Abstract
Phytoremediation is a good technique for removing cadmium (Cd) from farmland soils. To remove Cd from these soils effectively, it is necessary for Cd ions to be transported to the shoot organs for later harvest. However, the mechanism of Cd translocation to shoot organs via xylem vessels has not yet been elucidated. We selected oilseed rape plants (Brassica napus L.) and established a method to collect xylem exudates from these plants. After 3 days of Cd treatment (10 µmol L−1 and 30 µmol L−1) the Cd concentrations in the xylem exudates were approximately 6.5 µmol L−1 and 16 µmol L−1, respectively. The detection of Cd in the xylem exudate indicated that Cd was moving to shoot organs via xylem vessels. The effect of these Cd treatments on the amino acid, organic acid and protein composition of xylem exudates from oilseed rape plants was investigated. The level of amino acids and organic acids detected was enough to bind Cd transported via the xylem. Sodium dodecylsulfate-polyacrylamide gel electrophoresis analysis revealed that proteins with molecular weights of 36 kDa and 45 kDa clearly increased in the exudates with Cd treatment. The possibility that these compounds are binding Cd in the xylem exudates was discussed.
INTRODUCTION
Cadmium (Cd) is a toxic heavy metal. The accumulation of Cd in soils has occurred through human agricultural, industrial or urban activities (CitationMcLaughlin et al. 1999). When plants absorb Cd that has accumulated in soils, several physiological processes are inhibited (inhibition of ribonuclease activity [CitationShah and Dubey 1995]; inhibition of nitrate reductase activity [CitationHernández et al. 1996]; decrease of total chlorophyll content [CitationLarsson et al. 1998]). These inhibited physiological processes result in decreasing productivity of the crop plants (CitationFlorijin and Van Beusichem 1993). In addition, Cd causes serious human health problems when it enters the food chain (CitationObata and Umebayashi 1997). To sustainably produce food with safe levels of Cd, it is necessary for the Cd to be removed from the farmland. However, it is difficult to develop techniques to effectively remove Cd from polluted soils. Established practices (exchanging contaminated soil with fresh soil or covering contaminated soil with fresh soil) are time consuming and expensive.
Phytoremediation is a new technique that involves removing pollutants from the environment or rendering them harmless by green plants (CitationRaskin et al. 1994). Phytoremediation can be applied to the removal of both organic and inorganic pollutants present in the soil. This technique is low cost and is not stressful for the environment, unlike other established practices. Cadmium can be removed from contaminated soils by concentrating the Cd in the harvestable parts of plants grown in the contaminated soils. However, most Cd ions absorbed by plants are retained in the roots, and only small amounts of Cd are transported to the shoots (CitationCataldo et al. 1983). Cadmium concentrations in the roots are at least 10-fold greater than those in the shoots (CitationChaney et al. 1997). To remove Cd from soils more effectively, it is necessary for more Cd ions to be transported to the shoot organs for later harvest. To date, studies have shown that the translocation of Cd from shoots to grains occurs via sieve tubes in rice plants (CitationTanaka et al. 2007). In contrast, translocation of Cd from roots to shoot organs is thought to be via the xylem vessels. The mechanism of Cd translocation in xylem vessels has been investigated in several plant species (indian mustard [CitationSalt et al. 1995] and maize [CitationFlorijin et al. 1993]); however, little is known about the physiological and molecular mechanism of Cd transport from roots to shoots.
Cellular Cu and Zn are bound to ligands and their ligands can be exchanged when Cu and Zn are moving in the cell (CitationClemens et al. 2002). In the case of Cu, some distributors have been identified as the metallochaperones (CitationO’Halloran and Culotta 2000). As Cd is also a heavy metal similar to Cu and Zn, Cd is thought to be bound to a ligand when it is present in the cell or when it is transported. In reality, when Cd enters the root system, it reaches the xylem in the form of a complex bound with a ligand, such as an organic acid (CitationSenden et al. 1992). In long-distance translocation of Cd via the xylem vessels, Cd might also bind to a ligand. In buckwheat, aluminum (Al) is transported from the roots to the shoot in the form of an Al–citrate complex (CitationMa and Hiradate 2000). In Alyssum, translocation of nickel (Ni) via the xylem vessels occurred by forming a complex with histidine (CitationKrämer et al. 1996). These results support the hypothesis that Cd is transported via the xylem vessels in the form of some type of complex. If these ligands that are binding with Cd directly can be detected, the mechanism of Cd movement in the xylem vessels will be elucidated.
