Abstract
Variability in the emission factors of nitrous oxide (N2O) associated with the application of chemical fertilizer (EFF) and organic matter (EFO) were analyzed in two onion fields (GL, Gray Lowland soil [Gleysol; Food and Agriculture Organization/UNESCO]; BL, Brown Lowland soil [Fluvisol; Food and Agriculture Organization/UNESCO]) in Mikasa, Hokkaido, Japan. Nitrous oxide flux was measured using a closed chamber technique in four treatments (FOP, chemical nitrogen fertilization and organic matter application, with plants; F, chemical nitrogen fertilization only, without plants; OP, organic matter application only, with plants; C, control, no fertilization or organic matter application, without plants) for 4 years in GL (2000, 2003–2005) and for 1 year in BL (2005). The application rate of chemical fertilizer nitrogen ranged from 237 to 242 kg N ha−1 year−1 in GL and was 284 kg N ha−1 year−1 in BL; organic matter nitrogen ranged from 81 to 117 kg N ha−1 year−1 in GL and was 181 kg N ha−1 year−1 in BL. The emission factors (EF) were calculated using the equations: EFF (%) = (N2O emission in FOP–N2O emission in OP)/(applied chemical nitrogen fertilizer) × 100 and EFO (%) = (N2O emission in FOP–N2O emission in F)/(applied organic matter nitrogen) × 100. The annual N2O emissions for treatments FOP, F, OP and C were 7.2–17, 5.7–17, 3.2–9.9 and 2.0–12 kg N ha−1 year−1, respectively, in GL and 5.6, 2.8, 1.9 and 1.8 kg N ha−1 year−1, respectively, in BL. The EFF ranged from 1.3% to 5.5% in GL and was 1.3% in BL. The EFF was positively correlated with the mean annual air temperature (P < 0.01), suggesting that N2O emission derived from chemical nitrogen fertilizer increases as air temperature rises. The EFO, however, differed greatly between GL (ranging from −5.2% to 9.1%) and BL (1.5%). The EFO was positively correlated with the mean annual relative humidity, although the correlation was not significant (P = 0.23). This finding suggests that much wetter climatic conditions may increase N2O emissions derived from organic matter nitrogen. The estimated N2O emissions based on these EF values and the rate of nitrogen application coincided well with the measured N2O emissions in the FOP treatment in both soils.
INTRODUCTION
Nitrous oxide (N2O) is a major greenhouse gas. It has a 296-fold higher greenhouse effect than that of carbon dioxide (CO2), and its concentration in the air is increasing at a rate of 0.8 p.p.b. per year (CitationIntergovernmental Panel on Climate Change 2001). The global emission of N2O was estimated to be 16.2 Tg N year−1, and the emission from agricultural soils accounted for 24% of all emissions (CitationIntergovernmental Panel on Climate Change 2001; CitationMosier et al. 1998).
The simplest way to estimate N2O emission from agricultural soil is to use the emission factor (EF) proposed by the CitationIntergovernmental Panel on Climate Change (2006) and fertilizer nitrogen application rate described to statistical data (e.g. published by the Japanese government). The EF is the ratio of the N2O emission divided by the amount of nitrogen fertilizer applied. The Intergovernmental Panel on Climate Change has proposed a default EF value of 1.0%, although if an EF value based on actual measurements is available, that value is preferred to the default value. In Japan, the EF was estimated to be 0.62% for all fertilized upland fields (CitationAkiyama et al. 2006). The N2O emission from agricultural soils usually increases with increases in the application of chemical fertilizer, manure and crop residue (CitationBouwman 1996; CitationCochran et al. 1997; CitationMogge et al. 1999; CitationMori et al. 2005; CitationTokuda and Hayatsu 2004; CitationZou et al. 2005), and EF values differ among the various types of fertilizers (e.g. 1.65% for chemical fertilizer and 0.15–0.59% for residues in winter wheat fields; CitationZou et al. 2005). In addition, because several types of amendments are usually applied to an agricultural field, N2O emissions from the soil would be affected by their interactions. Thus, to more accurately assess N2O emissions from agricultural fields receiving chemical fertilizer, manure and crop residue, each of these EF values needs to be calculated.
Several studies have reported annual variation in EF values, which may be the result of climatic differences. For example, N2O emissions ranged from 3.5 to 15.6 kg N ha−1 year−1 and the EF ranged from 1.1 to 6.4% in an onion field in Hokkaido, Japan (CitationKusa et al. 2002). CitationDobbie et al. (1999) reported N2O emissions of 1.0–18.4 kg N ha−1 year−1 and EF values of 0.3–5.8% in grasslands in Scotland. CitationTakakai et al. (2006) reported N2O emissions of 7.1–256 kg N ha−1 year−1 and the EF ranged from 1.8 to 36% in agricultural peat soil in Indonesia. CitationSmith et al. (1998) noted that the EF calculated in Scottish agricultural soils was lower than 1.25% because of lower temperatures than those recorded by CitationBouwman (1996).
