Abstract
Cucumber (Cucumis sativus L.) plants were grown with hydroponics in a greenhouse and the absorption and translocation of nitrogen in the plants were investigated after the first fruit harvest using 15N-labeled nitrogen. The 15N-labeled nutrient solution, which contained 13 mmol L−1 nitrate, was supplied for 7 days from first fruit harvest on 21 September, 2005. Following 7 days of 15N treatment, these plants received the same composition of non-labeled nitrogen for 16 days (chase period). Commercial-sized fruits, approximately 20 cm in length, were harvested every day. The whole plants were harvested three times at the beginning (21 September) and at the end (28 September) of the 15N treatment period, and at the end of the chase period on 14 October. The average nitrogen absorption rate was 196 mg plant−1 per day during the 15N treatment period and 181 mg plant−1 per day during the chase period. The labeled nitrogen was detected in the fruits within 1 day of the 15N treatment, and the percentage of nitrogen from labeled nitrogen in the harvested fruits increased up to approximately 50% at the end of the 15N treatment period. The percentage of labeled nitrogen in the fruits quickly decreased after changing from 15N solution to the non-labeled conditions during the chase period. At the end of the 15N treatment period on 28 September, the percentage distribution of labeled nitrogen was 46% in the leaves, 24% in the harvested fruits, 13% in the stems, 9% in the roots and 9% in the small fruits. After 16 days of chase period, the distribution of labeled nitrogen decreased in the leaves (33%), stems (6.5%), roots (3.6%) and small fruits (1%), but 56% of labeled nitrogen was distributed in the harvested fruits. Based on the result obtained, the nitrate absorbed from the medium is rapidly transported to young fruits as well as to leaves and stems. The nitrogen once assimilated or stored in the leaves, stems and roots is gradually redistributed to the fruits for supporting their rapid growth. To maximize the yield of cucumber fruits, it is necessary to supply sufficient levels of nitrogen continuously after the first harvest.
Key words:
INTRODUCTION
Cucumber (Cucumis sativus L.) is a popular vegetable and widely cultivated in the world. Cucumber fruits are used not only as fresh vegetables, for example, in salads, but also as pickling and cooking materials. In Japan, cucumber is one of the most important fruit vegetables along with tomatoes and strawberries, and the planting area in Japan was 13,700 ha in 2004. One of the characteristics of cucumber growth is that vegetative growth and reproductive growth proceed at the same time for a long period. Cucumber plants continue to produce new branches, new flowers and new fruits for a long time. It is very important to maintain a proper balance of vegetative growth and reproductive growth during the harvest period to maximize the fruit yield by pinching the stem bud and nutrient supply.
Plants absorb the mineral nutrients that are necessary for growth from the roots and produce a photosynthate, mainly in the leaves, by using solar energy, water and carbon dioxide. Enlargement of the cucumber fruit depends on translocation of the water, the photosynthate and various nutrients from the leaves and roots. Several investigators have reported the role of the leaves in the translocation and partitioning of the photosynthate on fruit development in some vegetables, such as tomato (CitationBertin 1995; CitationHeuvelink 1997; CitationHeuvelink and Buiskool 1995; CitationHikasa and Imada 1993; CitationShishido and Hori 1991), cucumber (CitationMurakami et al. 1982), strawberry (CitationKumakura and Shishido 1994) and green pepper (CitationSteer and Pearson 1976). Furthermore, in the cucumber, CitationKanahama and Saito (1988) reported that bent cucumber fruit occurs when translocation of the photosynthate to the fruit is limited. The quality of cucumber fruit is usually classified based on shape, uniformity, defects and skin color; therefore, the yield of commercial fruits decreases with the occurrence of bent fruit. The control of cultivation to accelerate the efficient distribution and accumulation of the photosynthate to the fruit is important to obtain high-quality fruits.
The plant vigor of the cucumber is affected by temperature, humidity, irrigation, fertilization and training. In particular, fertilization is very important for controlling plant vigor, including photosynthetic activity. Nitrogen is one of the most important nutrients to influence the growth of plants. Plants assimilate absorbed nitrate into nitrite via nitrate reductase (NR; EC 1.6.6.1) and ammonium via nitrite reductase (NiR; EC 1.6.6.4) in roots and leaves, and the ammonium is assimilated into amino acids through the glutamine synthetase (GS; EC 6.3.1.2) and glutamete synthase (GOGAT; EC 1.4.1.14) pathways. Then plants synthesize various proteins from the assimilated nitrogen that have various physiological functions.
It is recognized that leaves play a role not only in supplying the photosynthate, but also in the main part of nitrogen metabolism. In the case of soybean plants, most of the nitrate absorbed from the medium in the reproductive stage is transported to the leaves and redistributed from the leaves to the pods and seeds, mainly in the form of asparagine (CitationOhyama 1983, Citation1984; CitationOhyama and Kawai 1983). In cucumber, the role of the leaves as a source of nitrogen has not been elucidated.
