Abstract
Iron (Fe) deficiency stress is a widespread problem in agriculture and must be overcome to increase crop yields, particularly in calcareous soils. Unlike barley, rice, one of the three major crops in the world, is very susceptible to low Fe availability because of a low capacity to secrete mugineic acid family phytosiderophores (MAs), which are Fe chelators secreted by graminaceous plants. We tested three transgenic rice lines possessing three barley genes involved in MAs synthesis in a field experiment on a calcareous soil under paddy conditions. Two rice lines, one with a barley gene encoding nicotianamine synthase (NAS) and the other with a barley gene encoding a dioxygenase, referred to as Fe-deficiency specific clone no. 3 (IDS3), showed higher tolerance to low Fe availability under these conditions. The rice line with the IDS3 gene also had increased concentrations of Fe and zinc (Zn) in the grains. These results show that introducing barley genes involved in the synthesis of MAs into rice is an effective and practical method to improve agricultural productivity in calcareous soils.
INTRODUCTION
Iron (Fe) deficiency is a widespread agricultural problem that is common in calcareous soils, which account for approximately 30% of the world's cultivated soils (CitationMori 1999). Although Fe is abundant in the soil, Fe availability in calcareous soils is very low because of a high soil pH, which reduces the solubility of ferric iron. Graminaceous plants have a specific uptake mechanism for precipitated ferric iron in soils (CitationMarschner et al. 1986); they secrete metal chelators known as mugineic acid family phytosiderophores (MAs) into the rhizosphere (CitationTakagi 1966, Citation1976; CitationTakagi et al. 1984) and absorb the Fe(III)–MAs complex from the soil through the Fe(III)–MAs complex transporter YS1 (CitationCurie et al. 2001).
Although rice is one of the most important crops in the world, it is much more susceptible to low Fe availability than other graminaceous plants because of a low MAs-secretion capacity (CitationMori et al. 1991). CitationTakagi (1976) discovered MAs by observing that chlorosis of paddy rice was intensified by increasing soil moisture (CitationTakagi 1966). He hypothesized that a certain Fe-chelating substance diffuses from the rhizosphere under submerged conditions.
Rice is well adapted to waterlogged soils in which the concentration of soluble ferrous iron increases with the decrease in soil redox potential. Recently, CitationIshimaru et al. (2006) demonstrated that rice plants are able to take up ferrous iron directly through a ferrous iron transporter, OsIRT1. Therefore, severe Fe deficiency is relatively rare in irrigated rice systems (CitationDobermann and Fairhurst 2000). However, symptoms of Fe deficiency occur even under submerged conditions if farmers do not consider Fe-deficiency-sensitive rice traits or use reclaimed virgin calcareous soils or soils with high salinity.
The MAs are synthesized from methionine (CitationKawai et al. 1988; CitationMori and Nishizawa 1987; CitationShojima et al. 1990; ). The genes that encode all enzymes in the biosynthetic pathway of MAs from barley roots have previously been isolated: HvSAMS (CitationTakizawa et al. 1996), HvNAS1-7 (CitationHiguchi et al. 1999), HvNAAT-A, HvNAAT-B (CitationTakahashi et al. 1999), HvDMAS1 (CitationBashir et al. 2006), HvIDS2 (CitationOkumura et al. 1994), and HvIDS3 (CitationNakanishi et al. 1993).
Figure 1 Biosynthesis pathway of mugineic family phytosiderophores (MAs). DMA, 2′-deoxymugineic acid; DMAS, deoxymugineic acid synthase; epiHDMA, epi-hydroxydeoxymugineic acid; epiHMA, epi-hydroxymugineic acid; HMA, hydroxymugineic acid; IDS2 (IDS3), iron deficiency specific clone no. 2 (3); MA, mugineic acid; NAAT, nicotianamine aminotransferase; NAS, nicotianamine synthase; SAMS, S-adenosyl-methionine synthetase.