Nitrous oxide emissions from agricultural soils originate not only from applied nitrogen but also from the nitrogen derived from soil organic matter. The purpose of this study was to calculate EF values for chemical fertilizer and added organic matter and to investigate the annual variation in EF values for agricultural soils in Mikasa, Hokkaido, Japan.
MATERIALS AND METHODS
Site description
Two onion fields with Gray Lowland (GL) soil (Gleysol; Food and Agriculture Organization/UNESCO) and Brown Lowland (BL) soil (Fluvisol; Food and Agriculture Organization/UNESCO) in Mikasa, Hokkaido, Japan, were investigated (GL, 43°14.4′Ν, 141°50′Ε; BL, 43°13.6′Ν, 141°49.1′Ε). Previous crops were onion and onion has been cultivated for more than 10 years at both sites. The distance between the GL and BL sites was 2.7 km. The study was conducted in 2000 and 2003–2005 in GL and in 2005 in BL. The mean annual temperature and precipitation were 7.4°C and 1,155 mm, respectively, over the past 30 years (observed at Iwamizawa weather station, 43°12.6′Ν, 141°47.3′E). Soils at both sites were covered with snow, but did not freeze, in winter. lists the soil chemical properties at both sites.
Management of both fields (e.g. application rate and method of fertilizer) treated as farmer's practices around the fields. At the beginning of May at site GL, 242 kg N ha−1 year−1 (NH+ 4, 187; NH− 4, 55) of chemical fertilizer was applied in 2000, 2003 and 2004 and 237 kg N ha−1 year−1 (NH+ 4, 183; NH− 4, 54) was applied in 2005. At the end of April 2005 at site BL, 204 kg N ha−1 (NH+ 4, 45; NH− 4, 159) was applied and 80 (Nitrolime) kg N ha−1 was applied in mid October (total 284 kg N ha−1 year−1). Chemical fertilizers were applied to the surface of the soil afterwhich the soil was plowed to 0–5 or 10 cm depth. At site BL, organic fertilizer was applied in the form of manure made by sludge at a rate of 45 kg N ha−1 year−1 at the end of March and as rice brain at a rate of 38 kg N ha−1 year−1 in mid October. After the harvest, onion residue was left on the field at both sites until plowing. At site GL, the amounts of
Table 1 Chemical properties of the soil layers in Gray Lowland soil (GL) and Brown Lowland soil (BL)
Treatments
We set up four treatments in both GL and BL: FOP, chemical nitrogen fertilization and organic matter application, with plants; F, chemical nitrogen fertilization only, without plants; OP, organic matter application only, with plants; and C, control, no fertilization or organic matter application, without plants. Organic matter application included onion residue for treatments FOP and OP at both sites, and also included organic fertilizer at the BL site. The area of each treatment plot was 40 m2 (5 m × 8 m) at GL and 8 m2 (2 m × 4 m) at BL. Each treatment was set up at the beginning of April (one replicate at each site) and management (e.g. ploughing) was the same for each treatment. The treatment plot at site GL was created at the same place throughout the investigation period.
N2O and NO flux measurements
N2O and NO fluxes were measured using a closed chamber method (four replications) with two types of cylindrical stainless steel chambers. In 2000, a chamber that was 30 cm in diameter and 35 cm in height was used (CitationKusa et al. 2002). In 2003, 2004 and 2005, the diameter of the chamber was 20 cm and the height was 25 cm (CitationToma and Hatano 2007). The cover of the chamber was made of acryl and was equipped with a sample collector, pressure regulating bag and a Tedlar bag (0.5 L). In 2000, 2003 and 2004, we put the chambers 2 or 3 cm into the soil and started measurements after 15 min. In 2005, however, the chamber had a base made of stainless steel with a diameter of 20 cm; the upper part of the base was equipped with a slight depression, which was filled with water to seal it during the measurement (CitationToma and Hatano 2007). The base was kept on the ground, except during harvesting and cultivation, and the base was reset 1 day before the next sampling. Onion plants and their living parts were removed from the chamber base, but the litter covering the base was left inside during the measurement.
We took the gas samples before and 15 min after closing the chamber. A 250-mL gas sample was taken using a syringe (25 mL) and was injected into a Tedlar bag (0.5 L). The bags were then brought to the laboratory and 20 mL of each gas sample was immediately transferred into a glass vial (10 mL). From these bag samples, NO was analyzed using a chemoluminescence nitrogen oxide analyzer (model 265P, Kimoto Electric, Osaka, Japan) within 12 h, and from the samples of vials N2O was analyzed with an ECD (Electron capture detector) gas chromatograph (model GC-14B, Shimadzu, Kyoto, Japan) within 1 month.