In the present study, we selected oilseed rape plants (Brassica napus L.) for removal of Cd from farmland. As the oilseed rape plant is a Brassicaceae, it is expected to be Cd tolerant and a Cd accumulator. The key role of the Brassicaceae plant family in phytoremediation was reviewed by CitationPalmer et al. (2001). Oilseed rape plants can also be used as a basic ingredient in vegetable oil and in compost after harvest. In addition, oilseed rape plants belong to the same taxonomic group as Arabidopsis thaliana. Genes of oilseed rape plants share high sequence identity with their Arabidopsis homologues (CitationCavell et al. 1998). These relationships allow us to use genetic techniques established in Arabidopsis. We attempted to collect exudate by cutting the stem of oilseed rape plants approximately 2 cm above ground level. First, we confirmed that the exudate was derived from xylem vessels. Xylem exudate was also collected from oilseed rape plants treated with Cd (10 µmol L−1 and 30 µmol L−1). In the xylem exudate, compounds such as amino acids, organic acids and proteins are expected to form a complex with Cd. The effects of Cd on the levels of these compounds in xylem exudate were investigated.
MATERIALS AND METHODS
Plant materials
Seeds of oilseed rape plants (Brassica napus L. cv. Nourin No. 16) were purchased from the Kaneko Seed Company (Gumma, Japan). Oilseed rape plants were grown in pots (9 cm internal diameter; 9 cm height) for approximately 6 weeks in a greenhouse under natural light. The temperature in the greenhouse was controlled at 24ºC during the day and at 18ºC at night. Until the collection of the exudate, plants were cultured using a nutrient solution. The nutrient solution contained 0.5 mmol L−1 NH4H2PO4, 3.0 mmol L−1 KNO3, 2.0 mmol L−1 Ca(NO3)2, 1.0 mmol L−1 MgSO4, 0.09 mmol L−1 Na2EDTA (ethylendiamine-N, N, N′, N′-tetraacetic acid disodium), 0.09 mmol L−1 FeSO4, 22.5 µmol L−1 H3BO3, 10 µmol L−1 MnSO4, 0.1 µmol L−1 (NH4)6Mo7O24, 0.35 µmol L−1 ZnCl2 and 0.20 µmol L−1 CuCl2. The pH of the solution was adjusted to 6.7 with 1.0 mol L−1 NaOH. Six-week old plants were transferred to nutrient solution containing 10 and 30 µmol L−1 Cd and treated for 24, 48 and 72 h. The Cd was added to the nutrient solution as CdCl2.
Collection of xylem exudate from oilseed rape plants
Xylem exudate samples were collected by cutting the stems approximately 2 cm above ground level. To avoid contamination from broken cells, exudate samples collected for the first 5 min after cutting the stems were not used in the analysis. For each experiment, xylem exudate samples were collected for 2 h between 13.00 and 15.00 hours to eliminate the effect of circadian rhythms on exudate composition. The pH of the xylem exudate was checked using pH test paper (Test Paper No. 20, pH range 5.0–8.0; Toyo Roshi Kaisha, Tokyo, Japan). Collected samples were frozen at –20ºC until subsequent analysis.
Sugars, organic acids, anion and amino acids determination in the xylem exudate from oilseed rape plants
Sugar concentrations in the exudate were analyzed using a high performance liquid chromatography (HPLC) system following the method of CitationNakamura et al. (2004). Xylem exudate (2 µL) was diluted with 198 µL of distilled water, filtered through a disposable syringe filter unit (DISMIC-3; Toyo Roshi Kaisha) and applied to an HPLC system for the analysis of sugar (LC20; Nippon Dionex K.K., Osaka, Japan).
