Abstract
The primers PCN280f and NEPCN398r were designed for the quantitative detection of the potato-cyst nematode Globodera rostochiensis using real-time polymerase chain reaction (PCR). One, five, 50, 125 and 250 individuals of the second-stage juveniles (J2) of G. rostochiensis were mixed with various stages of vermiform Caenorhabditis elegans to make a total of 500 individuals and DNA was extracted from the nematode mixture. There was a significant correlation (r 2 = 0.9355, P < 0.001) between the threshold cycle values and the number of G. rostochiensis added. When nematodes were extracted from soils artificially infested with G. rostochiensis to various degrees and real-time PCR was conducted using DNA templates from the nematodes extracted, there was a highly significant correlation in the numbers of G. rostochiensis J2 from the real-time PCR method and morphological identification. Real-time PCR sensitively detected a single G. rostochiensis J2 out of 1,000 individuals of free-living nematodes. Similarly, real-time PCR primers RKNf and RKNr were designed for the detection of the root-knot nematode Meloidogyne incognita. This study demonstrated that the real-time PCR assay for the potato-cyst nematode and the root-knot nematode provides a sensitive and reliable means for the rapid quantification of these vermiform pests.
INTRODUCTION
Plant-parasitic nematodes sometimes cause serious damage to different kinds of crops. It has been accepted for decades that the effective control of plant-parasitic nematodes is dependent on agrochemicals (CitationHague and Gowen 1987). However, there is a growing concern about the use of agrochemicals. As it is known that there is a significant correlation between the initial population density of plant-parasitic nematodes in soil and the degree of damage to the host (e.g. CitationBack et al. 2006; CitationChikaoka 1983; CitationGugino et al. 2006; CitationKoenning 2000; CitationOhbayashi 1989), reliable and rapid identification and counts of the causal agent is critically important for the successful management of nematode pests. CitationMadani et al. (2005) emphasized that precise identification and knowledge about the number of nematodes in field soil are necessary to develop effective integrated pest control and reported the real-time polymerase chain reaction (PCR) primers for Globodera pallida and Heterodera shachtii. Recently, the real-time PCR primers were reported for Meloidogyne chitwoodi and Meloidogyne fallax (CitationZijlstra and van Hoof 2006) and the root-lesion nematode Pratylenchus penetrans (CitationSato et al. 2007). However, real-time PCR primers are not available for other important plant-parasitic nematodes, such as the potato-cyst nematode Globodera rostochiensis and the root-knot nematode Meloidogyne incognita. Thus, the purpose of this study was to develop a rapid and precise method for the detection and quantification of G. rostochiensis and M. incognita from mixed nematode communities using real-time PCR.
MATERIALS AND METHODS
Soils and nematodes used
A soil naturally infested with the potato-cyst nematode G. rostochiensis was collected from a farmland in Hokkaido, Japan. From the soil, second-stage juveniles (J2) and cysts of G. rostochiensis were collected using the Baermann method and the double-layer centrifugation technique, respectively, based on our previous report (CitationSato and Toyota 2006). Caenorhabditis elegans was maintained on agar plates inoculated with Escherichia coli JM109 and used to adjust the number of nematodes in real-time PCR. Eggs of the root-knot nematode M. incognita were collected from the diseased roots of sweet potato grown in a field and individuals of M. incognita J2 were collected from the eggs using the Baermann method (20–25°C for 2 days). Pratylenchus penetrans J2 was collected from a soil naturally infested with the root-lesion nematode using the Baermann method and was used as a reference in the primer check.
Primers
Primers in real-time PCR should be designed to produce 100–200 bp (Takara Bio, Otsu, Japan). Specific primers designed for G. rostochiensis by CitationBulman and Marshall (1997) produce more than 250 bp of PCR product and those for M. incognita designed by CitationSaeki et al. (2003) produce more than 500 bp. Therefore, these primers are suitable for specific detection, but not for quantitative detection using real-time PCR. Thus, new specific primers were designed in the ITS1 region for G. rostochiensis (PCN280f [5′-GCGTCGTTGAGCGGTTGTT-3′]– PCN398r [5′-CCACGGACGTAGCACACAAG-3′]) and for M. incognita (RKNf [5′-GCTGGTGTCTAAGTGTTGCTGATAC-3′]– RKNr [5′-GAGCCTAGTGATCCACCGATAAG-3′]). According to the ITS sequences available in the database National Center for Biotechnology (NCBI), the primer sequences of PCN280f and PCN398r showed a perfect match to Globodera pallida, Globodera tabacum, Heterodera hordecalis, Heterodera latipons, Heterodera ustinovi, Heterodera zeae and those of RKNr and RKNr perfectly matched with Meloidogyne javanica and Meloidogyne arenaria. The specific primers for M. incognita were considered not to amplify Meloidogyne hapla because of a one base difference in the second position from the 3′ end of the forward primer. A preliminary experiment showed that the specific primers designed for G. rostochiensis (PCN280f and PCN398r) did not react to M. incognita J2 and P. penetrans J2 and those designed for M. incognita (RKNf and RKNr) did not react to P. penetrans J2 and G. rostochiensis J2.