Plant growth rate and fruit yield often depend on the nitrogen supply to form amino acids, proteins, nucleic acids and other cellular constituents. In the case of cucumber at the first fruit harvest stage, the growth of the stems and leaves still continues, together with the enlargement of the fruits. Therefore, nitrogen supply may be necessary during the fruit harvest period. A higher dose of nitrogen application shows a tendency to increase the growth rate and the fruit yield. However, an excess amount of nitrogen fertilization causes salt accumulation in the medium, resulting in poor fruit quality and decreases in the commercial yield. Furthermore, excess fertilization causes groundwater contamination by, for example, nitrate and phosphorus, and it will cause the emission of nitrous oxide, which is a global warming gas (CitationMiyazaki 2000; CitationNoda 2001; CitationYoh and Minami 1991).
Recently, consumers and growers have been seeking environmentally friendly agriculture, in which proper fertilization is very important. To minimize the loss of nitrogen by leaching or denitrification, the amount of fertilizer application should be optimized in accordance with the nutrient absorption pattern of the crop. To avoid excess fertilization, a nutrient management practice is necessary based on the nutrient absorption pattern of the cucumber, the nutrient state of the plant and the nitrogen concentration in the soil or medium. It has been reported that the commercial yield and quality of fruits responds to nitrogen supply (CitationRuiz and Romero 1999; CitationSaito and Enomoto 1988; CitationYanai 1975) or to the nitrate concentrations in the soil (CitationSakai et al. 2000). Therefore, a diagnosis of nitrogen nutrition in the cucumber has been developed based on an analysis of nitrate concentrations of petiole juice (CitationOtani 1980; CitationRoppongi 1991).
To optimize the fertilization rate from the viewpoint of saving resources, and to avoid salt accumulation and to take into consideration environmental conservation, it is necessary to investigate the absorption and transport of supplied nitrogen in plants. As for the movement of the supplied nitrogen in the soil, supplied nitrogen is absorbed not only by a crop, but also by the next crop cultivation (CitationYuasa et al. 1981). CitationYuasa et al. (1981) reported that the amount of absorption of nitrogen supplied during the cultivation accounted for only approximately 40% of the total nitrogen, and the remaining 60% was derived from nitrogen supplied to the previous crops.
The absorption and translocation of supplied nitrogen has been reported in various crops (CitationInanaga 1996; CitationOritani 1984; CitationOsaki et al. 1991; CitationTatsumi and Kono 1980; CitationTatsumi et al. 1993). However, there are few reports from fruit vegetables (CitationFukutoku et al. 2000; CitationKarino et al. 1981; CitationMasuda et al. 1996; CitationNakano et al. 2003; CitationTakano and Suzuki 1989), and no studies have examined cucumbers. In the present study, we investigated the absorption and translocation of nitrogen after the first fruit harvest in cucumber plants cultivated in hydroponics using 15N-labeled nitrogen.
MATERIALS AND METHODS
Plant cultivation
Seeds of the rootstock (Cucurbita moschata. cv. Hikari Power Gold) plant were sown on 30 July, 2005, and seeds of the cucumber (Cucumis sativus L. cv. HighGreen22) were sown the following day. A scion of cucumber was grafted onto a rootstock plant in rock-wool cubes (5 cm × 5 cm × 5 cm) on 6 August. On 18 August, when the third foliage leaves had expanded, the plant was transplanted to a Wagner pot (1/2000a) containing 11 L of nutrient solution in a greenhouse. Cubes were fixed in each pot with wires and the surface of each pot was covered with a silver polyethylene film. Yamazaki cucumber formulation (CitationItagi et al. 1995) was used as the culture solution. The major mineral nutrient composition of the nutrient solution was as follows: N 13 mmol L−1, P 1 mmol L−1, K 6 mmol L−1, Ca 3.5 mmol L−1, Mg 2 mmol L−1. The composition of nitrogen is 6 mmol L−1 KNO3 and 3.5 mmol L−1 Ca (NO3)2. The amount of nutrient solution in the pots was kept at 11 L by adding tap water every day. The electrical conductivity of the nutrient solution was adjusted so that it was between 2.0 dS m−1 and 1.8 dS m−1 by adding concentrated nutrient solution every 2–3 days. The nutrient solution was renewed every 2 weeks. The nutrient solution was aerated through air stones connected to an air compressor.
The terminal bud of the main stem was pinched at the 20th node and the lateral shoots on the main stem between the 6th and 20th nodes were pinched at the first node. The lateral shoots on the main stem between the 1st and 5th nodes had been removed previously. The leaves on the main stem between the 1st and 5th nodes were defoliated before the first fruit harvest.
