Abstract
We studied the effects of the application of organic matter (OM) and chemical fertilizer (CF) on soil alkaline phosphatase (ALP) activity and ALP-harboring bacterial communities in the rhizosphere and bulk soil in an experimental lettuce field in Hokkaido, Japan. The ALP activity was higher in soils with OM than in soils with CF, and activity was higher in the rhizosphere for OM than in the bulk soil. Biomass P and available P in the soil were positively related to the ALP activity of the soil. As a result, the P concentration of lettuce was higher in OM soil than in CF soil. We analyzed the ALP-harboring bacterial communities using polymerase chain reaction based denaturing gradient gel electrophoresis (DGGE) on the ALP genes. Numerous ALP genes were detected in the DGGE profile, regardless of sampling time, fertilizer treatment or sampled soil area, which indicated a large diversity in ALP-harboring bacteria in the soil. Several ALP gene fragments were closely related to the ALP genes of Mesorhizobium loti and Pseudomonas fluorescens. The community structures of the ALP-harboring bacteria were assessed using principal component analysis of the DGGE profiles. Fertilizer treatment and sampled soil area significantly affected the community structures of ALP-harboring bacteria. As the DGGE bands contributing to the principal component were different from sampling time, it is suggested that the major bacteria harboring the ALP gene shifted. Furthermore, there was, in part, a significant correlation between ALP activity and the community structure of the ALP-harboring bacteria. These results raise the possibility that different ALP-harboring bacteria release different amounts and/or activity of ALP, and that the structure of ALP-harboring bacterial communities may play a major role in determining overall soil ALP activity.
INTRODUCTION
A major problem with the use of phosphorus (P) fertilizer is the low recovery rate of P from the fertilizer to the plants; only 10–20% of applied P is available to plants in the year of application (CitationHolford 1997; CitationMishima et al. 2006). The majority of applied P is rapidly fixed to the soil, where it is poorly available to plant roots (CitationSanyal and De Datta 1991). Global consumption of P-based fertilizers currently exceeds 30 million tones of P2O5 annually and, thus, the world's known reserves of high-quality rock phosphate will be consumed within the next 80 years (CitationIsherwood 2000). Beyond this time, the production of P-based fertilizers will require the processing of lower-grade rock phosphates at significantly higher cost. Furthermore, as P losses from farm fields have serious environmental and ecological problems, it is necessary to develop better management of P in agricultural systems (CitationTunney et al. 1997). Soil microorganisms have been recognized as playing a major role in many processes involving P transformations in soil (CitationBirch 1961). Using microbial associations to improve the efficiency of plant access to P from soil would be of considerable economic and environmental benefit.
Organic P in soil generally accounts for 30–50% of total soil P (CitationDalal 1978). Plants are unable to take up organic P directly from the soil and phosphatases are required to mineralize organic P to release plant-available P (CitationRichardson 2001). Phosphatases (phosphoric monoester hydrolases) are classified as acid phosphatase or alkaline phosphatase (ALP) by their optimum pH. Plant roots are known to be major producers of acid phosphatase (CitationDinkelaker and Marschner 1992; CitationSpeir and Cowling 1991), but do not produce ALP (CitationNakas et al. 1987; CitationTarafdar and Claassen 1988). Alkaline phosphatase originates from soil bacteria, fungi and fauna (CitationNakas et al. 1987; CitationTarafdar and Claassen 1988). The application of organic matter to soil is known to increase ALP activity (CitationLee et al. 2004; CitationMandal et al. 2007; CitationYang et al. 2006), and CitationMandal et al. (2007) reported that significantly greater ALP activity in organic matter treated soils might be because of the enhanced diversity of phosphate-solubilizing bacteria. To date, there is no clear indication of the relationship between the diversity of ALP-producing bacteria and ALP activity in the soil.
Recently developed molecular biological methods enable the characterization of microbial communities in environmental samples without any culturing step (CitationDobrovol'skaya et al. 2001; CitationKennedy and Gewin 1997; CitationRondon et al. 1999). Denaturing gradient gel electrophoresis (DGGE) with polymerase chain reaction (PCR) amplified genes is now a well-established technique in environmental microbiology (CitationMuyzer 1999; CitationTorsvik et al. 1998). The most widely used target of PCR-DGGE is the 16S rRNA gene because it is essential to all living prokaryotes and the gene is helpful in tracing phylogenetic relationships by referring to a huge accumulated database (e.g. the Ribosomal Database Project II; http://rdp.cme.msu.edu. The PCR-DGGE of amplified 16S rRNA gene is a useful tool for studying the diversity of bacterial communities in the environment. However, as the 16S rRNA gene is not a direct marker for describing “function”, it can be difficult to connect the changes in DGGE profiles with the population dynamics of a certain functional community of bacteria. Furthermore, the limitation of using 16S rRNA gene PCR has been revealed by applying a metagenomic approach, as new taxa were found after the survey by 16S rRNA gene PCR (CitationBaker et al. 2006). Several new trials on PCR-DGGE targeting amplified “functional genes” (e.g. the ammonium monooxygenase gene, amoA; CitationOved et al. 2001) may reduce the inaccuracy of the codon usage. A precondition of using a “functional gene” of bacteria for PCR-DGGE is the dependence on developing a specific primer that can be used to amplify genes from a wide diversity. If an ALP gene is investigated using sequence-based separation techniques like PCR-DGGE on the 16S rRNA gene, the tool could provide important information about the ALP-specific genetic potential of the soil bacterial community and its impact on P turnover.