Organic acid concentrations in the xylem exudate were also analyzed using an HPLC system (Class 10VP system; Shimadzu, Kyoto, Japan). Ten microliters of xylem exudate sample was diluted with 990 µL of distilled water and filtered through the disposable syringe filter unit described above. The filtrates were applied to a column (TSK gel ODS-100V, 4.6 × 250 mmol L−1; Tosoh, Tokyo, Japan) and eluted with 25 mmol L−1 NH4H2PO4–H3PO4 (pH 2.5). Eluted organic acids were detected with an ultraviolet detector (wave length 200 nm). Identification of organic acids in the xylem exudate was achieved by comparison of retention times with those of authentic organic acid standards (Wako Pure Chemical Industries, Osaka, Japan).
Anion (nitrate, phosphate and sulfate) concentrations were also determined using an HPLC system (an anion analyzer) (DX-120; Nippon Dionex K.K.). Fifteen microliters of exudate was diluted with 135 µL of distilled water. After filtration through the syringe filter described above, samples were applied to a column (InoPac AS14; Nippon Dionex K.K.) and eluted with 3.5 mmol L−1 Na2CO3 and 1.0 mmol L−1 NaHCO3. Eluted anions were detected with an electrical conductance detector (Nippon Dionex K.K.).
The concentrations of amino acids in the xylem exudate were determined using an amino acid analyzer (L-8000; Hitachi, Tokyo, Japan). Fifteen microliters of xylem exudate was diluted with 135 µL of 0.02 mol L−1 HCl. After centrifugation (at 11,000 g at 4ºC for 10 min) the supernatant liquid was collected and filtered through the disposable syringe filter unit described above. The filtrates were applied to the analyzer.
Determination of inorganic cations and cd concentration in the xylem exudate from oilseed rape plants
To analyze the concentrations of inorganic cations and Cd in the xylem exudate from oilseed rape plants, 15 µL of exudate was diluted with 5,985 µL of 0.1 mol L−1 HNO3 (the grade of HNO3 used was for the Analysis of Poisonous Metals; Wako Pure Chemical Industries). The concentrations of inorganic cations and Cd were determined using inductively coupled plasma spectrometry (IRIS Duo; Nippon Jarrell-Ash, Kyoto, Japan).
Protein analysis
Soluble proteins were extracted from the roots, stems and leaves of approximately 3-week-old oilseed rape seedlings. These samples were frozen with liquid nitrogen in a cold mortar and crushed to a powder. Proteins were extracted with a buffer containing 100 mmol L−1 Tris-HCl (pH 7.5), 5 mmol L−1β-mercaptoethanol and 1 mmol L−1 phenylmethanesulfonyl fluoride (PMSF). Subsequently, these extracts were homogenized with a pestle and centrifuged at 18,300 g at 4°C for 10 min. After centrifugation, the supernatant liquid portions were collected as soluble protein fractions. Protein concentrations in the exudate and in the root, stem and leaf extracts were determined using the Bradford method (CitationBradford 1976).
The protein profiles were examined using sodium dodecylsulfate-polyacrylamide gel electrophoresis (SDS-PAGE). For SDS-PAGE analysis, proteins in the xylem exudate were concentrated following the methods of CitationTsugita et al. (1996) with a small modification. First, four volumes of a solution containing 12.5% (w/v) trichloroacetic acid (TCA) and 25 mmol L−1 dithiothreitol (DTT) in acetone were added to the exudates. The mixture was incubated on ice for 45 min. After centrifugation (11,000 g at 4ºC for 15 min), the precipitant was collected and washed twice with 500 µL of a washing solution containing 20 mmol L−1 DTT in acetone to remove TCA from the precipitant. After washing, the proteins in the xylem exudate were collected by centrifugation and dried using a centrifugal concentrator (CC-105; Tomy Seiko, Saitama, Japan). Concentrated proteins were used for SDS-PAGE analysis. The proteins were separated on the 15% polyacrylamide gels using a Tris-glycine buffer system and detected using the silver-staining method (Daiichi Pure Chemicals, Tokyo, Japan).
RESULTS
Collection of xylem exudate from oilseed rape plants
After vertically cutting the base of the stem (approximately 2 cm above ground level), we could collect exudates from the cut surface of 6-week-old oilseed rape plants for 2 h. These exudates were clear and colorless. The average exudation rate from the cut surface of these plants was approximately 300 µL h−1, calculated by measuring the amount of exudate collected and dividing by the collection time. The pH of the exudate was estimated using pH test paper to be approximately 5.6.