15N treatment
15N treatment was started on 21 September at the first fruit harvest. The pinching treatment of terminal buds of the main stem and the first lateral branch had been completed when the 15N treatment started. 15N-labeled nutrient solution was put into the pot after the non-labeled nutrient solution was thrown away and the root was fully washed using deionized water. 15N-labeled nutrient solution was made from K15NO3 (7.05 atom%15N) and Ca (15NO3)2 (7.14 atom%15N). The 15N abundance of 15NO3 was 7.10 atom%15N. The mineral nutrient composition of the 15N-labeled nutrient solution was the same as the non-labeled nutrient solution. The 15N treatment was conducted for 1 week from 21 September to 28 September, and the nutrient solution was replaced by non-labeled nutrient solution after 28 September. During the 15N treatment, the amount of the nutrient solution in the pots was kept at 11 L by adding deionized water every day. Moreover, the electrical conductivity of the nutrient solution was adjusted to 1.8 dS m−1 by adding concentrated 15N-labeled nutrient solution once on 24 September.
Plant sampling and analysis
Cucumber fruits grown to standard commercial size in Japan, approximately 20 cm in length, were harvested daily from 21 September to 14 October. On 21 and 28 September and 14 October, three plants were harvested and separated into roots, stems, leaves, the first lateral branch, the second lateral branch and small fruits under 20 cm in length. Further, each lateral branch was separated into leaves and stems. After the fresh weight was measured, each part was dried for 1 week at 50°C in a ventilation oven and the dry weight was measured. The dried samples were ground into a fine powder using a vibrating mixer mill. Each sample was digested using the Kjeldahl digestion method with salicylic-sulfuric acid (10 g of salicylic acid was dissolved in 300 mL of H2SO4) and hydrogen peroxide. The N concentration was determined by colorimetry using the indophenol blue procedure (CitationOhyama et al. 1991). An aliquot of the Kjeldahl digestion solution was put directly into a Pyrex glass tube (4 mm outside diameter) for the preparation of the discharge tube for 15N analysis. The 15N abundance (atom% excess) was analyzed by emission spectrometry using a JASCO N-150 15N analyzer (CitationOhyama et al. 2004). The percentage of nitrogen from 15N-labeled nitrogen (LN) and non-labeled nitrogen (NLN) was calculated.
RESULTS
shows the change in the dry weight of each part of the cucumber plants harvested on 21 and 28 September and 14 October. The growth stage of the cucumber plants at the start of the 15N treatment was the early growth of the secondary lateral branch. The dry weight of each part kept increasing until the end of the experiment, except for the small fruits. The total dry weight of leaves and stems increased approximately 50% from 21 September until 14 October. The dry weight of the harvested fruits on 14 October was approximately 80 g plant−1, and it accounted for approximately 70% of the increase in total dry weight, approximately 111 g plant−1.
Figure 1 Changes in the dry weight of various organs of the cucumber. MS, main stem; 1LB, first lateral branch; 2LB, secondary lateral branch; HF, harvest fruits; SF, small fruits. Error bars denote the standard deviation (n = 3).
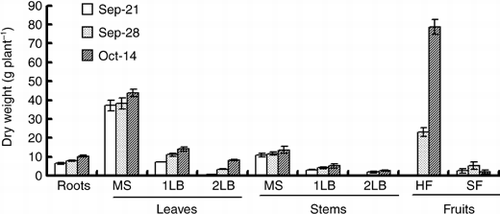
Figure 2 Changes in the nitrogen concentration of various organs of the cucumber. DW, dry weight; MS, main stem; 1LB, first lateral branch; 2LB, secondary lateral branch; HF, harvest fruits; SF, small fruits. Error bars denote the standard deviation (n = 3).
Table 1 Changes in the nitrogen content of various organs of the cucumber
shows the nitrogen concentration in each part. At the start of the 15N treatment, the nitrogen concentration of roots, leaves and stems was 46.5 g kg−1, 50.4–67.3 g kg−1 and 34.6–46.1 g kg−1, respectively. The nitrogen concentration of leaves and stems of the secondary lateral branch was 30% higher than that of the main stem. The nitrogen concentration of the leaves and stems decreased over the experimental period, whereas the nitrogen concentration was relatively constant in the roots. At the end of the chase period on 14 October, the nitrogen concentration was 45.2 g kg−1 in the roots, 44.6–56.9 g kg−1 in the leaves and 29.2–37.3 g kg−1 in the stems. The average nitrogen concentration of the harvested fruits was 39.3 g kg−1.
shows the changes in nitrogen content in each part of the cucumber plants. The total nitrogen content was 3.24 g plant−1 on 21 September, 4.61 g plant−1 on 28 September and 7.40 g plant−1 on 14 October, respectively. The average nitrogen absorption rate was 196 mg plant−1 per day from 21 to 28 September and 174 mg plant−1 per day from 28 September to 14 October, respectively. The total amount of LN absorbed from 21 September until 28 September in a plant was approximately 1,250 mg plant−1, indicating that nitrate in solution was not depleted because each plant was supplied more than 2,000 mg N nitrate in a pot.