Gas fluxes were calculated using the following equation:
Calculations of EFF, EFO and estimated N2O emission from the FOP treatment
If we assume that N2O emission is composed of N2O derived from applied chemical nitrogen fertilizer, applied organic matter and soil organic matter decomposition, N2O emission can be estimated as:
where EFF is the emission factor of N2O associated with the application of chemical nitrogen fertilizer, EFO is the emission factor of N2O associated with the application of organic nitrogen and [C] is N2O emission in the C treatment. EFF and EFO were calculated using the following equations:
where [FOP] is the N2O emission measured in the FOP treatment (kg N ha−1 year−1), [OP] is the N2O emission measured in the OP treatment (kg N ha−1 year−1) and [F] is the N2O emission measured in the F treatment (kg N ha−1 year−1). Onion residue might be decomposed during the snow-covered season because N2O fluxes were low after the snowmelt (CitationKusa et al. 2002). In addition, CitationToma and Hatano (2007) reported that approximately 45% of the carbon in onion residue was decomposed within 2 months. Therefore, we assumed that the onion residue was almost decomposed within the year.
Other measurements and sampling frequency
The soil temperature at 5 cm depth was measured five times in all treatments during the gas measurements. Disturbed soil samples were taken from depths of 0–5 cm, as a mixing soil sample from five places in each plot. Samples were extracted using distilled water, and NH+ 4 and NH− 4 concentrations were analyzed by colorimetry with indophenol-blue and ion chromatography (QIC analyzer, Dionex Japan, Osaka, Japan), respectively. Three replicate undisturbed soil samples were collected using a steel corer (100 mL) and the water-filled pore space (WFPS) was measured.
After fertilizer application, samples were collected three times per week from May to June, three times per month from July to August, and once or twice per month during the snow-covered season. From after snow melt until before fertilization, and from mid October until snow cover, sampling was conducted two or three times per month.
Statistical analysis
The relationships between the meteorological condition and N2O emission or EFs were determined using regression analyses. We used Excel Tahenryoukaiseki Ver.4.0 for Windows (Social Survey Research Information Company, Tokyo, Japan) for the regression analysis.
RESULTS
N2O flux
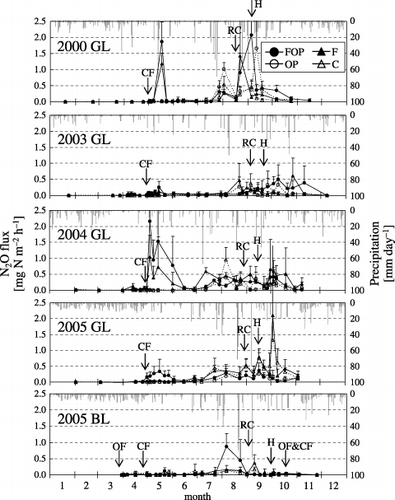
shows the seasonal variation in N2O flux at each site. Two peaks were observed each year at GL. The first peak was observed from May to June in the FOP and F treatments after chemical fertilizer application. The second peak occurred from late August to October in all treatments. At BL, only a negligible increase in N2O flux occurred in May in the FOP treatment, and the flux further increased during July and August. This was also found in the other treatments.
From May to June, at GL, the maximum N2O flux values in the FOP and F treatments in 2000 and 2004 were similar, and those in 2003 and 2005 resembled each other. From May to June in 2000 and 2004, the maximum N2O fluxes in FOP were 1.86 and 2.16 mg N m−2 h−1 and those in F were 1.19 and 1.04 mg N m−2 h−1, respectively. And in 2003 and 2005, the maximum N2O fluxes in FOP were 0.26 and 0.33 mg N m−2 h−1 and those in F were 0.13 and 0.16 mg N m−2 h−1, respectively. In BL, the maximum N2O flux was 0.12 mg N m−2 h−1 from May to June in 2005 in the FOP treatment.
From the end of August to the end of October each year, N2O flux in the FOP, F and OP treatments at GL ranged from 0.02 to 2.07, 0.02 to 2.10 and 0.01 to 1.65 mg N m−2 h−1, respectively, which was slightly higher than the ranges in the C treatment (0.01–1.33 mg N m−2 h−1). At BL, these values ranged from 0.001 to 0.44, –0.002 to 0.37 and 0.004 to 0.12 mg N m−2 h−1, respectively, and the values of C ranged from 0.01 to 0.17 mg N m−2 h−1.