In the present study we investigated ALP activity in the rhizosphere and established analytical techniques for bacterial communities with ALP activity using a PCR-DGGE method with newly designed primers. We examined the phylogenetic relationships of bacterial ALP genes and analyzed bacterial community structure changes with different soil management.
MATERIALS AND METHODS
Study site and field design
The study field was located at the Hokkaido Central Agricultural Experiment Station, Naganuma, Japan (43°3′Ν, 141°45′Ε). The soil type of the field was classified as Andosol. In 2003, an experimental site was established with the following treatments: chemical fertilizer plot (CF), 140 kg N ha−1 year−1, 52.2 kg P ha−1 year−1 and 100 kg K ha−1 year−1 applied as ammonium sulfate, double superphosphate and potassium sulfate, respectively; organic matter plot (OM), 25 Mg of manure (derived from crop residue) per hectare per year, 2 Mg of rice bran per hectare per year and 1,070 kg of fish meal per hectare per year. The same amount of total N, P and K applied in OM by the organic matter was applied to CF by chemical fertilizer. Each treatment was duplicated with plot sizes of 4.5 m × 6.0 m randomly distributed in the field. The present report is based on results obtained in the fourth year (2006). Fertilizer and organic matter were applied to CF and OM, respectively, on 26 May 2006. Seeds of lettuce (Lactuca sativa L. cv. Mizusawa) were sown on 12 May 2006 and transplanted on 6 June 2006 at 44,400 hills per hectare of planting density. Plants were sampled at 32 and 53 days after fertilizer application (DAF) and harvested on 18 July 2006. Plants were dried in an air-forced oven for 2 days at 65°C to obtain dry weight (DW), then ground for further analysis. Soil was sampled just before fertilizer application (BF) and at 11 DAF from the bulk soil, and at 32 and 53 DAF from both the rhizosphere and bulk soil. One composite soil sample consisting of three cores (15 cm of topsoil) was taken per plot, and subsamples were mixed by sieving. One composite rhizosphere sample was taken per plot and consisted of the roots of three randomly selected plants. The roots were shaken vigorously to separate soil not tightly adhering to the roots. The soil samples for gene analysis were stored at –80°C until the extraction of DNA. For soil chemical analysis, samples were passed through a 2-mm sieve and stored at 4°C. The chemical properties of the soils at BF are summarized in (CitationHokkaido Central Agricultural Experiment Station 1992).
Table 1 Chemical properties of the soil (0–15 cm) before fertilization
Soil ALP activity
The ALP activity was determined using the method described by CitationTabatabai and Bremner (1969). The soil was incubated in a solution with p-nitrophenyl phosphate (p-NPP) and the formation of p-nitrophenol was measured using a spectrophotometer U-3000 (Hitachi, Tokyo, Japan). The ALP activity was evaluated using a modified universal buffer (MUB) at pH 11.
Soil biomass P and available P
Biomass P (CitationBrookes et al. 1982) was calculated from the amount of inorganic P extracted by 0.5 mol L−1 NaHCO3 by subtracting the amount of P extracted from non-fumigated soil from the amount of P extracted from moist soil that had been fumigated with CHCl3 for 24 h. Phosphorus content was determined using the ammonium molybdate–ascorbic acid method (CitationMurphy and Riley 1962). Available P was determined by spectrophotometer (U-3000; Hitachi) using the method described by CitationTruog (1930).
Plant analysis
For the determination of total N and P content in plants, dried samples were digested with 4 mL H2SO4 and 300 g L−1 H2O2 in a Kjeldahl flask. Nitrogen was determined by spectrophotometer using the Indophenol blue method (CitationHorn and Squire 1966) and P was determined by colorimeter using the vanadomolybdate yellow method (CitationBertramson 1942).
DNA extraction
Soil DNA extraction was carried out using ISOIL for Beads Beating (Nippon Gene, Tokyo, Japan). The DNA extracts were purified with the Wizard DNA Clean-Up System (Promega, Madison, WI, USA) as recommended by the manufacturer. Purified DNA was stored at –20°C for PCR-DGGE analyses.
Design of the ALP gene primers
We designed primers to amplify partial fragments encoding ALP from soil DNA. The primers were designed based on an alignment of amino acid and nucleotide sequences encoding an ALP gene derived from various isolates: Bacillus subtilis 168 (database accession number AL009126), Nostoc sp. PCC7120 (BA000019), Caulobacter crescentus CB15 (AE005673), Pseudomonas aeruginosa PAO1 (CP000438), Sinorhizobium meliloti 1021 (AL591688), Mesorhizobium loti MAFF303099 (BA000012) and Corynebacterium glutamicum ATCC13032 (BA000036). The set of primers is ALPS-F730 (5′-CAGTGGGACGACCACGAGGT-3′; M. loti position 730–749) and ALPS-R1101 (5′-GAGGCCGATCGGCATGTCG-3′; M. loti position 1083–1101). A GC clamp (5′-CGC CCG CCG CGC CCCGCG CCC GTC CCG CCG CCC CCG CCC G -3′) was attached to the 5′ end of the ALPS-R1101 to improve the separation of the PCR fragments (CitationMyers et al. 1985).