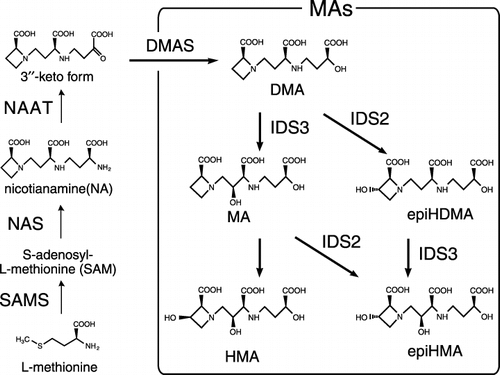
Among the major graminaceous crops, there is a positive correlation between the ability to secrete MAs and tolerance to low Fe availability (CitationRömheld and Marschner 1986; CitationTakagi 1976). In addition, the kind of MAs secreted from roots differs among species. For example, rice secretes 2′-deoxymugineic acid (DMA), whereas barley secretes not only DMA, but also mugineic acid (MA) and epi-hydroxymugineic acid (epiHMA) (CitationMori et al. 1991). Citationvon Wirén et al. (2000) showed that the more hydroxylated MAs have greater Fe(III)-complex stability at slightly acidic pHs. Because the pH of the outer-surface of the plasma membrane is locally acidified as a result of proton extrusion by H+-ATPase, hydroxylated MAs may be more effective at chelating ferric iron from the rhizosphere.
Our previous work has focused on the performance of transgenic rice lines under controlled conditions in a greenhouse. In this work, CitationTakahashi et al. (2001) produced a transgenic rice line possessing the barley NAAT gene with enhanced tolerance to low Fe availability through increased DMA; CitationKobayashi et al. (2001) showed that a transgenic rice line possessing the barley IDS3 gene secreted both DMA and MA; and CitationHiguchi et al. (2001a) produced a transgenic rice line possessing the barley HvNAS1 gene with enhanced NAS activity in Fe-deficient roots. We have also produced a transgenic rice line possessing both the HvNAS1 and HvNAAT genes. The focus of the present study was to evaluate the tolerance of these transgenic rice lines to low Fe availability in a calcareous soil under paddy conditions in the field.
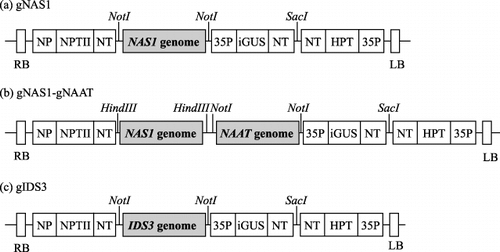
Figure 2 Constructs introduced into the rice lines. The gene region contained a promoter, the gene and a terminator. The NAAT gene contained both HvNAAT-A and HvNAAT-B. HPT, hygromycin phosphotransferase; iGUS, b-glucuronidase with intron; LB, left border; NP, nopaline promoter; NPTII, neomycin phosphotransferase II; NT, nopaline synthase terminator; RB, right border; 35P, cauliflower mosaic virus 35S promoter.
MATERIALS AND METHODS
Establishment of a calcareous paddy field
A calcareous subsoil from Toyama Prefecture (CitationMorikawa et al. 2004) containing fossil shells was used to establish a paddy field in the quarantine area of the Field Science Center of Tohoku University (Osaki, Miyagi, Japan) (39°N; 141°E). In brief, the chemical characteristics of the soil were: pH 9.2, CaCO3 383.5 g kg−1, electrical conductivity (EC) 0.048 dS m−1 (CitationMorikawa et al. 2004). The paddy field was 6 m long × 4 m wide and 0.5 m deep, and the external ridges were completely covered with a vinyl sheet to avoid contamination by the surrounding Andosol at the site. The experiment was conducted from April to October 2006.
