicca and Severini (1993). To obtain quantitative information, the spectra were divided into the following six regions (CitationFujitake and Kawahigashi 1999): alkyl C (5–50 p.p.m.), O-alkyl C (50–110 p.p.m.), aromatic C (110–145 p.p.m.), phenolic C (145–165 p.p.m.), carboxylic C (165–190 p.p.m.) and carbonyl C (190–220 p.p.m.). Aromaticity proposed by CitationHatcher et al. (1981) was calculated by expressing the aromatic and phenolic C (110–165 p.p.m.) as a percentage of the alkyl, O-alkyl, aromatic and phenolic C (5–165 p.p.m.).
The 1H NMR spectra of the stream HoA fractions were recorded under the following conditions: spectrometer frequency, 500.13 MHz; homo-gated decoupling; pulse width, 14.0 µs (90°); acquisition time 5.4 s; pulse delay 4.8 s; line-broadening factor, 5 Hz. The residual water (HOD) peak (4.8 p.p.m.) associated with water impurities was irradiated. Eight scans were accumulated for each sample. The 1H NMR data were integrated in five regions (CitationKawahigashi et al. 1996): 0.0–0.9 p.p.m. (Hγ, protons on terminal methyl groups attached to saturated aliphatic protons); 0.9–1.6 p.p.m. (Hβ, protons on methylene β attached to olefins or aromatic rings); 1.6–3.0 p.p.m. (Hα, protons on methyl and methylene α attached to aromatic carbons, carbonyl groups, ester groups and olefins); 3.0–4.3 p.p.m. (HC–O, protons on carbons attached to oxygen sugars, and on olefins and methoxyl groups); 6.0–9.0 p.p.m. (Har, protons attached to carbons of heteroaromatic and aromatic rings, and to carbonyl groups bonded to electronegative groups). Furthermore, a chemical shift region between 0 and 3.0 p.p.m. was assigned to aliphatic protons (Hal; Hγ + Hβ + Hα).
High performance size exclusion chromatography
Molecular weight distribution of the stream HoA fraction was measured using HPSEC. Details of the HPSEC system, the separation conditions and the sample preparation method have been previously reported (CitationAsakawa et al. 2006a). In brief, a Waters 600E system controller, a Waters 717 plus autosampler and a Waters 2487 dual wavelength absorbance detector (Waters Inc., Milford, MA, USA) were used. A Shodex OHpak SB-803 HQ column (Showa Denko, Tokyo, Japan; 8.0 mm internal diameter × 300 mm) protected by a Shodex OHpak SB-G guard column (8.0 mm internal diameter × 50 mm) was used for the size separations at 40°C. The injection volume was 20 µL, flow rate was 0.8 mL min−1, eluent was 10 mmol L−1 sodium phosphate buffer (pH 7.0), acetonitrile 3:1 (v/v) and the detection wavelength was 260 nm.
Two milligrams of the stream HoA fraction was dissolved in 2 mL of diluted NaOH (pH 8.0–9.0). An aliquot of the solution was diluted with eluent to a concentration of 20 mg L−1 and passed through a 0.2-µm membrane filter (DISMIC-13HP; Advantec). The total effective column volume (V 0 + V i) and void volume (V 0) were determined using acetone and Blue Dextran (2,000 kDa), respectively. Sodium polystyrene sulfonates (680, 356, 188, 86.5, 35.7, 15.8, 4.92, 1.37 kDa; Polyscience Inc., Warrington, PA, USA) and sodium p-styrenesulfonate (184 Da; Wako Chemical, Osaka, Japan) were used as standards. Molecular weight at peak maximum (Mp) was estimated using Waters Millenium 32 Chromatography Manager software (version 3.06).