PCR and DGGE
The PCR was carried out with a Thermal Cycler Dice (Takara Bio, Shiga, Japan) using Takara Ex-Taq (Takara Bio). The reaction mixture was prepared with template DNA (approximately 10 ng), 2 µmol L−1 of each primer, 1 × PCR buffer for Ex-Taq (which included 2 mmol L−1 of MgSO4), 0.2 mmol L−1 of each dNTP, and 0.5 U of Ex-Taq, in a final volume of 20 µL. The thermal cycle profile was as follows: initial denaturation at 94°C for 3 min, 35 cycles of denaturation at 94°C for 1 min, annealing at 57°C for 1 min, extension at 72°C for 2 min, final extension at 72°C for 7 min, and cooling at 4°C.
The DGGE analysis was carried out using a D-Code universal mutation system (Bio-Rad Laboratories, Richmond, CA, USA) according to the instruction manual. The conditions for separation were as follows: running at 90 V for 16 h in a 10% polyacrylamide gel with a denaturing gradient ranging from 40 to 60% at 60°C. After electrophoreses, gels were stained with SYBR Green I nucleic acid gel stain (Molecular Probes, Leiden, The Netherlands) following the manufacturer's instructions, and the bands were visualized with a LumiVisonPRO 400EX (Aishin-Seiki, Nagoya, Japan). Major bands on the gels were excised for sequencing.
Sequencing of DGGE bands
Excised pieces of DGGE gels were washed twice with 1 mL sterilized distilled water in a 1.5 mL tube. A portion of the gel piece (< 1 mm3) was used as the direct template for PCR to recover DNA fragments. The condition for recovering the ALP gene was the same as for the original PCR except that the reverse primer had no GC clamp attached. The PCR products were purified with the MinElute PCR purification kit (Qiagen, Hilden, Germany), ligated into the pGEM-T Vector (Promega) and transformed into ECOS Competent Escherichia coli XL1-Blue (Nippon Gene). The PCR amplification with primers SP6 and T7 was carried out directly on selected white colonies (presumed transformants). Sequencing reactions of selected clones were carried out with a GenomeLab DTCS–Quick Start Kit (Beckman Coulter, Fullerton, CA, USA), with primer T7, 20 µL reaction volume containing 50 fmol DNA template and 2 µmol sequencing primer. Thirty cycles of sequencing reactions (96°C for 20 s, 50°C for 20 s, 60°C for 4 min) were run and the reaction products were analyzed with a CEQ 8000 Genetic Analysis System (Beckman Coulter) after purification by ethanol precipitation.
Phylogenetic analysis
The nucleotide sequences that were determined were evaluated using the BLAST program (CitationAltschul et al. 1997) from the DNA Databank of Japan (DDBJ; Shizuoka, Japan). Sequences were aligned using ClustalW (DDBJ version; http://clustalw.ddbj.nig.ac.jp/top-j.html. Neighbor-joining (NJ) trees (CitationSaitou and Nei 1987) were constructed with ClustalW and drawn using NJplot (CitationPerrière and Gouy 1996; http://pbil.univ-lyon1.fr/software/njplot.html. The sequences generated in the present study have been deposited in the DDBJ database under accession numbers AB306951–AB306969.
Statistical analysis
Soil properties were compared using anova. To characterize the community structure of ALP-harboring bacteria, the values of the DGGE fragments were normalized. Principal component analysis (PCA) and multiple regression analysis were performed using JMP for Macintosh (SAS Institute, Cary, NC, USA).
RESULTS
ALP activity, soil chemical properties and plant analysis
The ALP activity was higher in the rhizosphere soil than in the bulk soil for OM. There was a significant difference in ALP activity between fertilizer application at 32 and 53 DAF ().
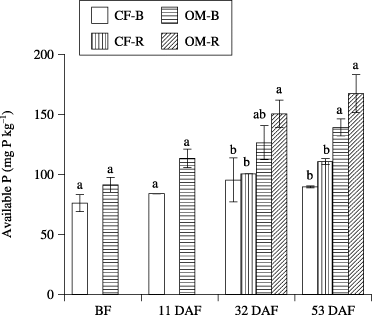
Biomass P generally increased with sampling time, and reached a peak at 32 DAF, and decreased thereafter. Although biomass P in OM was generally higher than in CF at 32 and 53 DAF, the difference was not significant (). Regardless of fertilizer application, available P of the rhizosphere tended to be higher than that of bulk soil. There were significant differences in available P between fertilizer application at 32 and 53 DAF (). There was a significant correlation between ALP activity and available P (r = 0.82, P < 0.001). In addition, there were positive correlations between ALP activity and biomass P (r = 0.42, P = 0.042) and between available P and biomass P (r = 0.51, P = 0.012).
Figure 1 Alkaline phosphatase activity in bulk soil (B) and rhizosphere soil (R) in the chemical fertilizer plot (CF) and organic matter plot (OM). Data are mean ± standard error (n = 2). BF, before fertilization; DAF, days after fertilization. Different letters indicate significant differences (P < 0.05).