NO flux and N2O-N/NO-N
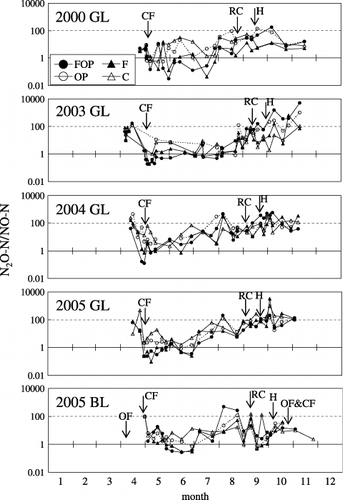
The maximum NO fluxes in the FOP and F treatments at GL were 3.30 and 0.78 mg N m−2 h−1, respectively, and these fluxes were observed from May to June in all years. At BL, the maximum NO flux in the FOP and F treatments was 0.12 mg N m−2 h−1 at the end of June and 0.48 mg N m−2 h−1 in early May. The NO fluxes in the OP and C treatments at both sites were low and stable throughout all seasons (data not shown). Based on these values, the ratio between N2O-N and NO-N (N2O-N/NO-N) decreased to less than 1.0 from May to June and increased to approximately 100 from September to October in the FOP and F treatments at GL (). At BL, N2O-N/NO-N decreased to less than 1.0 in June and increased to approximately 100 in August in the FOP and F treatments. In the OP and C treatments at both sites, the N2O-N/NO-N remained above 1.0 from May to June and the seasonal change in the ratio was small compared with that in FOP and F.
Soil temperature, WFPS, and soil NH− 4 and NO− 3 concentrations
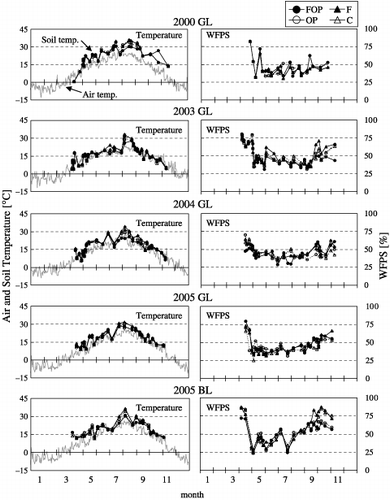
Soil temperature and WFPS are shown in . Seasonal variation in soil temperature was similar at both sites and resembled that of air temperature, with maximum values observed from July to August. At GL, a positive significant relationship between N2O flux and soil temperature was observed throughout all seasons in the OP and C treatments (OP, n = 104, r 2 = 0.13, P < 0.01; C, n = 104, r 2 = 0.07, P < 0.01). Considering only those periods when high N2O fluxes were observed (from May to June and from September to October), however, a positive significant relationship between N2O flux and soil temperature was observed only in OP (May–June, n = 32, r 2 = 0.12, P < 0.05; September–October, n = 30, r 2 = 0.26, P < 0.01). At BL, a positive significant relationship between N2O flux and soil temperature was observed throughout all seasons in the FOP, F and C treatments (FOP, n = 22, r 2 = 0.21, P < 0.05; OF, n = 20,
Figure 1 Seasonal variation in N2O flux at the Gray Lowland soil (GL) and Brown Lowland soil (BL) sites. The arrows indicate the time of chemical fertilizer application (CF), organic fertilizer application (OF), root cutting (RC) and harvest and residue application (H). The four treatments were: FOP, chemical nitrogen fertilization and organic matter application, with plants; F, chemical nitrogen fertilization only, without plants; OP, organic matter application, with plants; C, no fertilization or organic matter, and no plants. Error bars indicate standard deviation.
The WFPS in all treatments at GL and BL were high after snow melt, decreased to 25–50% in summer and increased again in autumn (). Beginning in August, the WFPS at BL increased rapidly from 25% to approximately 75%, whereas that at GL increased more slowly. Across each year there was no relationship between N2O flux and WFPS in any treatment at either site. However, N2O flux was positively correlated with WFPS in the F treatment at GL from May to June (n = 30, r 2 = 0.23, P < 0.01) and in the OP treatment at BL from September to October (n = 8, r 2 = 0.54, P < 0.05).
Maximum soil NH+ 4 concentrations in the FOP and F treatments at both sites were observed in May; after this time, however, NH+ 4 was not detected (). Throughout each year, there was no significant relationship between N2O flux and soil NH+ 4 concentration. However, a positive relationship was observed in the OP treatment at site GL from September to October (n = 20, r 2 = 0.31, P < 0.05).
Soil NH− 4 concentration increased after fertilizer application in the FOP and F treatments at both sites, and maximum concentration was observed from June to August (). Soil NH− 4 concentrations in the OP and C treatments also increased from June to August, but the concentrations were lower than those in FOP and F. In all treatments, soil NH− 4 concentration decreased in mid August. Throughout all seasons, a positive significant relationship between N2O flux and soil NH− 4 concentration was found in the C treatment at GL (n = 76, r 2 = 0.16, P < 0.01).
N2O emission, EFF and EFO
Annual N2O emissions differed greatly among treatments and years (). At GL, the annual N2O emissions ranged
Figure 2 Seasonal variation in N2O-N/NO-N at the Gray Lowland soil (GL) and Brown Lowland soil (BL) sites. See Fig. 1 for an explanation of the arrows and treatments.