RESULTS AND DISCUSSION
Seasonal changes in 13C NMR spectra of the HoA fraction in soil
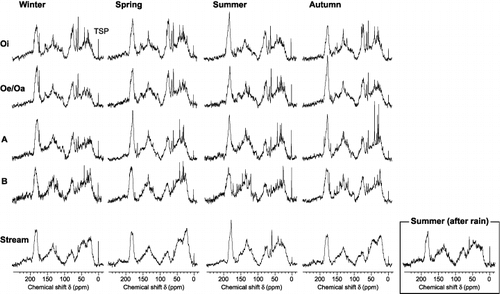
The 13C NMR spectra obtained for HoA fractions are shown in . The 13C NMR spectra of HoA fractions derived from organic horizons were similar to those of HoA fractions in seepage water from the organic horizon of a Norway spruce, Scots pine and European beech forest collected using a lysimeter (CitationGuggenberger et al. 1998; CitationKaiser et al. 2001; CitationVance and David 1991). This similarity in the chemical properties of the HoA fraction of DOM derived from different origins has been interpreted by CitationKaiser et al. (2001), indicating that the processes controlling their formation were largely similar. In addition, CitationKaiser et al. (2001) suggested that the chemical properties of HoA fractions collected using the water extraction method, operational conditions of which were selected from an earlier study (CitationAsakawa et al. 2006b), did not differ significantly from those collected using the lysimeter method.
Data from the integration of the signal areas of 13C resonance are shown in . In the Oi horizon samples, the O-alkyl C proportion slightly decreased from winter to summer, and increased in autumn. The HoA fractions from the other horizons also showed the lowest proportions of O-alkyl C, indicative of a carbohydrate structure in summer. This result is consistent with the 1H NMR spectroscopy in our previous study (CitationAsakawa et al. 2006a). Many studies on the decomposition of soil organic matter have suggested that microorganisms preferentially decompose the carbohydrate structures (CitationAlmendros and Dorado 1999; CitationBaldock et al. 1992; CitationKalbitz et al. 2003; CitationYanagi et al. 2003). Therefore, the lower O-alkyl C proportion in summer would be because of increased microbial activity.
Figure 2 Liquid-state 13C nuclear magnetic resonance spectra of hydrophobic acid fractions of water-extractable organic matter obtained from Oi, Oe/Oa, A and B horizons of Dystric Cambisol and of dissolved organic matter in a stream (aquatic humic substances) in 2004. TSP, sodium 3-trimethylsilylpropionate-2,2,3,3 D4.
In contrast to the O-alkyl C proportion, alkyl C proportions in the Oi, Oe/Oa and A horizon samples in summer were higher than those in winter (). This may be consistent with the observation that an accumulation of aliphatic (alkyl C) structures occurs after biodegradation of organic matter (CitationBaldock et al. 1990). CitationBaldock et al. (1990) indicated that utilization of carbohydrate carbon by soil microorganisms caused the synthesis of alkyl carbon. Furthermore, CitationCapriel et al. (1990) suggested that the stability of aliphatic structures is attributed to physical inaccessibility rather than recalcitrance. Accumulation of aliphatic structures appears to originate from both selective preservation and synthesis by microorganisms (CitationBaldock et al. 1992).
Several investigators observed the relative enrichment of aromatic compounds in soil organic matter during biodegradation in liquid culture and showed their stability (CitationAlmendros and Dorado 1999; CitationKalbitz et al. 2003; CitationYanagi et al. 2003). In the present study, the HoA fractions from A and B horizons, unlike the organic horizon samples, showed greater aromatic C proportions and aromaticity in summer (). This difference between organic and mineral horizon samples was possibly attributed to the difference in the contribution of microbial metabolic products.
Vertical changes in the 13C NMR spectra of the HoA fraction in soil
Changes in the 13C NMR spectra of HoA fractions with soil depth were more pronounced than seasonal changes (). Relative intensities of methoxyl (55 p.p.m.) and phenolic peaks (150 p.p.m.), which are indicative of lignin structure (CitationWilson 1987), decreased with increasing soil depth from Oi to B horizons. This result shows the degradation of lignin structure in mineral horizons.