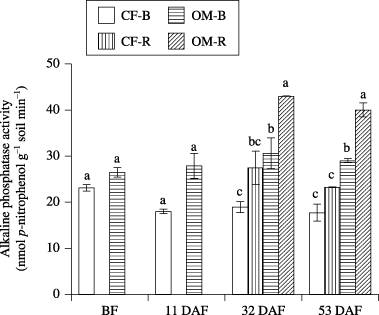
Figure 2 Biomass P in bulk soil (B) and rhizosphere soil (R) in the chemical fertilizer plot (CF) and organic matter plot (OM). Data are mean ± standard error (n = 2). BF, before fertilization; DAF, days after fertilization. Different letters indicate significant differences (P < 0.05).
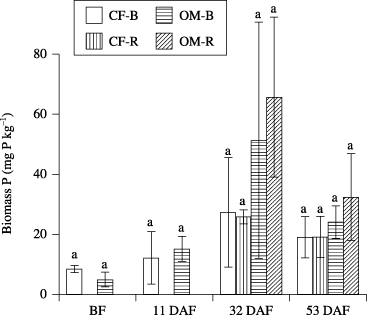
Figure 3 Available P in bulk soil (B) and rhizosphere soil (R) in the chemical fertilizer plot (CF) and organic matter plot (OM). Data are mean ± standard error (n = 2). BF, before fertilization; DAF, days after fertilization. Different letters indicate significant differences (P < 0.05).
There were significant differences in DW and P concentration of lettuce between fertilizer applications. However, there was no significant difference in N concentration of lettuce between fertilizer applications ().
DGGE analysis
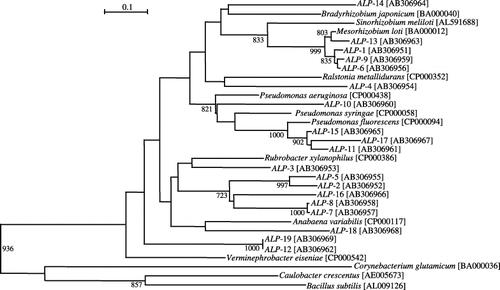
The PCR-DGGE profile of the ALP genes is shown in . From the DGGE profile, 19 bands (ALP-1–ALP-19) were excised and sequenced for phylogenetic analyses. Results of a BLAST search based on the deduced amino acid sequences, ALP-1, ALP-6, ALP-9 and ALP-13, showed a high similarity to the ALP of M. loti MAFF303099 (90, 90, 90 and 94% identity, respectively). The closest sequences to ALP-11, ALP-15 and ALP-17 were the ALP of Pseudomonas fluorescens PfO-1 (87, 88 and 81% identity, respectively). ALP-3, ALP-7, ALP-8 and ALP-18 were most closely related to the ALP gene isolated from Anabaena variabilis ATCC29413 (52, 55, 55 and 51% identity, respectively). ALP-2, ALP-5 and ALP-16 showed a similarity to the ALP of Rubrobacter xylanophilus DSM9941 (57, 58 and 61% identity, respectively). The closest sequences to ALP-12 and ALP-19 were the ALP gene isolated from Verminephrobacter eiseniae EF01–2 (55 and 55% identity, respectively). ALP-10 displayed the closest homology to the ALP gene derived from Pseudomonas syringae pv. phaseolicola 1448A (65% identity). ALP-4 was most closely related to the ALP gene derived from Ralstonia metallidurans CH34 (60% identity). And ALP-14 displayed the closest homology to the ALP gene derived from Bradyrhizobium japonicum USDA 110 DNA (56% identity). The NJ tree was drawn based on deduced amino acid sequences of the DGGE bands and related sequences obtained from the DNA data banks using ClustalW (). The result almost reflected the results of BLAST. With regard to the low identity value, another cluster partly showed it, but it was thought that this was based on a difference between the BLAST and ClustalW algorithms.
Table 2 Dry weight and the P and N concentrations of lettuce
Figure 4 Alkaline phosphatase gene denaturing gradient gel electrophoresis profiles of the bacterial communities using polymerase chain reaction from bulk soil (B) and rhizosphere soil (R) in the chemical fertilizer plot (CF) and organic matter plot (OM). BF, before fertilization; DAF, days after fertilization. Lines are duplicated. M, DGGE Marker II (Nippon Gene, Tokyo, Japan).
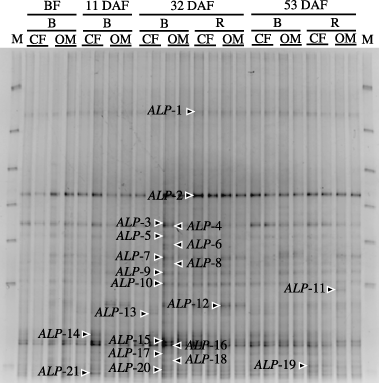
Figure 5 Phylogenetic tree based on alkaline phosphatase (ALP) amino acid sequences derived from ALP gene fragments. The tree was produced using a neighbor-joining algorithm. The bar indicates an estimated 10% sequence divergence. Bootstrap values are given for 1,000 replicate trees and values greater than 70% are indicated. The accession number for each sequence is enclosed in parentheses.