The nitrogen content of the roots, the leaves and the stems increased over the treatment period until 14 October. The increase rate of the nitrogen contents of the roots during the treatment period was approximately 60%, which was the same as the increase rate of the dry weight. Although the total nitrogen content in the leaves and stem of the main stem was relatively constant, these organs increased LN and decreased NLN during the 7-day 15N treatment period. The increase rate of the nitrogen contents in total leaves and total stems was approximately 30%, which was low in comparison with the increase rate of the dry weight in leaves (46%) and stems (53%). The NLN contents of the leaves and stems of the second lateral branch and the harvested fruits increased during the 7-day 15N treatment period. The nitrogen contents of the total fruits (harvested fruits plus young fruits) increased 3,091 mg plant−1 over the experiment period until 14 October. The increase in the nitrogen of the fruits accounted for 74% of the whole plant.
Figure 3 Changes in the nitrogen distribution rate of various organs of the cucumber. MS, main stem; 1LB, first lateral branch; 2LB, secondary lateral branch; HF, harvest fruits; SF, small fruits. Error bars denote the standard deviation (n = 3).
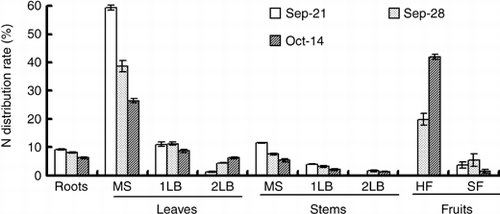
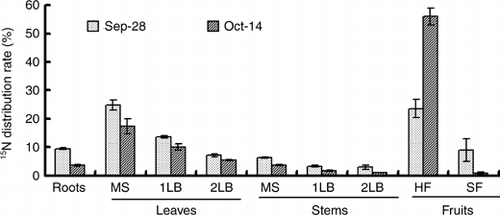
Figure 4 Changes in the 15N distribution rate of various organs of the cucumber. MS, main stem; 1LB, first lateral branch; 2LB, secondary lateral branch; HF, harvest fruits; SF, small fruits. Error bars denote the standard deviation (n = 3).
shows the distribution rate of nitrogen among each plant part. The distribution rate of nitrogen of the leaves and stems of the second lateral branch and the fruits at the end of the treatment on 14 October became higher than the rate at the start of the 15N treatment on 21 September. In contrast, the rate decreased in other parts. The distribution rate of nitrogen in the leaves of the main stem was 59% on 21 September. After that it decreased and it was 26% at the end of the chase period on 14 October. In contrast, the nitrogen distribution rate of the fruits was 3.7% on 21 September, 25% on 28 September and 43% on 14 October.
The 15N absorbed by a cucumber plant during the 15N treatment was 1,245 mg plant−1 (). At the end of the 7-day 15N treatment period approximately 23% of LN (293 mg plant−1) was distributed in the harvested fruits and approximately 9% (112 mg plant−1) was in the small fruits (). At the end of the 16-day chase period, approximately 56% of LN (707 mg plant−1) was distributed in the harvested fruits and approximately 1% (37 mg plant−1) was in the small fruits. From 21 September to 28 September, 405 mg of LN was distributed in the total fruits (including harvested fruits and small fruits), which accounted for 33% of absorbed LN. From 28 September to 14 October, 314 mg of LN was redistributed in the total fruits, which accounted for 24% of the absorbed LN. In the new growing organs, such as the leaf and stem of the second lateral branch and small fruit, the 15N content was low in comparison with other organs at the end of the 15N treatment (). However, the percentage of LN in the small fruits and the secondary lateral branch was higher than the older organs, and they were higher than 40% (). In contrast, the percentage of LN in the leaves of the main stem, whose 15N content was the highest, was the lowest at 17.3% in comparison with the other organs.
shows the nitrogen concentration in the leaves of each node of a main stem. The nitrogen concentration tended to become high in the upper leaves in comparison with the lower leaves, and it was 43.6–58.4 g kg−1 on 21 September, 36.5–50.7 g kg−1 on 28 September and 31.0–51.2 g kg−1 on 14 October. At the end of the 15N treatment on 28 September, the 15N content of the leaves on the main stem was higher in the upper leaves than the lower leaves, and it was 6.6 mg plant−1 on the 6th node and 37.5 mg plant−1 on the 20th node (). Moreover, a similar tendency was found in the 15N concentration, the 15N concentration of the leaves on the main stem was 11.8% on the 6th node and 21.9% on 20th node ().
Figure 5 Changes in the percentage of 15N-labeled nitrogen (LN) in total nitrogen of various organs of the cucumber. MS, main stem; 1LB, first lateral branch; 2LB, secondary lateral branch; HF, harvest fruits; SF, small fruits. Error bars denote the standard deviation (n = 3).
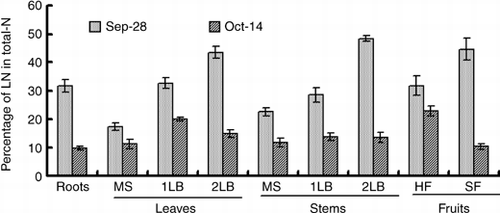
Figure 6 Changes in the nitrogen concentration in the leaves of each node of a main stem. Error bars denote the standard deviation (n = 3). DW, dry weight.