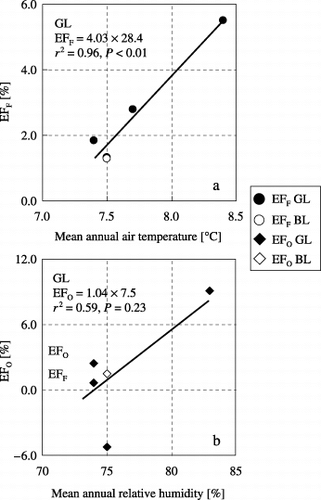
The EFF values, calculated using the annual N2O emission in the FOP and OP treatments according to EquationEq. 3, ranged from 1.32 to 5.50% at GL and the EFF value at BL was 1.30%. The EFO values, calculated using the annual N2O emission in the FOP and F treatments according to EquationEq. 4, ranged from −5.22% to 9.06% at GL and the EFO value at BL was 1.51% (). The EFF was positively correlated with mean annual temperature at GL (n = 4, r 2 = 0.96, P < 0.01; ), and the EFO increased as mean annual relative humidity increased at GL, although the relationship was not statistically significant (n = 4, r 2 = 0.59, P = 0.23; ). The EFF values at GL and BL in 2005
Figure 3 Seasonal variation in air and soil temperatures and water-filled pore space (WFPS) at the Gray Lowland soil (GL) and Brown Lowland soil (BL) sites. See Fig. 1 for an explanation of the treatments.
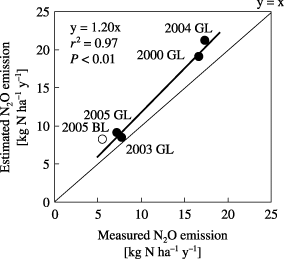
By assuming that N2O emission is composed of N2O derived from applied chemical nitrogen fertilizer, applied organic matter and soil organic matter decomposition, N2O emission was estimated according to EquationEq. 2. Chemical fertilizer induced N2O emissions were calculated by multiplying the EFF of the actual measurement by the chemical nitrogen application rate, and organic fertilizer induced N2O emissions were calculated by multiplying the EFO of the actual measurement by organic nitrogen application rate. N2O emissions in the C treatment were assumed to have originated from soil organic matter. According to our field measurements, the estimated N2O emission in the FOP treatment was approximately 20% larger than the measured emission ().
DISCUSSION
Emission factors of N2O associated with the application of chemical fertilizer (EFF)
The EF F estimated in this study showed high variability, ranging from 1.30 to 5.50% (); these values were larger than the 1.0% proposed by the CitationIntergovernmental Panel on Climate Change (2006) and the values reported in other studies (CitationAkiyama and Tsuruta 2002; CitationSmith et al. 1998; CitationZou et al. 2005). In this study, the EF F value was calculated by dividing the difference in N 2 O emissions between the FOP and OP treatments by the chemical nitrogen fertilizer application rate (EquationEq. 3). Because the amounts of applied chemical nitrogen fertilizer each year were nearly the same at GL, the annual variability in EF F was likely to be affected by the difference in the N 2 O emissions in the FOP and OP treatments
Figure 4 Seasonal variation in soil concentration and soil concentration at the Gray Lowland soil (GL) and Brown Lowland soil (BL) sites. See Fig. 1 for an explanation of the arrows and treatments.
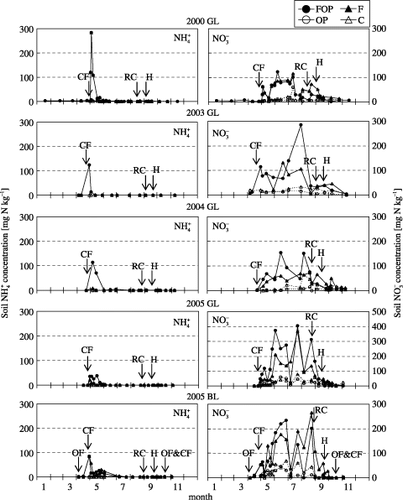
Table 2 Nitrogen application, N2O emission, EFF and EFO in Gray Lowland soil (GL) and Brown Lowland soil (BL)
Figure 5 Relationship between N2O emission and mean temperature and precipitation at the Gray Lowland soil (GL) site. (a) Relationship between the 2-month N2O emission and mean annual air temperature in the chemical nitrogen fertilization and organic matter application, with plants (FOP) and the chemical nitrogen fertilization only, without plants (F) treatments from May to June, (b) relationship between the 2-month N2O emission and precipitation in the FOP and the organic matter application, with plants (OP) treatments from September to October.