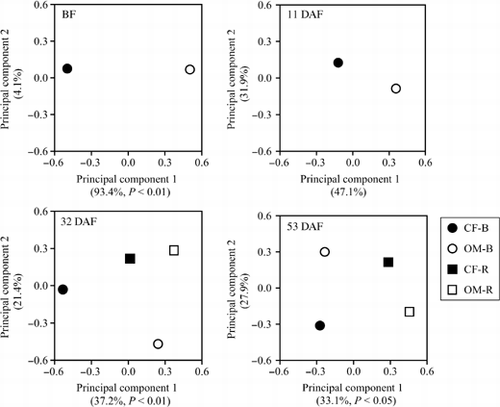
The community structures of ALP-harboring bacteria, based on DGGE profiles, were displayed using PCA (). The first two principal components explained 97.5, 89.0, 58.6 and 61.0% of the variance at BF, 11 DAF, 32 DAF and 53 DAF, respectively. Fertilizer treatment significantly affected the community structures of ALP-harboring bacteria at BF and 32 DAF in terms of principal component 1. In addition, when comparing bulk soil and rhizosphere soil in OM or CF, the community structures of ALP-harboring bacteria were significantly different between the rhizosphere and the bulk soil at 32 and 53 DAF in terms of principal component 1. From correlations between the principal component 1 and DGGE band intensity, the principal component 1 was highly influenced by ALP-2 (r = 0.85), ALP-11 (r = 0.82) and ALP-12 (r = –0.82) at 32 DAF, and by ALP-18 (r = –0.90) and ALP-1 (r = –0.88) at 53 DAF. To investigate the influence of community structure on soil ALP activity, the relationship between the ALP activity and the DGGE profiles of ALP-harboring bacteria was evaluated by multiple regression analysis. The ALP activity was used as the dependent variable, and principal components 1 and 2 of the PCA were used as explanatory variables. The multiple regression model at 32 DAF was significant (adj. R 2 = 0.81, P = 0.007) and was highly influenced by principal component 1 (principal component 1: P = 0.003, principal component 2: P < 0.175), whereas the model at 53 DAF was not significant (adj. R 2 = 0.09, P = 0.338).
DISCUSSION
Effects of organic matter and the rhizosphere on ALP activity
The addition of organic matter to the soil is known to increase the activity of various soil enzymes, such as dehydrogenase, nitrate reductase (CitationCrecchio et al. 2001), β-glucosidase, urease (CitationMadejón et al. 2001) and arginine deaminase (CitationKandeler et al. 1999). Alkaline phosphatase is also known to be induced by the addition of organic matter to the soil (CitationLee et al. 2004; CitationMandal et al. 2007; CitationYang et al. 2006). In this study, ALP activity in OM plots was higher than in CF plots (). Thus, the activity of ALP-harboring bacteria increased in response to organic matter application. There were also higher ALP activities in the rhizosphere for OM than in the bulk soil (); this result is similar to earlier studies (CitationKandeler et al. 2002; CitationTarafdar and Jungk 1987). In the rhizosphere, roots secrete organic compounds, such as sugars, organic acids and polysaccharides, that accumulate and provide energy sources for microbes (CitationWhipps 1990). Thus, increased ALP activity in the rhizosphere for OM suggests that the activity of ALP-harboring bacteria increased because of root exudation even under CF application.
Figure 6 Principal component analysis of denaturing gradient gel electrophoresis profiles of alkaline phosphatase (ALP) bacterial communities. BF, before fertilization; DAF, days after fertilization; B, bulk soil; R, rhizosphere soil. Symbols are the average values of two replicates. The level of variation explained by each principal component is indicated in parentheses. P values are given if there was a significant variety effect determined by anova.
Soil phosphatase activity (including ALP) can be a good indicator of the organic P mineralization potential and biological activity of soils (CitationChen 2003). There were significant positive correlations between ALP activity and the amount of biomass P, and between ALP activity and available P, suggesting that organic P was converted to biomass P and available P by ALP. In addition, the P concentration of lettuce was higher in OM than in CF (). This suggests increased mineralization of organic P from organic matter driven by increased microorganism ALP activity and, thus, plants could extract more available P from the rhizosphere. Part of this rhizosphere effect on ALP activity can be explained by significant negative correlations reported between organic P depletion and phosphatase activity in the rhizosphere (CitationAsmar et al. 1995; CitationTarafdar and Jungk 1987). Furthermore, the contribution of ALP is larger than that of acid phosphatase, although this depends on the soil type (CitationEivazi and Tabatabai 1977). In the present study, there was a tendency for an increase in available P in the rhizosphere, which is consistent with a higher rate of organic P mineralization than plant P uptake (CitationTarafdar and Jungk 1987; ), indicating that microbial biomass and/or activity may play an essential role in the mineralization of organic P, especially in the rhizosphere. Soil microbial biomass is a major sink and source of plant available P and plays a key role in the biochemical transformation of organic P (CitationStewart and Tiessen 1987). Active microbial biomass with a high turnover rate can be a slow-release, sustained source of available P (CitationOberson et al. 2001; CitationSeeling and Zasoski 1993). Biomass P in OM tended to be higher than in CF at 32 and 53 DAF, similar to an earlier study that showed that the microbial biomass P in a manured plot was considerably greater than that in a fertilizer-treated plot (CitationGhoshal and Singh 1995). There was also a positive correlation between biomass P and available P. These results suggest that the application of organic matter increased ALP activity and biomass P and contributed to the supply of available P in the soil.