Other notable differences were that the relative intensity of O-alkyl C (50–110 p.p.m.) decreased and that of aromatic C (110–145 p.p.m.) increased with increasing soil depth, especially in summer. These observations agree with our previous results using 1H NMR spectroscopy (CitationAsakawa et al. 2006a). represents vertical changes in the proportions of carbon in the HoA fraction. Clearly, the O-alkyl C proportion decreased from the Oi horizon to the A horizon, but it slightly increased in the B horizon in summer and autumn (). Changes in aromatic C proportion with soil depth were opposite to that of the O-alkyl C proportion (), and aromaticity was highest in A horizon samples (). A reduction in the O-alkyl C proportion in soil organic matter with soil depth has been
Table 2 Distribution of carbon species in hydrophobic acid fractions of water-extractable organic matter obtained from Dystric Cambisol and of dissolved organic matter in a stream (aquatic humic substances) in 2004 determined using liquid-state 13C nuclear magnetic resonance spectroscopy
Figure 3 Changes in the proportion of (a) O-alkyl C, (b) aromatic C, (c) alkyl C and (d) carboxylic C of hydrophobic acid fractions in water-extractable organic matter obtained from Oi, Oe/Oa, A and B horizons of Dystric Cambisol in 2004.
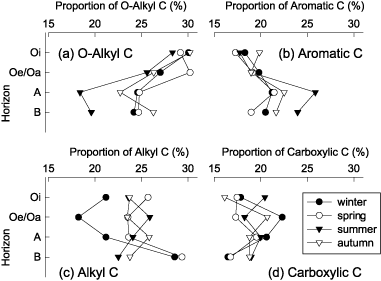
Alkyl C proportions of HoA fractions were higher in the B horizon compared with other horizons (), and carboxylic C proportions were lower () in winter and spring. This result may be consistent with the observation that sorption of the HoA fraction on subsoils caused a decrease in aromatic and carboxylic C proportions and an increase in the alkyl C proportion (CitationKaiser et al. 1997). Thus, it seems that vertical changes in the chemical properties of HoA fractions in the lower horizon may result from the sorption on mineral surfaces, especially in winter and spring. In summer and autumn, however, an increase in alkyl C proportion and a decrease in carboxylic C proportion in B horizon
Table 3 Water temperature, pH, concentrations of carbon in dissolved organic matter (DOC) and hydrophobic acid fractions (StHoA-C), and the proportions of StHoA-C in DOC in the stream water
Concentration and characteristics of HoA fractions in the stream
Water temperature, pH and concentrations of carbon in DOM (DOC) and HoA fractions (StHoA-C) of stream water are presented in . The DOC concentration ranged from 0.81 mg C L−1 in winter to 2.0 mg C L−1 in summer. The proportion of carbon in the HoA fraction was 44–55% of DOC, except in spring. These values are typical for alpine streams and are at the lower range for temperate surface waters (CitationMcKnight et al. 1997; CitationThurman 1985). In spring, the proportion of carbon in the HoA fraction of DOC was high (88%). This might be the result of effluent from the soil because the Oe/Oa, A, and B horizons represented the highest proportion of carbon in the HoA fraction of WEOC in spring too (CitationAsakawa et al. 2006a).
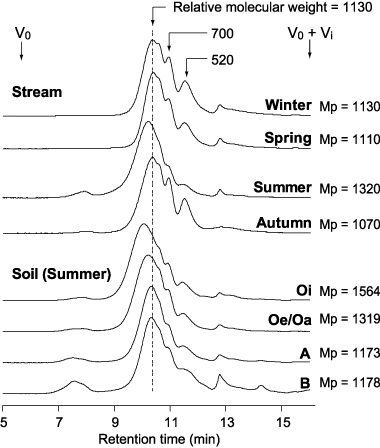
The HPSEC chromatograms of stream and soil HoA fractions are illustrated in . The chromatograms show a major broad peak with subtle shoulders and small subpeaks. These shoulders and subpeaks in the low molecular weight range are often observed in size exclusion chromatograms of DOM (CitationDawson et al. 1981), HoA fractions (CitationAsakawa et al. 2006a) and aquatic humic substances (CitationNagao et al. 2003). In addition, elution times of stream HoA fractions correspond to those of HoA fractions in each soil horizon (), indicating that HoA fractions in stream and soil consist of similar components.