Chemical composition of xylem exudate from oilseed rape plants
To confirm that the exudates collected from 6-week-old oilseed rape plants are derived from xylem vessels, the sugar, organic acid, anion, amino acid, inorganic cation and protein contents were investigated. Sucrose, fructose and glucose were detected in the exudates of these plants using HPLC analysis. The glucose concentration in the exudates was 0.30 mmol L−1. However, fructose and sucrose concentrations in the exudates were very low. Fructose concentration in the exudates was 0.087 mmol L−1 and only trace levels of sucrose were detected ().
The HPLC analysis of these exudates revealed that malic acid and citric acid were detected in the exudates (). The concentrations of malic acid and citric acid in the exudates were 0.74 mmol L−1 and 0.50 mmol L−1, respectively ().
The concentrations of anions (nitrate, sulfate and phosphate) are shown in . The concentrations of nitrate, sulfate and phosphate in the exudate were 15.6 mmol L−1, 6.8 mmol L−1 and 4.0 mmol L−1, respectively.
The results of free amino acid analyses are shown in and . The concentration of free amino acids in the exudates was 8.33 mmol L−1, averaged on three independently collected samples (). Our results indicated that glutamine was the predominant amino acid in the exudate, occurring at a concentration of 4.92 mmol L−1 (). Glutamine accounted for approximately 60% of the total amino acids present in the exudate ().
Table 1 Effects of the chemical composition of the xylem exudate from oilseed rape plants on Cd treatment
The concentrations of inorganic cations in the exudates are also presented in . Calcium, potassium and magnesium are the predominant cations. The concentrations of calcium, potassium, and magnesium were 7.56 mmol L−1, 6.37 mmol L−1 and 3.49 mmol L−1, respectively.
The concentrations of protein in the exudates are shown in . Protein concentrations in the exudates were approximately 13 µg mL−1. The SDS-PAGE analysis depicted in demonstrates that the protein composition in the xylem exudate was distinctly different from that of the extracts from roots, stems and leaves. Proteins with molecular weights of 13 kDa, 25 kDa and 45 kDa were characteristic of the xylem exudates from oilseed rape plants (arrowhead in ).
Cadmium concentration in the xylem exudate of oilseed rape plants
Xylem exudates were collected from the same age (6-week-old) oilseed rape plants that were treated with 10 µmol L−1 and 30 µmol L−1 Cd. Visual symptoms of Cd treatment, such as withering leaf and leaf chlorosis, were not observed under these experimental conditions. The exudation rate of the plants treated with Cd was approximately 240 µL h−1. The pH of these exudates was approximately 5.6. The Cd concentrations in the xylem exudate are shown in . After Cd treatment for 24 h, Cd was detected in the xylem exudate. The concentrations of Cd in the xylem exudate were 5.2 µmol L−1 (10 µmol L−1 Cd treatment) and 8.5 µmol L−1 (30 µmol L−1 Cd treatment). The Cd concentration in the xylem exudate increased with an increase in the Cd concentration of the hydroponic solution. In the case of the 10 µmol L−1 Cd treatment, the Cd concentration in the xylem exudate was approximately 5 µmol L−1 after 24 h of treatment (). The Cd concentration increased slowly after that and reached approximately 6.5 µmol L−1 when the plants were treated with 10 µmol L−1 Cd for 72 h. However, Cd concentration in the xylem exudate increased gradually up to approximately 16 µmol L−1 when the plants were treated with 30 µmol L−1 Cd for 72 h ().
Figure 1 Effect of Cd treatment on the protein concentration of the xylem exudates collected from oilseed rape plants. Plants were treated with 10 µmol L−1 and 30 µmol L−1 Cd-containing hydroponic solution for 48 h and the xylem exudates were collected after this time. Data are the mean ± standard deviation of three independent samples.
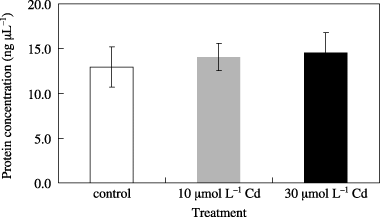
Table 2 Amino acid concentrations in the xylem exudate from oilseed rape plants treated with 0 (control), 10 or 30 µmol L−1 Cd for 48 h
Figure 2 Sodium dodecylsulfate-polyacrylamide gel electrophoresis (SDS-PAGE) analysis of proteins in the xylem exudates and proteins extracted from the roots, stems and leaves of oilseed rape plants. Three micrograms of protein was subjected to SDS-PAGE analysis and detected using silver staining in each lane.