Seed preparation
A total of five rice (Oryza sativa L. cv. Tsukinohikari) lines, four being transgenic (), were tested in the field experiment. Each transgene cassette was cloned to pBIGRZ1 (CitationAkiyama et al. 1997) and introduced into rice according to the method developed by CitationHiei et al. (1994). Here, the rice line possessing the 13.5-kb genome fragment of HvNAS1 gene (CitationHiguchi et al. 2001a) was defined as “gNAS1” the line possessing the 7.6-kb genome fragment of HvNAS1 gene and the 11-kb genome fragment of HvNAAT gene was defined as “gNAS1-gNAAT”, the 11-kb genome fragment of HvNAAT was the same as that used by CitationTakahashi et al. (2001); and the line possessing the 20-kb genome fragment of IDS3 gene (CitationKobayashi et al. 2001) was defined as “gIDS3”. The seeds of these transgenic lines used in the experiment were harvested in the previous year from an Andosol paddy field in the quarantine area of Tohoku University. Seeds of gNAS1, harvested from calcareous soil in the previous year, were also used and defined as “gNAS1cal.” Non-transgenic (NT) rice seeds harvested in the previous year from Andosols were used as controls. Seeds with a SG > 1.13 were used, except for those of gIDS3 (specific gravity (SG) > 1.06) because very few gIDS3 seeds were harvested in the previous year. The seeds of transgenic rice lines had been screened with an antibiotic (Hygromycin B; Roche (Basel, Switzerland)) for at least three generations.
Germination and transplanting
Seeds were soaked in a fungicide (Momi guard; Hokuko, Tokyo, Japan) solution overnight and then soaked in water at 20°C for 6 days to break seed dormancy. The incubation temperature was then increased to 30°C overnight to accelerate germination. Germinated seeds were sown in a nursery bed containing mountainous brown soil (pH 5.5) in a tray of 3-mL pots on 3 April 2006. Three seeds were sown per pot, covered with soil, and grown for 45 days in a greenhouse.
Healthy seedlings were transplanted (three per hill) into the calcareous paddy field on 18 May 2006 after gently removing them from the tray and washing them with water to remove the nursery bed soil. No significant differences (P < 0.05) were found among the rice lines at the time of transplanting with respect to plant height, leaf color or tiller number. Plant heights (average ± standard deviation; n = 10) were 21.3 ± 1.0 cm (NT), 21.3 ± 1.1 cm (gNAS1), 22.5 ± 0.3 cm (gNAS1-gNAAT), 22.0 ± 1.5 cm (gIDS3) and 22.9 ± 1.2 cm (gNAS1cal). The soil plant analysis development (SPAD) values used to assess leaf color were 32.4 ± 3.3 (NT), 33.3 ± 1.9 (gNAS1), 30.8 ± 4.1 (gNAS1-gNAAT), 33.5 ± 3.4 (gIDS3) and 33.8 ± 3.0 (gNAS1cal). No new tillers were observed at the time of transplanting (i.e. there were three tillers per hill at the time of transplanting). After transplanting the field was fertilized with 3.8 g of controlled-release NPK-type fertilizer (Long70; Asahi-Kasei Corporation, Tokyo, Japan), corresponding to 180 kg N ha−1, 141 kg P ha−1 and 180 kg K ha−1. Each plot contained five 1.2-m long rows of rice with 20 cm between rows and 15 cm between hills, corresponding to a stand of 33.3 hills m−2. The water in the paddy was maintained at > 6 cm deep throughout the experiment except for the 2 weeks before harvest.