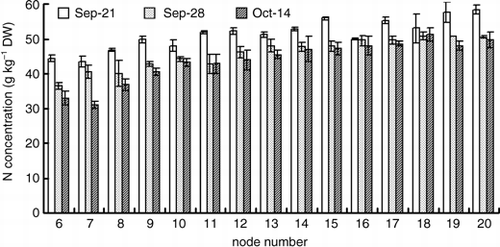
Figure 7 Changes in the percentage of 15N-labeled nitrogen (LN) in total nitrogen in the leaves of each node of the main stem. Error bars denote the standard deviation (n = 3).
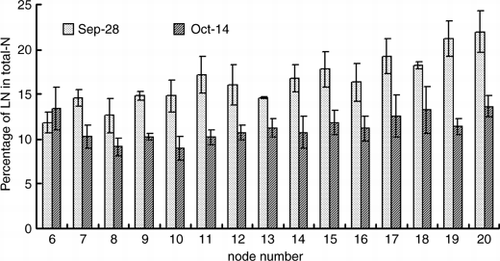
During the chase period after the 15N treatment, the 15N content of each part, except the fruits, decreased. The amount of 15N translocation to other parts was the highest in the leaves on the main stem, although the translocation rate of the 15N content was high in the roots and the stems of the second lateral branch. The amount of 15N translocation from the leaves on the main stem to other parts was greater in the upper leaves than the lower leaves. During the chase period, when non-labeled nitrogen was supplied, the NLN content of the leaves on the 8th–20th nodes of the main stem had increased, although the content of the 6th and 7th nodes did not increase ().
Table 2 Changes in the nitrogen content of the leaves of each node of the main stem
Figure 8 Changes in the percentage of 15N-labeled nitrogen (LN) in total nitrogen in the harvest fruits of cucumber. Error bars denote the standard deviation (n = 3–6).
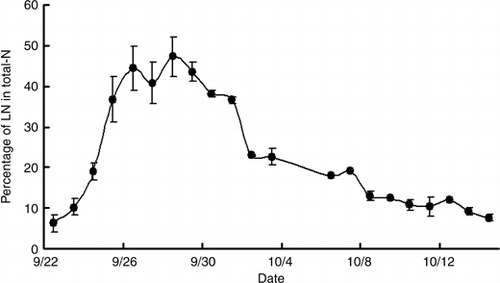
shows the changes in the percentage of LN in the harvested fruits. The number of days from flowering to harvest size was 7–12 in this period, the female flowers that bloomed when 15N treatment started were harvested on 28–30 September. The percentage LN of the harvested fruits one day after 15N treatment on 22 September was 6%. After that, the percentage LN of the harvested fruits increased over the 15N treatment period, and the percentage LN reached 47% at the end of the 15N treatment on 28 September. After the end of the 15N treatment, the percentage LN of the harvested fruits decreased to 43% on the next day (29 September). The percentage LN in harvested fruits decreased consistently after the culture solution was replaced with non-labeled solution, and it was as low as 7.4% when the experiment was finished on 14 October.
DISCUSSION
In an experiment about the absorption and translocation of nutriment like this experiment, it is important that the growth of the plant is normal and healthy. Sometimes, the emergence of lateral branches is poor in the glasshouse because of the low humidity (CitationAbe and Ota 1986). This experiment was cultivated without humidity control in the glasshouse; however, the emergence of the lateral branches was normal in this experiment. The distribution rate of the dry weight among organs was almost the same as the result shown by CitationYuasa et al. (1981). Therefore, it can be considered that cucumber growth in this experiment was normal and similar to commercial production in Japan.
In this experiment, the nitrogen concentrations of leaves and stems tended to decrease after the fruit harvest started (). Although the cultivation method was different in the present experiment, CitationYanai (1975) reported that the nitrogen concentration of the leaves and the stems decreases after the start of the fruit in the semi-forcing culture of cucumber. The nitrogen concentration of leaves and stems decreased after the fruits started to grow larger in eggplant, (CitationTakano and Suzuki 1989), melon (CitationFukutoku et al. 2000) and green pepper (CitationUesugi and Fujita 1972). Therefore, it was suggested that the nitrogen in leaves and stems is exported to a sink organ in the cucumber, which is similar to what happens in other fruit vegetables and other plants, such as rice (CitationOritani 1984), maize (CitationOsaki et al. 1991) and potato (CitationOsaki et al. 1991).
In general, cucumber fruits are harvested at an immature stage in Japan, and fruit harvest continues for a long time (approximately 3 months) after the fruit harvest starts. Because of this, the vegetative growth and the reproductive growth progress at the same time, and a balance in the two types of growth is very important to obtain optimum yield with good-quality fruits. In this experiment, the dry weight of the total leaves and total stems increased approximately 50% over the 23-day experimental period (). The harvested fruits accounted for approximately 70% of the amount of dry weight increase over this period (). Similar results were reported in eggplant (CitationTakano and Suzuki 1989). Fruits are shown to be a strong sink after flowering in the strawberry, and the increase in dry weight is much higher than the increase in the other organs (CitationForney and Breen 1985). The result of the present experiment in cucumber is in agreement with these reports.