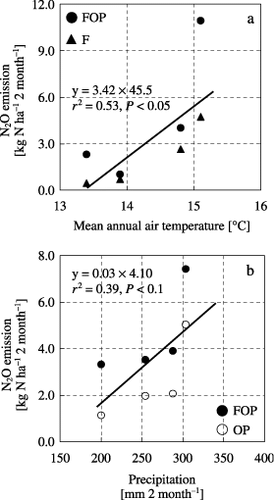
Figure 6 Relationships between emission factors (EF) and climatic conditions. (a) Relationship between the emission factor of N2O associated with the application of chemical nitrogen fertilizer (EFF) and mean annual air temperature, (b) relationship between the emission factor of N2O associated with the application of organic nitrogen (EFO) and mean annual relative humidity. Regression analysis carried out only on the Gray Lowland soil (GL) data.
Emission factors of N2O associated with applied organic matter (EFO)
In this study, we believe that the effect of nitrogen uptake by plants to the N2O emission in FOP and OP treatments may be small. Variation in nitrogen uptake by plants usually increases from June to July (CitationHayashi and Hatano 1999). In 2000 and 2003–2005, variation in nitrogen uptake by plants showed the same pattern as previous studies (data not shown). Although nitrogen uptake by plants increased after June, the variation in nitrate concentration in soil in the F treatment changed in a similar way to FOP. Moreover in the C treatment, soil nitrate concentration also changed in a similar way to OP (). It is clear that the plants assimilated the nitrogen in FOP and OP. However, we do not understand why the concentrations of nitrate in non-planted treatments did not differ from the planted treatments.
Figure 7 Estimated versus the measured N2O emissions in the chemical nitrogen fertilization and organic matter application, with plants (FOP) treatment. •, Gray Lowland soil (GL); ○: Brown Lowland soil (BL).
At GL and BL, EFO values ranged from −5.22 to 9.06% (). At GL, the coefficient of variation (CV; calculated from data in ) of the amount of the N in the applied organic matter was 16% and the CV of the N2O emission in the FOP treatment was 45%. Therefore, change in N2O emission may have had a greater effect on the fluctuation of EFO than did the variation in applied organic matter. In this study, we calculated the EFO based on the difference in N2O emissions in the FOP and F treatments (EquationEq. 4). The EFO appeared to be strongly affected by the N2O emission from the end of August to the end of October because N2O emission originating from chemical fertilizer was released from May to June (,). The N2O-N/NO-N in the FOP treatment was approximately 100 from early September to the end of October, and during this season N2O was likely to be produced mainly by the denitrification process (CitationBouwman 1990). Similar results were reported by CitationKusa et al. (2002, Citation2006). From September to October we found no correlations between N2O flux and soil environmental factors, such as soil temperature, WFPS or soil NH+ 4 and NH− 4 concentrations, although a positive correlation between N2O flux and WFPS has been reported in previous studies (e.g. CitationDobbie and Smith 2003). When the WFPS is approximately 60%, N2O might be produced in soil (CitationGranli and Bockman 1994). In this study, we found no relationship between N2O flux and WFPS, and the reason for this is unclear. However, from September to October, the 2-month N2O emission and 2-month precipitation tended to be positively related, indicating that the time lag between N2O production and diffusion disappeared (). This relationship between 2-month N2O emission and 2-month precipitation from September to October indicated that N2O production was affected by the water conditions of the soil. In addition, EFO and annual mean relative humidity tended to have a positive relationship, with EFO values increasing under wetter climatic conditions (). CO2 flux from soil is seen as an indicator of microbial activity and is affected by temperature, substrate and water conditions. CitationGulledge and Schimel (1998) reported that microbial respiration increased as the ratio of water to the water holding capacity (10–60%) increased. In addition, CitationLinn and Doran (1984) and CitationGulledge and Schimel (1998) reported that CO2 production by microorganisms showed maximum production at 60% WFPS. In this study, because WFPS increased to approxiately 60% from August to October, restriction of the water condition might be lifted and microbial activity, including nitrifier and denitrifier activity, might become high over this period. Therefore, wetter conditions increased the EFo because wetter conditions might not only lead to increases in microbial activity and the supply of nitrogen to the nitrifier and denitrifier, but also to the production of more N2O in the denitrification process.
The EFO differed between GL and BL (−5.22% and 1.51%, respectively) in 2005. One reason for this might be a difference in the denitrification activities between sites GL and BL. From August to September, N2O flux and N2O-N/NO-N did not increase at BL, whereas these values did increase at GL, indicating that the denitrification activity at BL might be weaker than that at GL (). Because organic matter was needed for the denitrification process, stronger denitrification activity at GL would reduce more N2O to N2 in the FOP and OP treatments than F and C treatments. At BL, however, weaker denitrification activity would produce mainly N2O in the denitrification process in all treatments. Furthermore, the difference in the carbon content of the soil between sites GL and BL might affect the denitrification activities. This explains why the N2O emission in the FOP and OP treatments at GL was lower than that in the F and C treatments, resulting in a negative EFO value. Another reason may be the effect of microorganisms in the rhizosphere on N2O production in the FOP and OP treatments. However, we could not confirm this effect because root cutting did not influence N2O fluxes in this study. A further reason might be a difference in the application methods and amounts of organic matter between the sites. Only onion residue was applied to the GL field, whereas at BL half of the applied organic matter was organic fertilizer. However, these effects on the EFO values could not be determined in the present study.