Experimental design and measured parameters
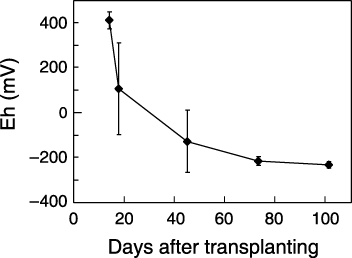
The experimental plots were arranged in a completely randomized design (see ), including four transgenic rice lines (gNAS1, gNAS1-gNAAT, gIDS3 and gNAS1cal) and one wild type (NT), with three replicates of each transgenic line and four replicates of NT. Plant height was measured 16, 25, 30, 37 and 42 days after transplanting (DAT). The leaf color (SPAD value) of the largest leaf was measured 16, 25, 37 and 42 DAT using a SPAD meter (KonicaMinolta, Tokyo, Japan). The number of tillers was counted 16, 25, 30 and 42 DAT. After harvesting, the number of grains, the 1000-grain weight and the grain yield were measured. The concentrations of Fe, Zn, Mn and Cu in the rice grains were also measured using inductively coupled plasma atomic emission spectrometry (SPS1200VR; Seiko, Tokyo, Japan) after digestion of 10 seeds with 1 mL HNO3 and 1 mL H2O2 (Wako, Osaka, Japan) at 200°C for 20 min by MARS XPRESS (CEM Corporation, Matthews, NC, USA). Six hills per plot of each line were used for the above measurements. Soil redox potential (Eh, mV) was measured at 16 points in the experimental field; there were no evident trends in Eh over the experimental site. As the paddy was inundated just after transplanting and the soil was kept submerged until the end of the experiment, Eh decreased over time (). At the time of first measurement (16 DAT), Eh was approximately +400 mV, decreasing to approximately 0 mV 28 DAT and to –200 mV 75 DAT. Differences among transgenic rice lines were tested using anova and subsequent post-hoc comparisons of means were tested using the least significant difference (LSD) test (P = 0.05).
RESULTS
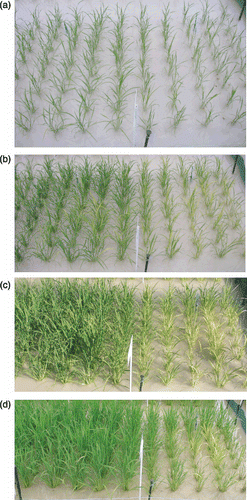
There was good growth of all lines during the first 16 DAT; thereafter, chlorosis was evident in some lines and these lines also grew less vigorously. By 42 DAT, the three transgenic rice lines were clearly superior to NT both in leaf color and growth (), although there were differences in performance in individual plots. Using gIDS3 as an example, there was no evident difference from NT at 16 DAT (), but chlorotic symptoms appeared in NT but not in gIDS3 30 DAT (). The clearest difference between gIDS3 and NT was evident 42 DAT (), but 1 week later (50 DAT), leaf chlorosis began to disappear, especially in NT plants located close to the plot boundary adjacent to the transgenic gIDS3 rice plants ().
Figure 3 Mean soil redox potential (Eh, mV) during the experimental period from 0 to 100 days after transplanting. Error bars represent the standard deviation.
The visible assessment of leaf color was confirmed by the measured SPAD values (). On 16 DAT, the SPAD value of all lines was approximately 30, indicative of green-colored leaves, with no significant difference in leaf color among the lines (P < 0.05). Nine days later (25 DAT), however, the SPAD value of NT decreased to < 25, and the SPAD value of all transgenic rice lines was significantly higher than that of NT. This effect was also evident at 37 DAT, and the SPAD value of the gNAS1-gNAAT line was the highest on both occasions. There was no significant difference in the SPAD value of the lines at 45 DAT, confirming the visual observation of partial recovery in leaf color of NT at 50 DAT ().
Although there was no significant difference (P < 0.05) in plant height among rice lines at 16 DAT (), gNAS1, gNAS1cal and gIDS3 plants were higher than those of NT from 25 DAT. These plants continued to increase in height (5.6–2.8 cm) until 42 DAT, unlike NT plants that did not increase and those of gNAS1-gNAAT increased by only 1.9 cm. At the time of grain harvest, there was no difference in height among the lines (data not shown). The number of tillers per plant 16 and 25 DAT differed among the lines tested, and the number of tillers of gIDS3 was significantly higher than that of the other transgenic rice lines and NT (). By 42 DAT, however, all lines had approximately 15 tillers per plant.
Figure 4 (a) Photograph (42 days after transplanting [DAT]) of the rice lines tested in a paddy field in the quarantine area of the Field Science Center of Tohoku University (Osaki, Miyagi, Japan) (39°N; 141°E) and (b) the field layout. Each population contained five 1.2-m long rows of rice with 20 cm between rows and 15 cm between hills. The box in the upper left indicates the two plots photographed on a number of occasions (Fig. 5). NT, non-transgenic.