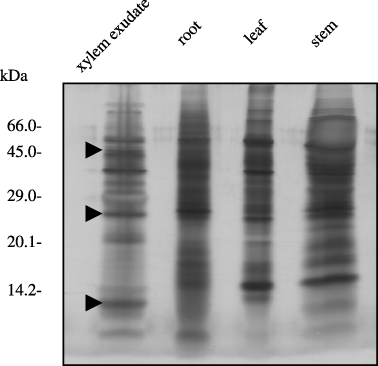
Figure 3 Cadmium concentration in the xylem exudate collected from oilseed rape plants. Plants were treated with 10 µmol L−1 (○) and 30 µmol L−1 (•) Cd for 24, 48 and 72 h. Data are the mean ± standard deviation of three independent samples.
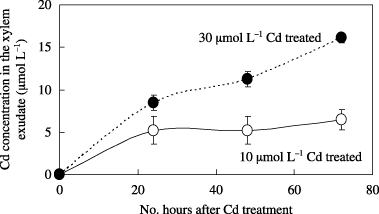
Table 3 Content of amino acids as a percentage of the total amino acids in the xylem exudate of oilseed rape plants
Response of the chemical compositions of the xylem exudate to Cd treatment
The effects of chemical components in the xylem exudates from 6-week-old oilseed rape plants on Cd treatment are shown in . Chemical compositions of the control plants were compared with those from Cd-treated plants (10 µmol L−1 Cd and 30 µmol L−1 Cd for 48 h). The glucose concentration in the xylem exudate decreased after Cd treatment. Meanwhile, the fructose concentration decreased after only 30 µmol L−1 Cd treatment.
The HPLC analysis of organic acids in the xylem exudate of oilseed rape plants revealed that the concentrations of malic acid and citric acid did not respond to Cd treatment ().
Nitrate and sulfate concentration in xylem exudates increased slightly with Cd treatment (). Phosphate concentration in the exudates decreased in the 30 µmol L−1 Cd treatment. However, we could not investigate significant differences among the anion concentrations in the xylem exudates from oilseed rape plants after Cd treatment.
Changes in the total amino acid concentration in the xylem exudate from oilseed rape plants are shown in . The total amino acid concentration in the xylem exudates was 8.33 mmol L−1 based on the average of three independent samples. The total amino acid concentration decreased after both Cd treatments (10 µmol L−1 and 30 µmol L−1). The concentration of total amino acids decreased to 6.4 mmol L−1 (10 µmol L−1 Cd treatment) and to 5.4 mmol L−1 (30 µmol L−1 Cd treatment) after 48 h of Cd treatment. We could not see a dose-dependency for total amino acid concentration. The concentrations of total amino acid decreased when plants were treated with either 10 µmol L−1 or 30 µmol L−1 Cd. Glutamine is the predominant amino acid in the xylem exudate (,). Aspartic acid, asparagine and threonine levels are also prominent, but less so than glutamine (,). The composition of amino acids in the xylem exudate was also compared. After 48 h of Cd treatment (10 µmol L−1), the ratio of aspartic acid to the total amino acids decreased by sixth part of the level in control plants. Meanwhile, this ratio decreased to approximately 50% of the level in the control plants when plants were treated with 30 µmol L−1 Cd. Cysteine and proline were detected in the xylem exudate after Cd treatment (,).
The effects of Cd treatment on almost every inorganic cation in the xylem exudates were not significant (). However, copper concentration in the xylem exudates increased after Cd treatment.