Stream HoA fractions in summer had higher molecular weights at peak maximum (Mp) compared to the samples from other seasons (). As shown in , Mp values of samples from winter, spring and autumn (1,070–1,130 Da) were similar to those of HoA fractions in the B horizon (924–1178 Da), whereas that of the summer sample (1,320 Da) was within the range of Mp values of HoA fractions from the Oe/Oa horizon (1319–1444 Da) (CitationAsakawa et al. 2006a).
Figure 4 Size exclusion chromatograms of hydrophobic acid fractions of dissolved organic matter in a stream (aquatic humic substances) collected in 2004 and of hydrophobic acid fractions of water-extractable organic matter obtained from Oi, Oe/Oa, A and B horizons of Dystric Cambisol in summer 2004. Mp, relative molecular weight at peak maximum (DA). V0, void volume; VD+ Vi, total effective column volume.
displays 1H NMR spectra of HoA fractions in the stream, Oi and B horizon soil. The spectra of stream HoA fractions in winter, spring and autumn show large signal abundances of Hal (0–3.0 p.p.m.), and especially those of Hβ (1.0–1.6 p.p.m.). The stream spectrum closely resembles those of HoA fractions of B horizon soil () and of humic substances isolated from stream water, as presented by CitationMalcolm (1990). In summer, the spectrum of the stream HoA fraction is similar to that of HoA fractions in the Oi and Oe/Oa
Figure 5 1H nuclear magnetic resonance spectra of (a) hydrophobic acid fractions of dissolved organic matter in a stream (aquatic humic substances) collected in 2004 and (b) of hydrophobic acid fractions of water-extractable organic matter obtained from Oi (summer 2004) and B horizons (winter 2004) of Dystric Cambisol. HOD; TSP, sodium 3-trimethylsilylpropionate-2,2,3,3 D4.
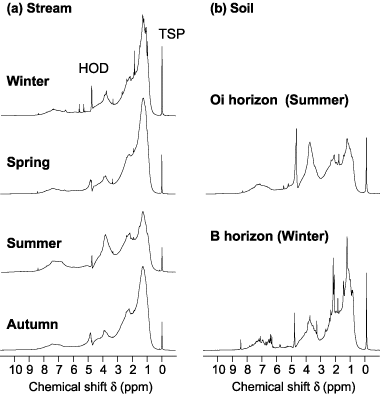
The distribution of each type of proton species in stream HoA fractions is shown in . The sample from summer had a considerably low proportion of Hal (62.9%) compared with that in other seasons (77.4–81.0%). In contrast, the proportions of HC–O and Har in the summer sample were higher than those in the samples from other seasons. We have previously reported that the proportion of Hal in HoA fractions in the soil significantly increased from 50.1–59.7% in organic horizons to 64.4–74.0% in the B horizon (CitationAsakawa et al. 2006a). Therefore, it seems that high proportions of Hal in the winter, spring and autumn samples may be attributed to the chemical properties of HoA fractions in the B horizon.
shows the liquid-state 13C NMR spectra of HoA fractions in the stream. Samples from winter, spring and autumn showed large signal abundances in the peaks at chemical shift (δ) values of 25 and 45 p.p.m. These spectra were similar to those of fluvic acids in the stream with low DOC concentrations (CitationMcKnight et al. 1992). The spectrum of the summer sample exhibited methoxyl (55 p.p.m.) and phenolic peaks (150 p.p.m.), which is the signature typical of lignin-derived compounds, whereas these peaks were almost absent in the spectra of samples from other seasons. Relatively strong signals for lignin were observed in spectra of HoA fractions from upper organic soil horizons, and these signals were almost absent in HoA fractions from the B horizon (). This result strongly suggests that the stream HoA fraction in summer was derived from HoA fractions in organic soil horizons.