![Figure 4 (a) Photograph (42 days after transplanting [DAT]) of the rice lines tested in a paddy field in the quarantine area of the Field Science Center of Tohoku University (Osaki, Miyagi, Japan) (39°N; 141°E) and (b) the field layout. Each population contained five 1.2-m long rows of rice with 20 cm between rows and 15 cm between hills. The box in the upper left indicates the two plots photographed on a number of occasions (Fig. 5). NT, non-transgenic.](/cms/asset/a8d316a4-60f7-408a-9016-d44e02c4b7a4/tssp_a_10382561_o_f0004g.gif)
The heading stage occurred at approximately 100 DAT (25 August) and the plants were harvested on 152 DAT (23 October). The number of grains in gNAS1 was significantly higher (P < 0.05) than the number of grains in NT, and the number of grains tended to be higher in transgenic rice lines compared with NT (). Among all lines, the proportion of fully matured grains ranged from 70 to 80%, with no significant difference among lines (P < 0.05). The 1000-grain weights of both gNAS1 and gNAS1cal were higher than the weight of NT, whereas the weights of gNAS1-gNAAT and gIDS3 were similar to NT. The grain yield of gNAS1 and gNAS1cal tended to be higher than that of NT, but not significantly so (P < 0.05).
The concentrations of Fe and Zn in the rice grains of gIDS3 were significantly higher (P < 0.05) compared with the concentrations in NT (). The Zn concentration of gNAS1-gNAAT was significantly higher than that of NT (P < 0.05), whereas a decrease in Cu concentrations was found in gNAS1-gNAAT compared with NT. There were no significant differences among lines for Mn concentration in the grains.
DISCUSSION
Transgenic rice lines possessing three barley genes involved in MA synthesis that improve Fe nutrition clearly grew better than NT after transplanting into a calcareous paddy field. This was especially evident during early growth (i.e. until 42 DAT) (). Plant heights of gIDS3, gNAS1 and gNAS1cal, the SPAD value of gNAS1-gNAAT, and the tiller number of gIDS3 were higher than those of NT (). During early growth soil Eh was > 0 mV (); the critical redox potential for Fe reduction and consequent dissolution has been reported to be –100 mV at pH 8 (CitationGotoh and Patrick 1974). The calcareous soil used in this experiment had a pH of 9.2; thus, the available ferrous iron may have been insufficient for plant growth. Nevertheless, the transgenic rice grew without exhibiting severe symptoms of chlorosis, suggesting that the MAs secreted from transgenic rice roots contributed to the absorption of ferric iron as an Fe(III)–MAs complex. This is in keeping with the findings of CitationHiguchi et al. (2001a), who showed that gNAS1 secreted more DMA relative to NT under Fe deficiency, and CitationKobayashi et al. (2001), who showed that gIDS3 secreted not only DMA but also MA, which forms a more stable complex with ferric iron than does DMA.
Figure 6 (a) The SPAD value, (b) plant height and (c) tiller number from 15 to 42 days after transplanting of rice lines tested in a paddy field in the quarantine area of the Field Science Center of Tohoku University (Osaki, Miyagi, Japan) (39°N; 141°E). Asterisks indicate statistical significance between non-transgenic (NT) and the transgenic rice lines (P = 0.05).
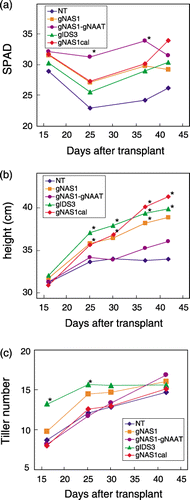
Table 1 Grain yield and yield components of rice lines tested in a paddy field in the quarantine area of the Field Science Center of Tohoku University (Osaki, Miyagi, Japan) (39°N; 141°E)
Table 2 Concentrations of Fe, Zn, Mn and Cu in grains of rice lines tested in a paddy field in the quarantine area of the Field Science Center of Tohoku University (Osaki, Miyagi, Japan) (39°N; 141°E)
Aside from improved Fe uptake, the barley genes introduced into the rice may have increased the translocation of Fe within the plant. CitationHiguchi et al. (2001b) found that expression of the introduced HvNAS1 gene was also inducible in Fe-deficient shoots. Furthermore, NA synthesized by the NAS enzyme plays a role as a carrier in the translocation of ferrous iron (CitationKoike et al. 2004; CitationTakahashi et al. 2003).