Response of proteins in the xylem exudate to Cd treatment
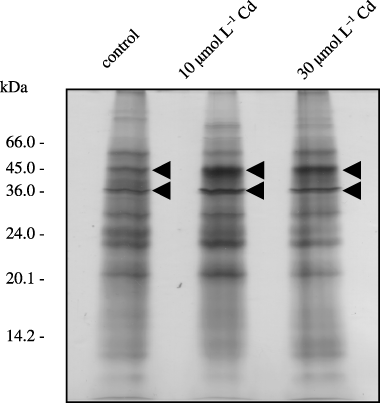
After Cd treatment, xylem exudate protein (XEP) concentrations were 14.1 µg mL−1 (10 µmol L−1 Cd treatment for 48 h) and 14.6 µg mL−1 (30 µmol L−1 Cd treatment for 48 h). Although the XEP concentration was slightly higher in the 30 µmol L−1 Cd treatment, these differences were not statistically significant when analyzed using a t-test (). These results indicate that Cd treatment does not affect protein concentration in the xylem exudate. The effect of Cd on the XEP profiles was investigated. After Cd treatment (10 µmol L−1 for 48 h and 30 µmol L−1 for 48 h), we confirmed that the XEP composition had changed (). Two proteins with molecular weights of 36 kDa and 45 kDa clearly increased in the xylem exudate as a result of Cd treatment (arrowheads in ). Proteins that decreased in abundance as a result of Cd treatment could not be detected on these silver-stained gels.
Figure 4 Analysis of xylem exudate proteins using sodium dodecylsulfate-polyacrylamide gel electrophoresis (SDS-PAGE). Xylem exudate samples were collected from control plants and 6-week-old plants were transferred to a hydroponic solution containing 10 µmol L−1 and 30 µmol L−1 Cd (CdCl2) and treated for 48 h. Three micrograms of protein was subjected to SDS-PAGE analysis and detected using silver staining in each lane.
DISCUSSION
Collection of xylem exudate from oilseed rape plants
Analysis of the pH and chemical components of the exudates collected from the cut end of oilseed rape plants revealed that the exudate is derived from xylem vessels. The pH of the exudate was estimated (using pH test paper) to be approximately 5.6, a value similar to that reported for the xylem exudate of other plants (sugar beet [CitationLópez-Millán et al. 2000]; caster bean plants [CitationSchurr and Schulze 1995]; tree tobacco [CitationHocking 1980]). The exudating rate derived from the xylem vessels was approximately 300 µL h−1. We confirm that the variation of this rate between plants was large in our experiment.
The HPLC analysis revealed that glucose, fructose and sucrose are present in the exudate as free sugars. The major sugar form in the exudate was glucose (). The low level of sucrose found in the exudate is similar to xylem sap from squash plants (CitationSatoh et al. 1992), caster bean plants (CitationSchurr and Schulze 1995) and tree tobacco plants (CitationHocking 1980). In oilseed rape plants approximately 100 mmol L−1 of sucrose is present in the cytosolic compartment of the whole leaf (CitationLohaus and Moellers 2000). Because sucrose and fructose concentrations in the exudate are at trace levels, the possibility that some substances are contaminating the exudate is very low.
The results of the organic acid analysis are shown in . The concentrations of organic acids that were detected in the exudate by the HPLC analysis were approximately 1.2 mmol L−1. This value is lower than that in the xylem sap of maize plants (CitationCanny and McCully 1988). In our HPLC analysis, oxalic acid was not detected because chloride and nitrate in the xylem exudate were disturbing the detection of oxalic acid. Oxalic acid was detected in the xylem sap of maize plants (CitationCanny and McCully 1988) as one of the predominant organic acids. The difference in total organic acid concentrations between these results may diminish if we could detect oxalic acid in the exudates from oilseed rape plants.
Anion analysis revealed that the exudate contained nitrate, phosphate and sulfate (). Our results showed that the concentrations of these anions were higher than those in the hydroponic solution. CitationCanny and McCully (1988) demonstrated that on a molecular basis, nitrogen was almost equally divided between amino acids and nitrate in the xylem sap of maize plants. Our results indicated that the ratio of nitrate to total amino acids was 1.34, a value similar to that in the xylem sap of maize plants. These results also confirm that these exudates were derived from the xylem vessels.
The concentration of total amino acids in the exudate was 8.3 mmol L−1 (). The amount of free amino acids in the exudate was similar to that found in tree tobacco plants (CitationHocking 1980) and maize plants (CitationCanny and McCully 1988). Our results indicated that glutamine was the predominant amino acid in the exudates of oilseed rape plants (). Glutamine was also the predominant amino acid in the xylem exudate of the plants mentioned above. These results further support the assumption that the exudate obtained from the cut end of the stem of oilseed rape plants was mostly derived from xylem vessels.