Estimation of N2O emission
The N2O emission estimated using EquationEq. 2 coincided well with the measured values (). Based on data from CitationDobbie et al. (1999), CitationFlynn et al. (2005) estimated the N2O emission from agricultural soils in Scotland using chemical nitrogen fertilizer application rate and the EF as a function of average monthly air temperature or monthly precipitation. In addition, CitationDobbie and Smith (2003) reported that the EF changed with crop type: in grassland EF ranged from 0.4 to 6.5%, while in upland it ranged from 0.5 to 1.5%. CitationHuang et al. (2004) also reported that the EF for N2O emissions that originated from residues was positively affected by the residue C/N ratio. Therefore, it should be noted that our results may be specific to onion fields. Moreover, in this study there was variation in the N2O emission from the C treatment (). The effect of previous cultivation or management of the C plot on N2O emission was not clear. Therefore, further study of the variation of N2O emission from bare soil is necessary to more accurately estimate N2O emissions from agricultural soils based on EF values (CitationAkiyama et al. 2006). In addition, further research into the relationships between EF and meteorological conditions in different land uses would enable estimation of N2O emission at a regional scale and would lead to a better understanding of temporal and spatial variations in N2O emissions.
Conclusions
N2O emissions from soil cultivated with onion showed large temporal variability. In this study, we examined the emission factors for N2O emissions from plots receiving applied chemical nitrogen fertilizer (EFF) or applied organic matter (EFO) and N2O emissions from soil organic matter (control, bare soil). The EFF estimated based on 4 years of measurements in two soil types ranged from 1.3 to 5.5%, which is larger than the emission factor recommended by the Intergovernmental Panel on Climate Change (1.0%). In contrast, the EFO ranged from −5.22 to 9.06%. The two soil types had similar EFF values, but different EFO values. N2O emission derived from applied chemical nitrogen fertilizer appeared to be influenced by nitrification, whereas that derived from applied organic matter appeared to be influenced by denitrification. The EFF was correlated significantly with mean annual temperature and there was a tendency for higher mean annual relative humidity to increase EFO. Thus, it is important to consider denitrification activity in soil when estimating N2O emissions derived from applied organic matter as well as N2O emission associated with soil organic matter decomposition.
ACKNOWLEDGMENTS
This paper was presented at the International Workshop on Monsoon Asia Agricultural Greenhouse Gas Emissions (MAGE-WS), 7–9 March 2006, Tsukuba, Japan. This work was financially supported by the Global Environmental Research Program of the Ministry of the Environment of Japan (No. S-2). The authors thank Takeshi Morimoto and Masahiro Yamazaki for their permission to use their onion fields for this study.
REFERENCES
- Akiyama , H and Tsuruta , H . 2002 . Effect of chemical fertilizer form on N2O, NO and NO2fluxes from Andisol field . Nutr. Cycl. Agroecosyst , 63 : 219 – 230 .
- Akiyama , H , Yan , X and Yagi , K . 2006 . Estimations of emission factors for fertilizer-induced direct N2O emissions from agricultural soils in Japan: summary of available data . Soil Sci. Plant Nutr , 52 : 774 – 787 .
- Anderson , IC and Levine , JS . 1986 . Relative rates of nitric oxide and nitrous oxide production by nitrifiers, denitrifiers, and nitrate respirers . Appl. Environ. Microbiol , 51 : 938 – 945 .
- Bouwman , AF . 1990 . “ Nitric oxide and nitrogen dioxide ” . In Soils and the Greenhouse Gas Effect , Edited by: Bouwman , AF . 120 – 124 . London : John Wiley & Sons .
- Bouwman , AF . 1996 . Direct emission of nitrous oxide from agricultural soils . Nutr. Cycl. Agroecosyst , 46 : 53 – 70 .
- Cochran , VL , Sparrow , EB , Schlentner , SF and Knight , CW . 1997 . Long-term tillage and crop residue management in the subarctic: fluxes of methane and nitrous oxide . Can. J. Soil Sci , 77 : 565 – 570 .
- Dobbie , KE , McTaggart , IP and Smith , KA . 1999 . Nitrous oxide emissions from intensive agricultural systems: variations between crops and seasons, key driving variables, and mean emission factors . J. Geophys. Res , 104 ( D21 ) : 26891 – 26899 .
- Dobbie , KE and Smith , KA . 2003 . Nitrous oxide emission factors for agricultural soils in Great Britain: the impact of soil water-filled pore space and other controlling variables . Glob. Change Biol , 9 : 204 – 218 .