In the present experiment, NT initially grew poorly, but growth improved after the soil Eh fell below 0 mV (). This confirmed the observation of CitationMorikawa et al. (2004) that NT grown alone in the same soil used in the present study was severely chlorotic; indeed many seedlings died in the early stages of growth before the Eh fell below 0 mV. In calcareous paddy field soils, therefore, it is crucial for rice to survive in the early stage of growth. It is precisely during this period that increased MAs production and secretion are effective through absorption of ferric iron as an Fe(III)–MAs complex in transgenic lines. Furthermore, MAs may diffuse out of the rhizosphere of individual plants under waterlogged conditions (CitationTakagi 1966). The NT plants growing near gIDS3 were greener than those distant from gIDS3 (), which suggests that NT utilized the MAs secreted by the transgenic rice and this may explain how NT survived in the present experiment. The decrease in soil redox potential with time () suggests that the overall improvement in color of plants of all lines after 42 DAT resulted from the absorption of ferrous iron via the OsIRT1 transporter (CitationIshimaru et al. 2006). This resulted in a similar grain yield of all lines (), despite the clearly inferior performance of NT during early growth.
Interestingly, the concentrations of Fe and Zn in gIDS3 grains were higher than the concentrations in NT grains (), two essential micronutrients often deficient in human nutrition (CitationWelch 2005). Our results suggest that MA synthesized by IDS3 contributed not only to improved Fe absorption from the soil but also to increased translocation to the grain.
In conclusion, we have shown that a transgenic approach to increase the tolerance of rice to low Fe availability is a practical way to improve agricultural productivity in calcareous paddy soils. This strategy may also apply to other graminaceous crops such as upland rice, maize and sorghum that have reduced amounts of MAs secretion. Further opportunities for improving Fe nutrition of crops involve the recently reported novel bHLH transcription factor induced by Fe deficiency (IRO2) in rice and barley (CitationOgo et al. 2006, Citation2007) and the synthetic ferric chelate reductase gene (refre1-372) in rice that has been selected for improved activity at high pH (CitationIshimaru et al. 2007; CitationOki et al. 2004).
ACKNOWLEDGMENTS
We thank Ms Rie Yamamoto for assistance with field management and Dr Seiji Nagasaka, Ms Reiko Itai Nakanishi, Dr Haruhiko Inoue, Dr Yasuhiro Ishimaru, Dr Tomoko Nozoye, Dr Takashi Tsukamoto, Ms Yuko Ogo, Mr Yasuaki Wada, Mr Takahiro Aoyama, Mr Yusuke Kakei, Mr Ippei Ogawa and Ms Kanako Usuda for assistance with the field research. We thank Mr Hiroshi Masuda for measurement of metal concentrations, Dr Bashir Khurram for advice on statistical analysis, and Dr Takanori Kobayashi and Dr Pax Blamey for careful reading of the manuscript.
Notes
These authors contributed equally to this work
REFERENCES
- Akiyama , K , Nakamura , S , Suzuki , T , Wisniewska , I , Sasaki , N and Kawasaki , S . 1997 . Development of a system of rice transformation with long genome inserts for their functional analysis for positional cloning . Plant Cell Physiol. Suppl , 38 : s94
- Bashir , K , Inoue , H Nagasaka , S . 2006 . Cloning and characterization of deoxymugineic acid synthase genes from graminaceous plants . J. Biol. Chem , 43 : 32395 – 32402 .
- Curie , C , Panavience , Z , Loulergue , C , Dellaporta , SL , Briat , JF and Walker , EL . 2001 . Maize yellow stripe1 encodes a membrane protein directly involved in Fe(III) uptake . Nature , 409 : 346 – 349 .