The translocation rate of nitrogen from the leaves to the fruits is higher than the changes in the distribution rate of the dry matter in the fruit enlargement stage of the eggplant (CitationTakano and Suzuki 1989). CitationFukutoku et al. (2000) reported that 76% of nitrogen absorbed after pollination was distributed in the fruits of melon. When the sink activity of the fruit increased, the assimilated nutrients were transported more preferentially to the fruit than to the vegetative organs in tomato (CitationHeuvelink 1997; CitationHeuvelink and Buiskool 1995). In the present experiment, the increase rate of the nitrogen content of the fruit was higher than the increase rate of the dry matter, and the increase in the nitrogen content of the fruit accounted for 74% of the whole plant (, ). Furthermore, in the 7 days from the harvest-starting day (over the 15N treatment), approximately 31% of nitrogen that existed in the organ except for the fruit was translocated to the fruits, including harvested fruits and young fruits (). In that period, the leaves of the main stem had the strongest translocation activity of nitrogen compared with other organs, 23% of nitrogen contained in the leaves of the main stem translocated to other organs (). This quantity was equivalent to approximately 70% of the amount of increase of nitrogen in the fruits (). Over the 7 days of the 15N treatment period, approximately 33% of LN was distributed in the fruits, and in the 16-day chase period approximately 24% of LN was redistributed to fruits from leaves, stems and roots (). The results of this experiment suggest that nitrogen in the leaf was translocated mainly and preferentially to the fruits. Therefore, fruits are a strong sink in fruit enlargement terms in cucumber, and all the vegetative organs, especially the leaves, are working as a source of nitrogen.
In the cucumber, when harvest begins, many parts can become a sink in addition to the fruit, for example, the female flower and the new leaf. However, the sink strength of the specific fruit is especially strong during the fruit enlargement term. Thus, the photosynthate is translocated intensively to the fruit, and translocation to other organs decreases (CitationMurakami et al. 1982). In this experiment, 15N treatment ran for 7 days after the first harvest. The 15N distribution rate in the fruits was higher than the distribution rate of total nitrogen (,). The female flowers that bloomed on the 15N treatment start day (21 September) were harvested on 28–30 September and the 15N concentration of fruits was 47.3% on 28 September (). It can be considered that approximately 50% of nitrogen contained in the harvest fruit of the cucumber is nitrogen absorbed after flowering. The nitrogen absorbed within 24 h before the harvest accounted for 6.1% of all nitrogen in the harvest fruit (). Some part of the absorbed nitrogen is translocated rapidly to the fruits; however, most of the nitrogen may be assimilated in the leaves and retranslocated to the fruits thereafter. CitationFukutoku et al. (2000) reported that the source–sink relationship of nitrogen is similar to that of the photosynthate in the melon. Although fruit set is different in the melon and the cucumber, it can be considered that the source–sink relationship of the photosynthate may be similar to the movement of nitrogen in the cucumber.
The nitrogenous source of the new leaf is not only newly absorbed nitrogen, but also nitrogen redistributed from old leaves in the vegetative growth of rice (CitationYoneyama and Sano 1978). In peanut plants, in which vegetative growth and reproductive growth progress at the same time, the nitrogen translocated to the pods from other vegetative organs is more important than the nitrogen newly absorbed from the soil (CitationInanaga 1996). In this experiment, nitrogen absorbed before the harvest began was translocated to the secondary lateral branch that newly appeared in the fruit enlargement term. It can be considered that the nitrogen absorbed in the vegetative growth phase is important in the occurrence of the lateral branch in the fruit enlargement term, and that the condition of nitrogenous nutrition before harvest influences the occurrence of the lateral branch.
In this experiment, the 15N content of the leaves of the main stem was higher in the upper leaves than the lower leaves at the end of the 15N treatment (). The amount of 15N translocation to other organs was higher in the upper leaves than in the lower leaves (). The leaves of the 6th and 7th nodes incorporated a small amount of LN and NLN after the supply of NLN was resumed. When 15N treatment was finished on 28 September, approximately 35 days passed after the leaf foliated from the 6th and 7th nodes. Thus, we considered that the assimilative capacity of these leaves declined markedly in comparison with the upper new leaves. Cucumber growers tend to defoliate the old leaves at about 40 days after the leaves are fully developed. The defoliation prevents light-interception among leaves and reduces the damages of disease and pest. The defoliation must be done after losing the assimilatory activity. The results of this experiment suggested that the 40 days may be the proper period for defoliation of cucumber leaves.