The distribution of carbon species in stream HoA fractions changed seasonally (). In summer, the proportion of alkyl C (27.6%) in the stream HoA fraction was lower than that in samples from other seasons (29.3–39.4%). Instead, proportions of O-alkyl (23.3%) and aromatic C (21.7%) in the summer sample were higher than those in winter, spring and autumn samples (O-alkyl; 18.5–22.3%, aromatic C; 15.9–17.6%). The summer sample also showed higher aromaticity (0.35) than the samples in other seasons (0.26–0.32). These results support the observation that the chemical properties of stream HoA fractions in summer differed from those of samples in other seasons. In addition, the high alkyl C proportion of stream HoA fractions, in particular spring and autumn samples, may be associated with the high alkyl C proportion in HoA fractions in the B horizon ().
Unlike our results, CitationMalcolm (1989) observed that solid-state 13C NMR spectra of fluvic and humic acids in large rivers were almost invariant with season. CitationClair et al. (1996)
Table 4 Distribution of proton species in hydrophobic acid fractions of dissolved organic matter in a stream (aquatic humic substances) collected in 2004 determined using 1H nuclear magnetic resonance spectroscopy
These results from HPSEC and 1H and 13C NMR spectroscopy suggest that stream HoA fractions in winter, spring and autumn were derived from HoA fractions in the B horizon, but that summer samples were derived from the organic horizon. This means that seasonal changes in the chemical properties of HoA fractions in small streams in a forested watershed mainly result from pathways of HoA fractions in soils to streams. Consequently, the chemical properties of stream HoA fractions in winter, spring and autumn were affected by vertical changes in the chemical properties of HoA fractions in the soil. In summer, it appears that the HoA fractions in leachate from the organic horizons may contribute to form the stream HoA fraction. Because we collected summer stream water during a drought, leachate from the deeper mineral horizons should have a negligible effect on stream water. Instead, we speculated that the stream HoA fraction in summer was derived from the HoA fraction leached from the organic horizons adjacent to the stream by dew and/or eluated from the leaves that had fallen into the stream. This speculation is supported by the similarity between the 13C NMR spectrum of the stream HoA fraction in summer and that of samples collected after a rainstorm in summer, which became surface runoff ().
Conclusions
This study demonstrated that the chemical properties of HoA fractions in Dystric Cambisol changed with season and soil depth. The seasonal changes were attributed to selective utilization and preservation by microorganisms. However, different patterns were observed between organic and mineral soil horizons. The variations in the chemical properties in HoA fractions with season were less significant than those with soil depth. Vertical changes in chemical properties of HoA fractions appeared to be a combination of microbial decomposition and sorption to mineral surfaces. From Oi to A horizons, the effect of microbial decomposition seems to be more prominent than that of sorption. It seems that sorption of HoA fractions to mineral surfaces may increase the proportion of aliphatic structures in B horizon samples.
These changes in the chemical properties of HoA fractions in soil would contribute to the chemical properties of HoA fractions of DOM (aquatic humic substances) in the adjacent stream. The stream HoA fractions would be derived from HoA fractions through the B horizon. However, in summer it is suggested that the stream HoA fraction originated from the HoA fraction of DOM in a near-stream surface organic soil horizon. Consequently, vertical changes in the chemical properties of HoA fractions in soil and hydrological properties are important factors in the characteristics of HoA fractions in streams in a forested watershed.
ACKNOWLEDGMENT
This research was supported by a Grant-in-Aid for Scientific Research (B-15380107, 2003-2005 and B-1530001, 2003-2005) from The Japan Society for the Promotion of Science.