- Dobermann , A and Fairhurst , TH . 2000 . Rice: Nutrient Disorders and Nutrient Management , Singapore : International Rice Research Institute and Potash and Phosphate Institute .
- Gotoh , S and Patric , WH . 1974 . Transformations of iron in a waterlogged soil as affected by redox potential and pH . Soil Sci. Soc. Am. Proc , 38 : 66 – 71 .
- Hiei , Y , Ohta , S , Komari , T and Kumashiro , T . 1994 . Efficient transformation of rice (Oryza sativa L.) mediated by Agrobacterium and sequence analysis of the boundaries of the T-DNA . Plant J , 6 : 271 – 282 .
- Higuchi , K , Suzuki , K , Nakanishi , H , Yamaguchi , H , Nishizawa , NK and Mori , S . 1999 . Cloning of nicotianamine synthase genes, novel genes involved in the biosynthesis of phytosiderophores . Plant Physiol , 119 : 471 – 479 .
- Higuchi , K , Takahashi , M , Nakanishi , H , Kawasaki , S , Nishizawa , NK and Mori , S . 2001a . Analysis of transgenic rice containing barley nicotianamine synthase gene . Soil Sci. Plant Nutr , 47 : 315 – 322 .
- Higuchi , K , Watanabe , S Takahashi , M . 2001b . Nicotianamine synthase gene expression differs in barley and rice under Fe-deficient conditions . Plant J , 25 : 159 – 167 .
- Ishimaru , Y , Kim , S Tsukamoto , T . 2007 . Mutational reconstructed ferric chelate reductase confers enhanced tolerance in rice to iron deficiency in calcareous soil . Proc. Natl Acad. Sci. USA , 104 : 7373 – 7378 .
- Ishimaru , Y , Suzuki , M Tsukamoto , T . 2006 . Rice plants take up iron as an Fe3+-phytosiderophore and as Fe2+ . Plant J , 45 : 335 – 346 .
- Kawai , S , Itoh , K , Takagi , S , Iwashita , T and Nomoto , K . 1988 . Studies on phytosiderophore: biosynthesis of mugineic acid and 2′-deoxymugineic acid in Hordeum vulgare L. var Minorimugi . Tetrahedron Lett , 29 : 1053 – 1056 .
- Kobayashi , T , Nakanishi , H , Takahashi , M , Kawasaki , S , Nishizawa , NK and Mori , S . 2001 . In vivo evidence that Ids3 from Hordeum vulgare encodes a dioxygenase that converts 2′-deoxymugineic acid to mugineic acid in transgenic rice . Planta , 212 : 864 – 871 .
- Koike , S , Inoue , H Mizuno , D . 2004 . OsYSL2 is a rice metal-nicotianamine transporter that is regulated by iron and expressed in the phloem . Plant J , 39 : 415 – 424 .
- Marschner , H , Römheld , V and Kissel , M . 1986 . Different strategies in higher plants in mobilization and uptake of iron . J. Plant Nutr , 9 : 695 – 713 .
- Mori , S . 1999 . Iron acquisition by plants . Curr. Opin. Plant Biol , 2 : 250 – 253 .
- Mori , S and Nishizawa , N . 1987 . Methionine as a dominant precursor of phytosiderophores in Graminaceae plants . Plant Cell Physiol , 28 : 1081 – 1092 .
- Mori , S , Nishizawa , N , Hayashi , H , Chino , M , Yoshimura , E and Ishihara , J . 1991 . Why are young rice plants highly susceptible to iron deficiency? . Plant Soil , 130 : 143 – 156 .
- Morikawa , CK , Saigusa , M , Nakanishi , H , Nishizawa , NK , Hasegawa , K and Mori , S . 2004 . Co-situs application of controlled-release fertilizers to alleviate iron chlorosis of paddy rice grown in calcareous soil . Soil Sci. Plant Nutr , 50 : 1013 – 1021 .