Phylogenetic analysis of the DGGE profile
We detected many bands in the DGGE profile of ALP genes regardless of sampling time, fertilizer treatment and sampled soil area (). This is consistent with a report that shows that almost half of all microorganisms in soil and on roots can mineralize organic P via phosphatases (CitationTarafdar and Claassen 1988). In addition, the sequenced bands in the profiles were homologous to ALP and they were distributed over a wide range on the phylogenetic tree. Annotated results from several sequenced bands show that they are closely related to M. loti and B. japonicum. These free-living rhizobia are known to respond to P stress by increasing ALP (CitationAl-niemi et al. 1997; CitationSmart et al. 1984). Several sequenced bands had a high similarity with P. fluorescens, which is an indigenous bacteria in the soil (CitationStanier et al. 1966) that is known to generate extracellular ALP (CitationFriedberg and Avigad 1967). Pseudomonas syringae was also annotated in this experiment and is also known to release extracellular ALP (CitationCheng et al. 1970). As all of the excised bands showed high homology with an alkaline phosphatase gene, these results indicate that the primer set used in the present study had specificity for amplifying ALP genes from soil. This is the first report to apply PCR-DGGE analysis to ALP genes in environmental samples. As the designed primer is based on a limited number of species, further verification of the primer set will be necessary in future. However, it is suitable to monitor bacterial communities containing ALP-harboring bacteria, and for discovering new ALP genes in natural environments.
Community structures of ALP-harboring bacteria
Organic matter is reported to alter bacterial communities (CitationMarschner et al. 2003; CitationSun et al. 2004); however, there are few reports about specific functions in bacterial communities. The PCA of the ALP gene DGGE profiles indicated that community structures were affected by fertilizer type (). The fertilizer treatment significantly affected bacterial community structures harboring ALP genes at 32 DAF. There were significant differences in ALP activity between CF and OM at 32 DAF, and it is possible that the community structures were involved. The community structures of ALP-producing bacteria were significantly different between the rhizosphere and the bulk soil at 32 and 53 DAF, whereas a significant difference in ALP activity was recognized only in the OM treatment between rhizosphere and bulk soil areas at both sampling times. Decreasing activity of ALP with increasing distance from the root surface may be associated with changes in bacterial community structure (CitationKandeler et al. 2002), and microbial communities in the rhizosphere are affected by root exudates (CitationDunfield and Germida 2003; CitationTesfaye et al. 2003). Thus, the present results also suggest a relationship between the type of fertilizer and root exudates. Further research is underway to determine whether the OM treatment changes the composition and/or quantity of root exudates. As the DGGE bands contributing to principal component 1 in 32 DAF were different from 53 DAF, it is suggested that major bacteria harboring an ALP gene changed with sampling time.
Changes in overall microbial community structure can be correlated with changes in certain functions (CitationAvrahami et al. 2003; CitationKandeler et al. 2002), and CitationMarschner et al. (2003, Citation2005) reported that bacterial community structure was correlated with ALP activity in the field. The link between microbial community structure and function is often weak because many ecosystem functions are carried out by a wide range of microbes that form substrate guilds (CitationZak et al. 1994). Multiple regression analysis between ALP activity and the PCA analysis of ALP gene DGGE profiles suggest that ALP activity is significantly explained by principal component 1 at 32 DAF, which contains the majority of the variance in the community structure. This raises the possibility that different ALP-harboring bacteria release different amounts and/or activities of ALP, and that the structure of ALP-harboring bacterial communities may play a major role in determining soil ALP activity. At 32 DAF, ALP activity and biomass P were at maximum values, indicating that microbial activity was also at a maximum.
This study showed that organic matter application had a major impact on ALP activity and the structure of ALP-harboring bacterial communities. The ALP-harboring bacterial community structure was in part significantly correlated with ALP activity. Further research on the characterization of the community structure will help explain the change in ALP activity.
ACKNOWLEDGMENTS
This research has been partly supported by a grant from the Northern Advancement Center for Science and Technology (NOASTEC Foundation).
REFERENCES
- Al-niemi , TS , Summers , ML , Elkins , JG , Kahn , ML and Mcdermott , TR . 1997 . Regulation of the phosphate stress response in Rhizobium meliloti by PhoB . Appl. Environ. Microbiol , 63 : 4978 – 4981 .
- Altschul , SF , Madden , TL Schäffer , AA . 1997 . Gapped BLAST and PSI-BLAST: a new generation of protein database search programs . Nucleic Acids Res , 25 : 3389 – 3402 .
- Asmar , F , Gahoonia , TS and Nielsen , NE . 1995 . Barley genotypes differ in activity of soluble extracellular phosphatase and depletion of organic phosphorus in the rhizosphere soil . Plant Soil , 172 : 117 – 122 .
- Avrahami , S , Liesack , W and Conrad , R . 2003 . Effects of temperature and fertilizer on activity and community structure of soil ammonia oxidizers . Environ. Microbiol , 5 : 691 – 705 .
- Baker , BJ , Tyson , GW Webb , RI . 2006 . Lineages of acidophilic archaea revealed by community genomic analysis . Science , 314 : 1933 – 1935 .
- Bertramson , BR . 1942 . Phosphorus analysis of plant material . Plant Physiol , 17 : 447 – 454 .
- Birch , HF . 1961 . Phosphorus transformations during plant decomposition . Plant Soil , 4 : 347 – 366 .
- Brookes , PC , Powlson , DS and Jenkinson , DS . 1982 . Measurement of microbial biomass phosphorus in soil . Soil Biol. Biochem , 14 : 319 – 329 .