To estimate the potential for other nematodes to be counted as G. rostochiensis in real-time PCR assay, nematodes were extracted from an agricultural soil in Kanagawa prefecture, which is considered not to contain G. rostochiensis, using the Baermann method, and real-time PCR was done to detect G. rostochiensis, using 200, 500 and 1,000 individual nematodes in triplicate as described below.
DNA extraction from nematodes
The DNA was extracted from approximately 200–1,000 individual nematodes using the modified method of CitationSato and Toyota (2006). Three hundred microliters of a nematode suspension was put into a 2-mL tube with 0.2 g of zirconia beads (0.1 mm in diameter) and then 30 µL of 10× TE (10 mM Tris-HCl (pH 8.0), 1 mM EDTA (pH 8.0)) buffer (pH 8.0) was added. The nematode suspension was treated with bead beading at 2,770 g for 90 sec two times (Bead-smash 12; Wakenyaku, Kyoto, Japan) and the tube was placed on ice. Fifty microliters of skim-milk solution (200 mg mL−1) was added to the tube, along with 30 µL of 3 mol L−1 sodium acetate, 200 µL of extraction buffer (5 mol L−1 NaCl, 0.5 mol L−1 Tris-HCl [pH 8.0], and 0.5 mol L−1 ethylenediaminetetraacetic acid [pH 8.0]) and 500 µL of chloroform and mixed well. After centrifugation for 15 min at 4°C at 20,350 g (3740; Kubota, Osaka, Japan), 400 µL of the supernatant was transferred to a new tube with 5 µL of glycogen solution (5 mg mL−1), 80 µL of 3 mol L−1 sodium acetate and 600 µL of isopropanol. Four hundred microliters of distilled water was added to the tube, mixed well and then centrifuged for 15 min at 4°C at 20,350 g. Four hundred microliters of this supernatant was combined with the first 400 µL of the supernatant. The DNA was precipitated by centrifugation for 15 min at 4°C at 20,350 g, washed with 70% ethanol once and air-dried. Finally, the DNA was dissolved in TE buffer to make a concentration of one individual nematode per 1 µL.
Real-time PCR
Real-time PCR was carried out using a Smart Cycler II (Cepheid; Takara Bio) in a final volume of 25 µL containing 12.5 µL of SYBR Premix Ex TaqTM (Perfect Real Time; Takara Bio), 5 µmol L−1 of each primer and 5 µL of template DNA using the manufacturer's recommended conditions (95°C for 10 s, [95°C for 5 s and 60°C for 30 s] × 45 cycles). A single real-time PCR was done in each DNA extract and means from three to six replicates of DNA extract were calculated as threshold cycle number (Ct) values. A negative control was also prepared in triplicate using distilled water instead of a DNA template.
Application of real-time PCR
Autoclaved Koganei soil (475 g non-infested soil [dry basis]) was put into a 1-L pot, mixed with 5% (w/w) of soil artificially infested with G. rostochiensis and 50 g (dry basis) of OC (Okara and coffee residue) compost was added (pH (H2O) (1:5), 6.0; electrical conductivity (1:10), 0.35 S m−1; water content, 353 g kg−1 fresh weight; ash content, 70.7 g kg−1; total N, 55 g kg−1; P2O5, 12 g kg−1; K2O, 16 g kg−1; CaO, 3.9 g kg−1; MgO, 2.9 g kg−1; Na2O, 0.2 g kg−1). The soil–compost mixture was adjusted so that the moisture content was 60% of the maximum water-holding capacity and incubated at 20°C (day : night = 16:8 h). Four replicate pots were prepared. After 3 weeks, nematodes were extracted using the Baermann method in triplicate per pot and the DNA was extracted based on the method described above from nematode suspensions in triplicate per pot.
Statistics
The significance of correlation coefficients and differences among mean values were analyzed using correlation analysis and anova protected by Fisher's range test (P < 0.05), respectively, using Excel statistics 2002 software (Social Survey Research Information, Tokyo, Japan).