It has been reported that nitrogen assimilated into leaves near the fruit-set node is preferentially translocated to the fruit in melons (CitationFukutoku et al. 2000). In contrast, in the case of cucumber, fruits can receive photosynthate from leaves that are located at a node far from the fruit node (CitationMurakami et al. 1982). In this experiment, a large amount of nitrogen was translocated from the upper young leaves (), in which the assimilative capacity might be high. In the melon, there is only one fruit in the node place in the middle. However, cucumber fruits set continuously on many nodes. This difference in fruit-set characteristics may be one of the reasons why the translocation pattern of nitrogen is different in the melon and the cucumber.
In conclusion, when the harvest began, the nitrogen that had already been absorbed and stored in leaves and stems was translocated preferentially to the fruits. The absorbed nitrogen after the harvest starting term was translocated rapidly to the leaves and assimilated there, and then translocated to the fruit. Approximately 50% of the nitrogen contained in the harvest fruit of the cucumber was nitrogen absorbed after flowering. To maximize the yield of cucumber fruits, it is necessary to supply a sufficient amount of nitrogen continuously after the first harvest to support reproductive and vegetative organs.
REFERENCES
- Abe , H and Ota , H . 1986 . Moisture control from room temperature and its effect for cucumber in glasshouse . Gunma J. Agric. Res. Series D , 2 : 1 – 5 .
- Bertin , N . 1995 . Competition for assimilates and fruit position affect fruit set in indeterminate greenhouse tomato . Ann. Bot , 75 : 55 – 65 .
- Forney , CF and Breen , PJ . 1985 . Dry matter partitioning and assimilation in fruiting and deblossomed strawberry . J. Am. Soc. Hort. Sci , 110 : 181 – 185 .
- Fukutoku , Y , Teraoka , Y , Koto , S and Kubo , K . 2000 . Nitrogen absorption and distribution of muskmelons (Cucumis melo L.) at different growth stages using hydroponics . Jpn. J. Soil Sci. Plant Nutr , 71 : 72 – 81 .
- Heuvelink , E . 1997 . Effect of fruit load on dry matter partitioning in tomato . Scientia Hortic , 69 : 51 – 59 .
- Heuvelink , E and Buiskool , RPM . 1995 . Influence of sink source interaction on dry matter production in tomato . Ann. Bot , 75 : 381 – 389 .
- Hikasa , Y and Imada , S . 1993 . Effect of nitrogen concentration of nutrient solution on the behavior of 14C-photosynthates in tomato . Jpn. J. Soil Sci. Plant Nutr , 64 : 377 – 384 .
- Inanaga , S . 1996 . Translocation and distribution of N within peanut plant . Jpn. J. Soil Sci. Plant Nutr , 67 : 662 – 667 .
- Itagi , T , Sasaki , K and Udagawa , Y . 1995 . Practical Technique for Hydroponics , Tokyo : Japan Association of Agricultural Electrification . (in Japanese)
- Kanahama , K and Saito , T . 1988 . Carbohydrate distribution and 14C-photosynthates uptake in the curved fruits of cucumber . J. Japan. Soc. Hort. Sci , 57 : 448 – 453 .
- Karino , H , Kagohashi , S and Kageyama , M . 1981 . Relationship between organ growth and nitrogen accumulation in muskmelon . J. Japan. Soc. Hort. Sci , 50 : 317 – 325 .
- Kumakura , H and Shishido , Y . 1994 . Effect of diurnal temperatures and leaf position on the translocation and distribution of 14C-photosynthates in fruiting strawberry cv. Morioka-16 . J. Jpn. Soc. Hort. Sci , 62 : 833 – 838 .
- Masuda , M , Hasegawa , H and Nomura , M . 1996 . Diurnal translocation of nitrogen (15N) and calcium (45Ca) from tomato roots to fruiting trusses . J. Jpn. Soc. Hort. Sci , 65 : 571 – 577 .
- Miyazaki , N . 2000 . Studies on the actual and qualitative condition of underground water in the areas in southern Tochigi Prefecture that plant upland crops . Bull. Tochigi Agr. Exp. Stn , 49 : 47 – 57 .
- Murakami , T , Inayama , M and Kobayashi , H . 1982 . Translocation and distribution of 14C-photosynthates in cucumber plants . Bull. Nat. Inst. Agr. Sci. Series D , 33 : 235 – 275 .
- Nakano , Y , Watanabe , S , Okano , K and Tatsumi , J . 2003 . Absorption and distribution of 15N by divided tomato roots; part immersed in a nutrient solution and part in a humid atmosphere . J. Jpn. Soc. Hort. Sci , 72 : 156 – 161 .
- Noda , S . 2001 . Application and use of fertilizers for the reduction of nitrous oxide emissions from upland soils . Soil Sci. Plant Nutr , 72 : 575 – 581 .
- Ohyama , T . 1983 . Comparative studies on the distribution of nitrogen in soybean plants supplied with N2 and NO-3 . Soil Sci. Plant Nutr , 29 : 133 – 145 .