- Chen , H . 2003 . Phosphatase activity and P fractions in soils of an 18-year-old Chinese fir (Cunninghamia lanceolata) plantation . For. Ecol. Manage , 178 : 301 – 310 .
- Cheng , K-J , Ingram , JM and Costerton , JW . 1970 . Release of alkaline phosphatase from cells of Pseudomonas aeruginosa by manipulation of cation concentration and of pH . J. Bacteriol , 104 : 748 – 753 .
- Crecchio , C , Curci , M , Mininni , R , Ricciuti , P and Ruggiero , P . 2001 . Short-term effects of municipal solid waste compost amendments on soil carbon and nitrogen content, some enzyme activities and genetic diversity . Biol. Fertil. Soils , 34 : 311 – 318 .
- Dalal , RC . 1978 . Organic phosphorus . Adv. Agron , 29 : 83 – 117 .
- Dinkelaker , B and Marschner , H . 1992 . In vivo demonstration of acid phosphatase activity in the rhizosphere of soil-grown plants . Plant Soil , 144 : 199 – 205 .
- Dobrovol'skaya , TG , Lysak , LV , Zenova , GM and Zvyagintsev , DG . 2001 . Analysis of soil bacterial diversity: methods, potentiality, and prospects . Microbiol , 70 : 119 – 132 .
- Dunfield , KE and Germida , JJ . 2003 . Seasonal changes in the rhizosphere microbial communities associated with field-grown genetically modified canola (Brassica napus . Appl. Environ. Microbiol , 69 : 7310 – 7318 .
- Eivazi , F and Tabatabai , MA . 1977 . Phosphatase in soils . Soil Biol. Biochem , 9 : 167 – 172 .
- Friedberg , I and Avigad , G . 1967 . Some properties of alkaline phosphatase of Pseudomonas fluorescens . Eur. J. Biochem , 1 : 193 – 198 .
- Ghoshal , N and Singh , KP . 1995 . Effects of farmyard manure and inorganic fertilizer on the dynamics of soil microbial biomass in a tropical dryland agroecosystem . Biol. Fertil. Soils , 19 : 231 – 238 .
- Hokkaido Central Agricultural Experiment Station . 1992 . Diagnostic Standard of Soil and Crop Nutrition , 2nd edn , Naganuma : Hokkaido Central Agricultural Experiment Station . (in Japanese)
- Holford , ICR . 1997 . Soil phosphorus: its measurement, and its uptake by plants . Aust. J. Soil Res , 35 : 227 – 239 .
- Horn , DB and Squire , CR . 1966 . The estimation of ammonia using the indophenol blue reaction . Clin. Chim. Acta , 14 : 185 – 194 .
- Isherwood , KF . The state of the fertilizer industry past, present and future . Proc. of the 68th International Fertilizer Industry (IFA) Annual Conference . May 22–25 2000 , Oslo, Norway. pp. 1 – 2 . Annual General Meeting
- Kandeler , E , Marschner , P , Tscherko , D , Gahoonia , TS and Nielsen , NE . 2002 . Microbial community composition and functional diversity in the rhizosphere of maize . Plant Soil , 238 : 301 – 312 .
- Kandeler , E , Stemmer , M and Klimanek , E-M . 1999 . Response of soil microbial biomass, urease and xylanase within particle size fractions to long-term soil management . Soil Biol. Biochem , 31 : 261 – 273 .
- Kennedy , AC and Gewin , VL . 1997 . Soil microbial diversity: present and future considerations . Soil Sci , 162 : 607 – 617 .
- Lee , JJ , Park , RD Kim , YW . 2004 . Effect of food waste compost on microbial population, soil enzyme activity and lettuce growth . Bioresour. Technol , 93 : 21 – 28 .
- Madejón , E , Burgos , P , López , R and Cabrera , F . 2001 . Soil enzymatic response to addition of heavy metals with organic residues . Biol. Fertil. Soils , 34 : 144 – 150 .
- Mandal , A , Patra , AK , Singh , D , Swarup , A and Masto , RE . 2007 . Effect of long-term application of manure and fertilizer on biological and biochemical activities in soil during crop development stages . Bioresour. Technol , 98 : 3585 – 3592 .
- Marschner , P , Grierson , PF and Rengel , Z . 2005 . Microbial community composition and functioning in the rhizosphere of three Banksia species in native woodland in Western Australia . Appl. Soil Ecol , 28 : 191 – 201 .
- Marschner , P , Kandeler , E and Marschner , B . 2003 . Structure and function of the soil microbial community in a long-term fertilizer experiment . Soil Biol. Biochem , 35 : 453 – 461 .
- Mishima , S , Taniguchi , S and Komada , M . 2006 . Recent trends in nitrogen and phosphate use and balance on Japanese farmland . Soil Sci. Plant Nutr , 52 : 556 – 563 .
- Murphy , J and Riley , JP . 1962 . A modified single solution method for the determination of phosphate in natural waters . Anal. Chim. Acta , 27 : 31 – 36 .
- Muyzer , G . 1999 . DGGE/TGGE a method for identifying genes from natural ecosystems . Curr. Opin. Microbiol , 2 : 317 – 322 .