RESULTS AND DISCUSSION
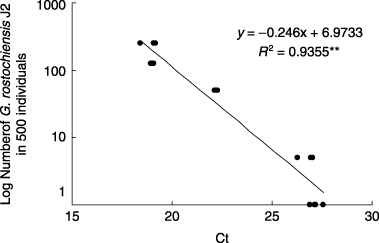
Although the Ct value (±standard deviation [SD]) in distilled water was 36.8 ± 0.6, the values in nematode suspensions of 500 individuals containing 1, 5, 50, 125 and 250 individuals of G. rostochiensis J2 were 27.2 ± 0.3, 26.8 ± 0.4, 22.2 ± 0.1, 19.0 ± 0.1 and 18.9 ± 0.4, respectively (). There was a highly significant correlation (r 2 = 0.9355, P < 0.01) between the Ct values and the number of G. rostochiensis J2. A single J2 of G. rostochiensis was mixed with 500 and 1,000 individuals of various stages of vermiform C. elegans and DNA was extracted from the mixtures. Although the Ct value of 200 individuals of C. elegans was 34.4 ± 0.4, the values in 500 and 1,000 individuals of C. elegans containing a single J2 of G. rostochiensis were 27.2 ± 0.3 and 27.6 ± 0.6, respectively, and the difference in the Ct values between the presence and absence of a single G. rostochiensis was significant (P < 0.05). This sensitive detection was further supported by the melting temperatures for amplicons, 86.4 ± 0.1°C, in nematode samples containing an individual of G. rostochiensis J2 compared with the temperatures, 82.9 ± 0.2°C, in nematode samples consisting of only C. elegans. The Ct values of 200, 500 and 1,000 individuals of nematode communities in a soil were 42.6 ± 1.2, 36.1 ± 0.7 and 34.7 ± 1.9, respectively. The Ct values tended to be lower with higher numbers of nematodes, but were markedly greater in samples containing a single J2 of G. rostochiensis (27.2–27.6). Thus, it was considered that nematodes other than G. rostochiensis may not be counted as G. rostochiensis in this real-time PCR assay.
Figure 2 Relationship between the numbers of Globodera rostochiensis J2 estimated from morphological identification and from real-time polymerase chain reaction (PCR). **P < 0.01.
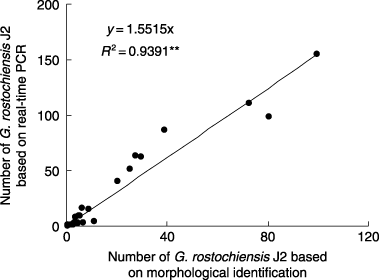
The number of G. rostochiensis J2 was estimated based on morphological identification and real-time PCR using the same nematode suspensions collected from soils differently infested with the nematode. There was a significant correlation (; r 2 = 0.9391, P < 0.01, n = 26) between the number of G. rostochiensis J2 estimated from the equation in in the real-time PCR assay and the number based on morphological identification, although the slope was 1.6 rather than 1. One of the possible reasons for this is a difference in the body size of G. rostochiensis J2. Although nematodes with a larger size showed lower Ct values (CitationSato et al. 2007), we regard a nematode, irrespective of the body size. Another possible reason is experimental error because the correlation equation in is based on log-transformed data on both the x-axis and the y-axis. Therefore, the equation may better describe the difference in orders of magnitude, but may not be sensitive enough for smaller differences. However, the exact reason for the discrepancy between the estimates using real-time PCR and morphological identification remains to be identified.
Figure 3 Real-time polymerase chain reaction assay for 500 individuals in nematode mixtures containing different numbers of Meloidogyne incognita J2 and various stages of Caenorhabditis elegans. Ct, threshold cycle value.
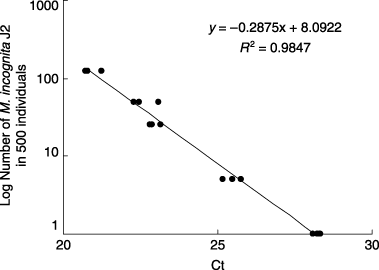
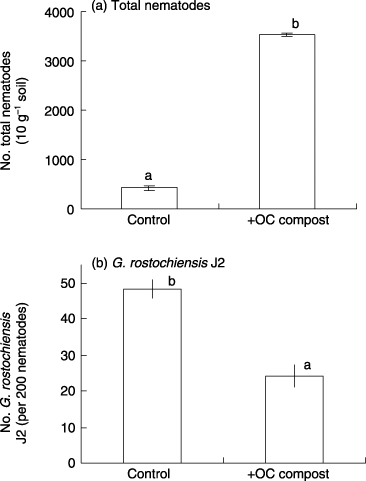
Figure 4 Effect of okara/coffee extraction residue compost on the numbers of (a) total nematodes and (b) Globodera rostochiensis J2 estimated from real-time polymerase chain reaction. Data are the means of four replicates (±standard deviation). Data with different letters are significantly different (P < 0.05).