- Flynn , HC , Smith , J , Smith , KA , Wright , J , Smith , P and Massheder , J . 2005 . Climate- and crop-responsive emission factors significantly alter estimates of current and future nitrous oxide emissions from fertilizer use . Glob. Change Biol , 11 : 1522 – 1536 .
- Granli , T and Bockman , OC . 1994 . Nitrous oxide from agriculture . Norw. J. Agric. Sci , 12 : 34 – 39 .
- Gulledge , J and Schimel , JP . 1998 . Moisture control over atmospheric CH4consumption and CO2production in diverse Alaskan soils . Soil Biol. Biochem , 30 : 1127 – 1132 .
- Hayashi , Y and Hatano , R . 1999 . Annual nitrogen leaching to subsurface drainage water from a clayey aquic soil cultivated with onions in Hokkaido, Japan . Soil Sci. Plant Nutr , 45 : 451 – 459 .
- Huang , Y , Zou , J , Zheng , X , Wang , Y and Xu , X . 2004 . Nitrous oxide emissions as influenced by amendment of plant residues with different C:N ratios . Soil Biol. Biochem , 36 : 973 – 981 .
- Intergovernmental Panel on Climate Change2001Climate Change 2001: Synthesis Report WG I, Technical SummaryAvailable from URL: http://www.ipcc.ch/pub/syreng.htm
- Intergovernmental Panel on Climate Change . 2006 . N2O emissions from managed soils, and CO2emissions from lime and urea application In 2006 IPCC Guidelines for National Greenhouse Gas Inventories , Vol. 4 , Agriculture, Forestry and Other Land . Use Available from URL: http://www.ipcc-nggip.iges.or.jp/public/2006gl/vol4.htm
- Kusa , K , Hu , R , Sawamoto , T and Hatano , R . 2006 . Three years of nitrous oxide and nitric oxide emissions from silandic andosols cultivated with maize in Hokkaido, Japan . Soil Sci. Plant Nutr , 52 : 103 – 113 .
- Kusa , K , Sawamoto , T and Hatano , R . 2002 . Nitrous oxide emissions for 6 years from a gray lowland soil cultivated with onions in Hokkaido, Japan . Nutr. Cycl. Agroecosyst , 63 : 239 – 247 .
- Linn , DM and Doran , JW . 1984 . Effect of water-filled pore space on carbon dioxide and nitrous oxide production in tilled and nontilled soils . Soil Sci. Soc. Am. J , 48 : 1267 – 1272 .
- Lipschultz , F , Zafiriou , OC , Wofsy , SC , McElroy , MB , Valois , FW and Watson , SW . 1981 . Production of NO and N2O by soil nitrifying bacteria . Nature , 294 : 641 – 643 .
- Mogge , B , Kaiser , E-A and Munch , J-C . 1999 . Nitrous oxide emissions and denitrification N-losses from agricultural soils in the Bornhöved Lake region: influence of organic fertilizers and land-use . Soil Biol. Biochem , 31 : 1245 – 1252 .
- Mori , A , Hojito , M , Kondo , H , Matsunami , H and Scholefield , D . 2005 . Effects of plant species on CH4and N2O fluxes from a volcanic grassland soil in Nasu, Japan . Soil Sci. Plant Nutr , 51 : 19 – 27 .
- Mosier , A , Kroeze , C , Nevison , C , Oenema , O , Seitzinger , S and van Cleemput , O . 1998 . Closing the global N2O budget: nitrous oxide emissions through the agricultural nitrogen cycle . Nutr. Cycl. Agroecosyst , 52 : 225 – 248 .
- Smith , KA , McTaggart , IP , Dobbie , KE and Conen , F . 1998 . Emissions of N2O from Scottish agricultural soils, as a function of fertilizer N . Nutr. Cycl. Agroecosyst , 52 : 123 – 130 .
- Takakai , F , Morishita , T Hashidoko , Y . 2006 . Effects of agricultural land-use change and forest fire on N2O emission from tropical peatlands, Central Kalimantan, Indonesia . Soil Sci. Plant Nutr , 52 : 662 – 674 .
- Tokuda , S and Hayatsu , M . 2004 . Nitrous oxide flux from a tea field amended with a large amount of nitrogen fertilizer and soil environmental factors controlling the flux . Soil Sci. Plant Nutr , 50 : 365 – 374 .
- Toma , Y and Hatano , R . 2007 . Effect of crop residue C:N ratio on N2O emissions from Gray Lowland soil in Mikasa, Hokkaido, Japan . Soil Sci. Plant Nutr , 53 : 198 – 205 .
- Zou , J , Huang , Y , Lu , Y , Zheng , X and Wang , Y . 2005 . Direct emission factor for N2O from rice-winter wheat rotation systems in southeast China . Atmos. Environ , 39 : 4755 – 4765 .