- Myers , RM , Fischer , SG , Lerman , LS and Maniatis , T . 1985 . Nearly all single base substitutions in DNA fragments joined to a GC-clamp can be detected by denaturing gradient gel electrophoresis . Nucleic Acids Res , 13 : 3131 – 3145 .
- Nakas , JP , Gould , WD and Klein , DA . 1987 . Origin and expression of phosphatase activity in a semi-arid grassland soil . Soil Biol. Biochem , 19 : 13 – 18 .
- Oberson , A , Friesen , DK , Rao , IM , Bühler , S and Frossard , E . 2001 . Phosphorus transformations in an oxisol under contrasting land-use systems: The role of the soil microbial biomass . Plant Soil , 237 : 197 – 210 .
- Oved , T , Shaviv , A , Goldrath , T , Mandelbaum , RT and Minz , D . 2001 . Influence of effluent irrigation on community composition and function of ammonia-oxidizing bacteria in soil . Appl. Environ. Microbiol , 67 : 3426 – 3433 .
- Perrière , G and Gouy , M . 1996 . WWW-query: an on-line retrieval system for biological sequence banks . Biochimie , 78 : 364 – 369 .
- Richardson , AE . 2001 . Prospects for using soil microorganisms to improve the acquisition of phosphorus by plants . Aust. J. Plant Physiol , 28 : 897 – 906 .
- Rondon , MR , Goodman , RM and Handelsman , J . 1999 . The earth's bounty: Assessing and accessing soil microbial diversity . Trends Biotechnol , 17 : 403 – 409 .
- Saitou , N and Nei , M . 1987 . The neighbor-joining method: a new method for sequencing phylogenetic trees . Mol. Biol. Evol , 4 : 406 – 425 .
- Sanyal , SK and De Datta , SK . 1991 . Chemistry of phosphorus transformations in soil . Adv. Soil Sci , 16 : 1 – 120 .
- Seeling , B and Zasoski , RJ . 1993 . Microbial effects in maintaining organic and inorganic solution phosphorus concentrations in a grassland topsoil . Plant Soil , 148 : 277 – 284 .
- Smart , JB , Dilworth , MJ and Robson , AD . 1984 . Effect of phosphorus supply on phosphate uptake and alkaline phosphatase activity in Rhizobia . Arch. Microbiol , 140 : 281 – 286 .
- Speir , TW and Cowling , JC . 1991 . Phosphatase activities of pasture plants and soils: relationship with plant productivity and soil P fertility indices . Biol. Fertil. Soils , 12 : 189 – 194 .
- Stanier , RY , Palleroni , NJ and Doudoroff , M . 1966 . The aerobic pseudomonads: a taxonomic study . J. Gen. Microbiol , 43 : 159 – 271 .
- Stewart , JWB and Tiessen , H . 1987 . Dynamics of soil organic phosphorus . Biogeochemistry , 4 : 41 – 60 .
- Sun , HY , Deng , SP and Raun , WR . 2004 . Bacterial community structure and diversity in a century-old manure-treated agroecosystem . Appl. Environ. Microbiol , 70 : 5868 – 5874 .
- Tabatabai , MA and Bremner , JM . 1969 . Use of p-nitrophenyl phosphate for assay of soil phosphatase activity . Soil Biol. Biochem , 1 : 301 – 307 .
- Tarafdar , JC and Claassen , N . 1988 . Organic phosphorus compounds as a phosphorus source for higher plants through the activity of phosphatase produced by plant roots and microorganisms . Biol. Fertil. Soils , 5 : 308 – 312 .
- Tarafdar , JC and Jungk , A . 1987 . Phosphatase activity in the rhizosphere and its relation to the depletion of soil organic phosphorus . Biol. Fertil. Soils , 3 : 199 – 204 .
- Tesfaye , M , Dufault , NS , Dornbusch , MR , Allan , DL , Vance , CP and Samac , DA . 2003 . Influence of enhanced malate dehydrogenase expression by alfalfa on diversity of rhizobacteria and soil nutrient availability . Soil Biol. Biochem , 35 : 1103 – 1113 .
- Torsvik , V , Daae , FL , Sandaa , R-A and Øvreås , L . 1998 . Novel techniques for analysing microbial diversity in natural and perturbed environments . J. Biotechnol , 64 : 53 – 62 .
- Truog , E . 1930 . The determination of readily available phosphorus in soils . J. Am. Soc. Agr , 23 : 874 – 882 .
- Tunney , H , Breeuwsma , A , Withers , PJA and Ehlert , PAI . 1997 . “ Phosphorus fertilizer strategies: Present and future ” . In Phosphorus Loss from Water to Soil , Edited by: Tunney , H , Carton , OT , Brookes , PC and Johnston , AE . 117 – 203 . Wallingford : CAB International Publications .
- Whipps , JM . 1990 . “ Carbon economy ” . In The Rhizosphere , Edited by: Lynch , JM . 59 West Sussex : Wiley & Son .
- Yang , C , Yang , L and Jianhua , L . 2006 . Organic phosphorus fractions in organically amended paddy soils in continuously and intermittently flooded conditions . J. Environ. Qual , 35 : 1142 – 1150 .
- Zak , JC , Willig , MR , Moorhead , DL and Wildman , HG . 1994 . Functional diversity of microbial communities: a quantitative approach . Soil Biol. Biochem , 26 : 1101 – 1108 .