- Ohyama , T . 1984 . Comparative studies on the distribution of nitrogen in soybean plants supplied with N2 and NO-3 at the pod filling stage, II. Assimilation and transport of nitrogenous constituents . Soil Sci. Plant Nutr , 30 : 219 – 229 .
- Ohyama , T and Kawai , S . 1983 . Nitrogen assimilation and transport in soybean leaves: Investigation by petiole girdling treatment . Soil Sci. Plant Nutr , 29 : 227 – 231 .
- Ohyama , T , Ito , M Kobayashi , K . 1991 . Analytical procedure of N, P, K contents in plants and manure materials using H2SO4–H2O2Kjeldahl digestion method . Bull. Fac. Agric. Niigata Univ , 43 : 111 – 120 .
- Ohyama , T , Tewari , K Abdel-Latif , S . 2004 . Direct analysis of 15N abundance of Kjeldahl digested solution by emission spectrometry . Bull. Fac. Agric. Niigata Univ , 57 : 33 – 40 .
- Oritani , T . 1984 . Studies on nitrogen metabolism in crop plants. 20. Translocation and accumulation into sink of 15N top-dressed at different growth stages in the rice plant . Jpn. J. Crop Sci , 53 : 276 – 281 .
- Osaki , M , Yoshimura , A and Tanaka , A . 1991 . Comparison of the behavior of carbon and nitrogen compounds during ripening between maize and potato . Jpn. J. Soil Sci. Plant Nutr , 62 : 282 – 290 .
- Otani , H . 1980 . On the simple diagnosis of nutritive condition of cucumber by density of juice . Bull. Shiga Agric. Exp. Stn , 22 : 50 – 55 .
- Roppongi , K . 1991 . Studies on nutritional diagnosis in fruit vegetables. 1 The diagnosis of nitrogen nutrition in cucumber on nitrate density of petiole juice . Bull. Saitama Hort. Exp. Stn , 18 : 1 – 15 .
- Ruiz , JM and Romero , L . 1999 . Cucumber yield and nitrogen metabolism in response to nitrogen supply . Scientia Hortic , 82 : 309 – 316 .
- Saito , K and Enomoto , M . 1988 . Studies on soil management and improvement of fertilizer application in vegetable field. IV. Effects of the supplemental nitrogen fertilizer on the concentration of nitrate nitrogen and total sugar in cucumber plants . Bull. Fukushima Agric. Exp. Stn , 27 : 21 – 27 .
- Sakai , K , Kuroyanagi , N , Yamamoto , Y and Fujita , A . 2000 . Effect of nitrate nitrogen concentration in the soil on yield and quality of forcing cultured cucumber . Bull. Fukuoka Agric. Res. Cent , 19 : 49 – 54 .
- Shishido , Y and Hori , Y . 1991 . The role of leaf as affected by phyllotaxis and leaf histology on the development of the fruit in tomato . J. Jpn. Soc. Hort. Sci , 60 : 319 – 327 .
- Steer , B and Pearson , CJ . 1976 . Photosynthate translocation in Capsicum annuum . Planta , 128 : 155 – 162 .
- Takano , T and Suzuki , K . 1989 . Changesin nutrient uptake rate and translocation of nutrients in the developmental stage of eggplant . Sci. Rept Fac. Agr. Meijo Univ , 25 : 17 – 25 .
- Tatsumi , J and Kono , Y . 1980 . Nitrogen uptake and transport by the intact root system of rice plants . Jpn. J. Crop Sci , 49 : 349 – 358 .
- Tatsumi , J , Okano , K and Kono , Y . 1993 . Translocation of carbon and nitrogen from mature leaves to the root system of vegetative wheat: Simultaneous feeding of gaseous 13CO2and 15NH3to single leaves . Sci. Rept Fac. Agr. Kobe Univ , 20 : 149 – 160 .
- Uesugi , I and Fujita , Y . 1972 . Comparison the growth and nutrient absorption and yield of sweet pepper usually received heavy fertilizer with it received light fertilizer on sandy soil in vinyl house . Bull. Kochi Inst. Agr. Forest Sci , 4 : 1 – 8 .
- Yanai , T . 1975 . Effect of nitrogenous fertilizer on the growth of cucumber plants in relation to soil type and application method in a vinyl house . Bull. Kochi Inst. Agr. Forest Sci , 7 : 29 – 41 .
- Yoh , M and Minami , K . 1991 . Emission of biogenic gas compounds from soil ecosystem and their effects on global environment . Soil Sci. Plant Nutr , 62 : 654 – 661 .
- Yoneyama , T and Sano , C . 1978 . Nitrogen nutrition and growth of the rice plant. II. Considerations concerning the dynamics of nitrogen in rice seedlings . Soil Sci. Plant Nutr , 24 : 191 – 198 .
- Yuasa , M , Ito , T and Aoba , T . 1981 . Responses of greenhouse cucumber to nitrogen fertilizer with respect to nitrogen transformations in the soil . Tech. Bull. Fac. Hort. Chiba Univ , 29 : 1 – 7 .