Notes
Present address: Faculty of Horticulture, Minamikyushu University, Miyazaki 884-0003, Japan.
REFERENCES
- Almendros , G and Dorado , J . 1999 . Molecular characteristics related to the biodegradability of humic acid preparations . Eur. J. Soil Sci , 50 : 227 – 236 .
- Anderson , HA , Hepburn , A , Miller , JD , Stewart , M , Ferrier , RC and Walker , TAB . 1990 . Humic substances of surface waters: Litter and soil throughflow relationships in two forested ecosystems . Anal. Chim. Acta , 232 : 3 – 10 .
- Asakawa , D , Mochizuki , H , Yanagi , Y , Suzuki , T , Nagao , S and Fujitake , N . 2006a . Changes in elemental composition, molecular weight, and 1H NMR spectrum of water-extractable hydrophobic acids in Cambisols with season and soil depth . Soil Sci. Plant Nutr , 52 : 361 – 370 .
- Asakawa , D , Mochizuki , H , Yanagi , Y , Suzuki , T , Nagao , S and Fujitake , N . 2006b . Effects of operational conditions for extraction and sample storage on the structural properties of water-extractable humic substances in soil . Humic Subst. Res , 3 : 15 – 24 .
- Baldock , JA , Oades , JM , Vassallo , AM and Wilson , MA . 1990 . Solid-state CP/MAS 13C N.M.R. analysis of bacterial and fungal cultures isolated from a soil incubated with glucose . Aust. J. Soil Res , 28 : 213 – 225 .
- Baldock , JA , Oades , JM , Waters , AG , Peng , X , Vassallo , AM and Wilson , MA . 1992 . Aspects of the chemical structure of soil organic materials as revealed by solid-state 13C NMR spectroscopy . Biogeochemistry , 16 : 1 – 42 .
- Candler , R , Zech , W and Alt , HG . 1988 . Characterization of water-soluble organic substances from a Typic Dystrochrept under spruce using GPC, IR, 1H NMR, and 13C NMR spectroscopy . Soil Sci , 146 : 445 – 452 .
- Capriel , P , Beck , T , Borchert , H and Harter , P . 1990 . Relationship between soil aliphatic fraction extracted with supercritical hexane, soil microbial biomass and soil aggregate stability . Soil Sci. Soc. Am. J , 54 : 415 – 520 .
- Christ , MJ and David , MB . 1996 . Temperature and moisture effects on the production of dissolved organic carbon in a Spodosl . Soil Biol. Biochem , 28 : 1191 – 1199 .
- Clair , TA , Sayer , BG , Kramer , JR and Eaton , DR . 1996 . Seasonal variation in the composition of aquatic organic matter in some Nova Scotian brownwaters: a nuclear magnetic resonance approach . Hydrobiol , 317 : 141 – 150 .
- Dawson , HJ , Hrutfiord , BF , Zasoski , RJ and Ugolini , FC . 1981 . The molecular weight and origin of yellow organic acids . Soil Sci , 132 : 191 – 199 .
- Food and Agriculture Organization . 1998 . World Reference Base For Soil Resources , World Soil Resources Reports, 84 Rome : Food and Agriculture Organization .
- Franco , I , Catalano , L , Contin , M and De Nobili , M . 2001 . Interactions between organic model compounds and pesticides with water-soluble soil humic substances . Acta Hydrochim. Hydrobiol , 29 : 88 – 99 .
- Fujitake , N and Kawahigashi , M . 1999 . 13C NMR spectra and elemental composition of fractions with different particle sizes from an Andosol humic acid . Soil Sci. Plant Nutr , 45 : 359 – 366 .
- Gressel , N , McColl , JG , Preston , CM , Newman , RH and Powers , RF . 1996 . Linkages between phosphorus transformations and carbon decomposition in a forest soil . Biogeochemistry , 33 : 97 – 123 .