When the specific primers for M. incognita were applied to a total of 500 individuals containing different numbers of M. incognita J2 and C. elegans, the Ct value in the control (no M. incognita) was 34.5 ± 2.8 and the value in nematode suspensions with a single M. incognita J2 was 28.2 ± 0. 1, and this difference was significant (P < 0.05). There was a highly significant correlation (r 2 = 0.9847, P < 0.01) between the Ct values and the number of M. incognita J2 ().
The total number of nematodes was greatly increased by the addition of OC compost () and the majority of nematodes were free-living based on their morphologies. The number of G. rostochiensis J2 estimated from a real-time PCR assay showed a significant decrease with OC compost when the number was expressed as the number of G. rostochiensis J2 per 200 individuals. In the morphological identification, to find a single G. rostochiensis from a large number of nematodes, like those in the OC-compost treatment (more than 3,000 individuals per 10 g), is tedious work, but real-time PCR was able to detect a single G. rostochiensis J2 from 1,000 nematodes. Therefore, quantification of G. rostochiensis or M. incognita using real-time PCR may be a powerful tool for samples containing a large number of nematodes.
ACKNOWLEDGMENTS
We thank Hajime Takeda for providing us with okra-coffee extraction residue compost and Hiroaki Okada for the kind gift of C. elegansand for useful suggestions. We also thank Atsuhiko Kushida for useful suggestions. This work was partly supported by a Grant-in-Aid for Scientific Research from the Ministry of Education, Science, Sports and Culture of Japan (No.17380048) and by the eDNA project, Ministry of Agriculture, Forestry and Fisheries, Japan.
REFERENCES
- Back , M , Haydock , P and Jenkinson , P . 2006 . Interactions between the potato cyst nematode Globodera rostochiensis and diseases caused by Rhizoctonia solani AG3 in potatoes under field conditions . Eur. J. Plant Pathol , 114 : 215 – 223 .
- Bulman , SR and Marshall , JW . 1997 . Differentiation of Australian potato cyst nematode (PCN) populations using the polymerase chain reaction (PCR) . NZ J. Crop Hort. Sci , 25 : 123 – 129 .
- Chikaoka , I . 1983 . Studies on damage of crops by the root lesion nematode, Pratylenchus penetrans COBB, and control measures especially by utilization of marigold (Tagets spp.) . Bull. Kanagawa Hort. Exp. Stn , 125 : 1 – 72 . (in Japanese with English summary)
- Gugino , BK , Abawi , GS and Ludwig , JW . 2006 . Damage and management of Meloidogyne hapla using oxamyl on carrot in New York . J. Nematol , 38 : 483 – 490 .
- Hague , NGM and Gowen , SR . 1987 . “ Chemical control of nematodes ” . In Principals and Practices of Nematode Control in Crops , Edited by: Brown , RH and Kerry , BR . 131 – 178 . Sydney : Academic Press .
- Koenning , SR . 2000 . Density-dependent yield ofHeterodera glycines-resistant and -susceptible cultivars . J. Nematol , 32 : 502 – 507 .
- Madani , M , Subbotin , SA and Moens , M . 2005 . Quantitative detection of the potato cyst nematode, Globodera pallida, and the beet cyst nematode, Heterodera schachtii, using real-time PCR with SYBR green I dye . Mol. Cell. Probe , 19 : 81 – 86 .
- Ohbayashi , N . 1989 . Studies on the methods for controlling the root-lesion nematode, Pratylenchus penetrans COBB infecting on Japanese radish . Bull. Kanagawa Hort. Exp. Stn , 39 : 1 – 90 . (in Japanese with English summary)
- Saeki , Y , Kawano , E , Yamashita , C , Akao , S and Nagatomo , Y . 2003 . Detection of plant parasitic nematodes, Meoidogyne incognita and Pratylenchus coffeae, by multiplex PCR using specific primer . Soil Sci. Plant Nutr , 49 : 291 – 295 .
- Sato , E and Toyota , K . 2006 . Application of PCR-DGGE into community structure analysis of soil nematodes . Jpn J. Soil Sci. Plant Nutr , 77 : 157 – 163 . (in Japanese with English summary)
- Sato , E , Min , YY , Shirakashi , T , Wada , S and Toyota , K . in press . Quantitative detection of the root-lesion nematode, Pratylenchus penetrans, from a nematode community using real-time PCR . Japanese Journal of Nematology ,
- Zijlstra , C and Van Hoof , RA . 2006 . A multiplex real-time polymerase chain reaction (TaqMan) assay for the simultaneous detection of Meloidogyne chitwoodiand M. fallax . Phytopathol , 96 : 1255 – 1262 .