- Nakanishi , H , Okumura , N , Umehara , Y , Nishizawa , NK , Chino , M and Mori , S . 1993 . Expression of a gene specific for iron deficiency (Ids3) in the roots of Hordeum vulgare . Plant Cell Physiol , 34 : 401 – 410 .
- Ogo , Y , Itai , RN Nakanishi , H . 2006 . Isolation and characterization of IRO2, a novel iron-regulated bHLH transcription factor in graminaceous plants . J. Exp. Bot , 57 : 2867 – 2878 .
- Ogo , Y , Itai , RN Nakanishi , H . 2007 . The rice bHLH protein OsIRO2 is an essential regulator of the genes involved in Fe uptake under Fe-deficient conditions . Plant J , 51 : 366 – 377 .
- Oki , H , Kim , S Nakanishi , H . 2004 . Directed evolution of yeast ferric reductase to produce plants with tolerance to iron deficiency in alkaline soils . Soil Sci. Plant Nutr , 50 : 1159 – 1165 .
- Okumura , N , Nishizawa , NK Umehara , Y . 1994 . A dioxygenase gene (Ids2) expressed under iron deficiency conditions in the roots of Hordeum vulgare . Plant Mol. Biol , 25 : 705 – 719 .
- Römheld , V and Marschner , H . 1986 . Evidence for specific uptake system for iron phytosiderophores in roots of grasses . Plant Physiol , 80 : 175 – 180 .
- Shojima , S , Nishizawa , NK , Fushiya , S , Nozoe , S , Irifune , T and Mori , S . 1990 . Biosynthesis of phytosiderophores: in vitro biosynthesis of 2′-deoxymugineic acid from L-methionine and nicotianamine . Plant Physiol , 93 : 1497 – 1503 .
- Takagi , S . 1966 . Studies on the physiological significance of flooded soil condition in rice plant growth – with special reference to flooding-induced chlorosis of rice seedlings , Bulletin of the Institute for Agricultural Research Tohoku University Vol. 18 , 1 – 158 . (in Japanese with English abstract)
- Takagi , S . 1976 . Naturally occurring iron-chelating compounds in oat- and rice-root washings . Soil Sci. Plant Nutr , 22 : 423 – 433 .
- Takagi , S , Nomoto , K and Takemoto , S . 1984 . Physiological aspect of mugineic acid, a possible phytosiderophore of graminaceous plants . J. Plant. Nutr , 22 : 423 – 433 .
- Takahashi , M , Nakanishi , H , Kawasaki , S , Nishizawa , NK and Mori , S . 2001 . Enhanced tolerance of rice to low iron availability in alkaline soils using barley nicotianamine aminotransferase genes . Nature Biotechnol , 19 : 466 – 469 .
- Takahashi , M , Terada , Y Nakai , I . 2003 . Role of nicotianamine in the intracellular delivery of metals and plant reproductive development . Plant Cell , 15 : 1263 – 1280 .
- Takahashi , M , Yamaguchi , H , Nakanishi , H , Shioiri , T , Nishizawa , NK and Mori , S . 1999 . Cloning two genes for nicotianamine aminotransferase, a criticalenzyme in iron acquisition (Strategy II) in graminaceous plants . Plant Physiol , 121 : 947 – 956 .
- Takizawa , R , Nishizawa , NK , Nakanishi , H and Mori , S . 1996 . Effect of iron deficiency on S-adenosyl-methionine synthetase in barley roots . J. Plant. Nutr , 19 : 1189 – 1200 .
- von Wirén , N , Khodr , H and Hider , RC . 2000 . Hydroxylated phytosiderophore species possess an enhanced chelate stability and affinity for iron(III) . Plant Physiol , 124 : 1149 – 1158 .
- Welch , R . 2005 . “ Harvesting health – the future of agriculture ” . In Plant Nutrition for Food Security, Human Health and Environmental Protection , Edited by: Li , CJ , Zhang , FS Dobermann , A . 4 – 5 . Beijing : Tsinghua University Press .
- These authors contributed equally to this work