- Guggenberger , G , Kaiser , K and Zech , W . 1998 . Organic colloids in forest soils: 1. Biochemical mobilization in the forest floor . Phys. Chem. Earth , 23 : 141 – 146 .
- Guggenberger , G , Zech , W and Schulten , H-R . 1994 . Formation and mobilization pathways of dissolved organic matter: evidence from chemical structural studies of organic matter fractions in acid forest floor solutions . Org. Geochem , 21 : 51 – 66 .
- Haitzer , M , Hoss , S , Traunspurger , W and Steinberg , C . 1999 . Relationship between concentration of dissolved organic matter (DOM) and the effect of DOM on the bioconcentration of benzo[a]pyrene . Aquatic Toxicol , 45 : 147 – 158 .
- Hatcher , PG , Schnitzer , M , Dennis , LW and Maciel , GE . 1981 . Aromaticity of humic substances in soils . Soil Sci. Soc. Am. J , 45 : 1089 – 1094 .
- Johnsen , S , Kukkonen , J and Grande , M . 1989 . Influence of natural aquatic humic substances on the bioavailability of benzo(a)pyrene to Atlantic salmon . Sci. Total Environ , 81 ( 82 ) : 691 – 702 .
- Kaiser , K , Guggenberger , G , Haumaier , L and Zech , W . 1997 . Dissolved organic matter sorption on subsoils and minerals studied by 13C-NMR and DRIFT spectroscopy . Eur. J. Soil Sci , 48 : 301 – 310 .
- Kaiser , K , Guggenberger , G , Haumaier , L and Zech , W . 2001 . Seasonal variations in the chemical composition of dissolved organic matter in organic forest floor layer leachates of old-growth Scots pine (Pinus sylvestris L.) and European beech (Fagus sylvatica L.) stands in northeastern Bavaria, Germany . Biogeochemistry , 55 : 103 – 143 .
- Kalbitz , K , Schwesig , D Schmerwitz , J . 2003 . Changes in properties of soil-derived dissolved organic matter induced by biodegradation . Soil Biol. Biochem , 35 : 1129 – 1142 .
- Kawahigashi , M , Fujitake , N and Takahashi , T . 1996 . Structural information obtained from spectral analysis (UV-VIS, IR, 1H NMR) of particle size fractions in two humic acids . Soil Sci. Plant Nutr , 42 : 355 – 360 .
- Kukkonen , J , MacCarthy , JF and Oikari , A . 1990 . Effects of XAD-8 fractions of dissolved organic carbon on the sorption and bioavailability of organic micropollutants . Arch. Environ. Contam. Toxicol , 19 : 551 – 557 .
- Leenheer , JA . 1981 . Comprehensive approach to preparative isolation and fractionation of dissolved organic carbon from natural waters and wastewaters . Environ. Sci. Technol , 15 : 578 – 587 .
- Malcolm , RL . 1989 . “ Applications of solid-state 13C NMR spectroscopy to geochemical studies of humic substances ” . In Humic Substances II: In Search of Structure , Edited by: Hayes , MHB , MacCarthy , P , Malcolm , RL and Swift , RS . 339 – 372 . Chichester : John Wiley & Sons .
- Malcolm , RL . 1990 . The uniqueness of humic substances in each of soil, stream and marine environments . Anal. Chim. Acta , 232 : 19 – 30 .
- McCarthy , JF , Roberson , LE and Burrus , LW . 1989 . Association of benzo(a)pyrene with dissolved organic matter: prediction of Kdomfrom structural and chemical properties of the organic matter . Chemosphere , 19 : 1911 – 1920 .
- McDowell , WH and Likens , GE . 1988 . Origin, composition and flux of the dissolved organic carbon in the Hubbard Brook valley . Ecol. Monogr , 58 : 177 – 195 .
- McKnight , DM , Bencala , KE , Zellweger , GW , Aiken , GR , Feder , GL and Thorn , KA . 1992 . Sorption of dissolved organic carbon by hydrous aluminum and iron oxides occurring at the confluence of Deer Creek with the Snake River, Summit Country, Colorado . Environ. Sci. Technol , 26 : 1388 – 1396 .
- McKnight , DM , Harnish , R , Wershaw , RL , Baron , JS and Schiff , S . 1997 . Chemical characteristics of particulate, colloidal, and dissolved organic material in Loch Vale Watershed, Rocky Mountain National Park . Biogeochemistry , 36 : 99 – 124 .
- Nagao , S , Matsunaga , T , Suzuki , Y , Ueno , T and Amano , H . 2003 . Characteristics of humic substances in the Kuji River waters as determined by high-performance size exclusion chromatography with fluorescence detection . Water Res , 37 : 4159 – 4170 .
- Preston , CM and Blackwell , BA . 1985 . Carbon-13 nuclear magnetic resonance for a humic and a fulvic acid: signal-to-noise optimization, quantitation, and spin-echo techniques . Soil Sci , 139 : 88 – 96 .
- Qualls , RG , Haines , BL and Swank , WT . 1991 . Fluxes of dissolved organic nutrients and humic substances in a deciduous forest . Ecology , 72 : 254 – 266 .
- Raber , B and Kögel-Knabner , I . 1997 . Influence of origin and properties of dissolved organic matter on the partition of polycyclic aromatic hydrocarbons (PAHs) . Eur. J. Soil Sci , 48 : 443 – 455 .
- Ricca , G and Severini , F . 1993 . Structural investigations of humic substances by IR-FT, 13C-NMR spectroscopy and comparison with a maleic oligomer of known structure . Geoderma , 58 : 233 – 244 .
- Schnitzer , M and Preston , CM . 1986 . Analysis of humic acids by solution and solid-state carbon-13 nuclear magnetic resonance . Soil Sci. Soc. Am. J , 50 : 326 – 331 .
- Thorn , KA , Folan , DW and MacCarthy , P . 1989 . Characterization of the International Humic Substances Society: standard and reference fulvic and humic acids by solution state carbon-13 (13C) and hydrogen-1 (1H) nuclear magnetic resonance spectrometry , Water Resources Investigations Report 89–4196 1 – 93 . Denver : US Geological Survey .
- Thurman , EM . 1985 . Organic Geochemistry of Natural Waters , Boston : Nijhoff/Junk Publishers .
- Thurman , EM and Malcolm , RL . 1981 . Preparative isolation of aquatic humic substances . Environ. Sci. Technol , 15 : 463 – 466 .
- Ussiri , DAN and Johnson , CE . 2003 . Characterization of organic matter in a northern hardwood forest soil by 13C NMR spectroscopy and chemical methods . Geoderma , 111 : 123 – 149 .
- Vance , GF and David , MB . 1991 . Chemical characteristics and acidity of soluble organic substances from a northern hardwood forest floor, central Maine, USA . Geochim. Cosmichim. Acta , 55 : 3611 – 3625 .
- Wilson , MA . 1987 . NMR Techniques and Applications in Geochemistry and Soil Chemistry , New York : Pergamon Press .
- Wilson , MA , Heng , S , Goh , KM , Pugmire , RJ and Grant , DM . 1983 . Studies of litter and acid insoluble soil organic matter fractions using 13C-cross polarization nuclear magnetic resonance spectroscopy with magic angle spinning . J. Soil Sci , 34 : 83 – 97 .
- Yanagi , Y , Hamaguchi , S , Tamaki , H , Suzuki , T , Otsuka , H and Fujitake , N . 2003 . Relation of chemical properties of soil humic acids to decolorization by white rot fungus Coriolus consors . Soil Sci. Plant Nutr , 49 : 201 – 206 .
- Present address: Faculty of Horticulture, Minamikyushu University, Miyazaki 884-0003, Japan.