The concentrations of inorganic cations in the exudate are presented in . Calcium, potassium, magnesium and sodium predominated. These results were similar to those in the xylem sap of the tobacco tree (CitationHocking 1980).
The exudate from oilseed rape plants contained 13.0 µg mL−1 proteins (). The protein concentration in the exudate is approximately threefold lower than that found in cucumber and pumpkin (CitationBuhtz et al. 2004) and similar to that found in squash plants (CitationSatoh et al. 1992). Comparison of the bands of protein detected on the silver-stained gel established that the xylem exudate contained characteristic proteins (). The pattern of visible protein bands on the SDS-PAGE gel was similar to that of Brassicaceae (CitationBuhtz et al. 2004).
We conclude that the exudate collected from the cut end of oilseed rape plants is derived from xylem vessels based on its: (1) pH, (2) sugar content, (3) organic acid composition, (4) anion and amino acid composition, (5) inorganic cation content, (6) protein concentration and composition.
Response of the chemical composition of the xylem exudate of oilseed rape plants to Cd treatment
Cadmium treatment (10 µmol L−1 and 30 µmol L−1 for 24–72 h) did not substantially affect the exudation rate of xylem exudates in oilseed rape plants. The Cd concentration in the xylem exudate of these plants increased with increasing Cd levels in the hydroponic solution (). However, the Cd concentration in the xylem exudate reached approximately 50% of that in the hydroponic solution after 72 h of Cd treatment. In maize and tomato plants, Cd accumulates in their roots and transport to the shoot organs is restricted (CitationRauser 1986). In oilseed rape plants, a similar mechanism might function and control the transport of Cd to the shoot organs. Meanwhile, the Cd concentration in rice xylem exudates was approximately 19 µmol L−1 when rice plants were treated with 10 µmol L−1 Cd for 3 days (CitationTanaka et al. 2003). The Cd concentration in the xylem exudates was higher than that in the nutrient solution. Thus, a different mechanism for long-distance Cd transport might function in rice plants.
We could not find a response for organic acids (malic acid and citric acid) in the xylem exudate from oilseed rape plants to Cd treatment (). However, the concentration of malic acid and citric acid were enough to bind Cd transported via the xylem vessels. Further research is necessary to elucidate the function of organic acids in the xylem exudate.
The total amino acid concentration in the xylem exudate decreased as a result of Cd treatment (). In pea seedlings, nitrate reductase activity was inhibited by Cd treatment (CitationHernández et al. 1996). If nitrate reductase activity was inhibited in the roots of oilseed rape plants, the total amino acid concentration might decrease in the xylem exudate. Despite a decrease in the total amino acid concentration in the xylem exudate, the glutamine concentration in the sap remained at high levels and the ratio of glutamine to the total amino acids increased (,). In Polygonum thunberigii, glutamine was detected as a compound that bound Cd in the xylem (CitationShinmachi et al. 2003). Glutamine might be functioning as a Cd-binding compound in the xylem exudate. The concentration of glutamine in the xylem exudate from oilseed rape plants is stoichiometrically sufficient when Cd is binding with glutamine in the xylem vessels.
Proline accumulation was also induced in the xylem exudate by Cd treatment (,). Proline was reported to play an important role in ameliorating the toxicity of heavy metals in microalgae (CitationSiripornadulsil et al. 2002). Proline might be functioning as a modulator of heavy metal toxicity in the xylem vessels of oilseed rape plants. Cysteine levels also increased in the xylem exudate (,). Cysteine is an essential amino acid for phytochelatin, which is involved in the detoxification of heavy metals. Cadmium treatment might induce the synthesis of cysteine in the roots. In maize seedlings, the pools of cysteine in the roots did not rise significantly with Cd treatment (CitationRauser et al. 1991). Cysteine in the xylem exudate might be loaded positively into the xylem vessels with Cd treatment.
Aspartic acid concentration in the xylem exudate was drastically decreased by Cd treatment (). In the plant kingdom, asparagine, isoleucine, lysine, methionine and threonine are biosynthesized from aspartic acid. Concentrations of these amino acids were not changed by Cd treatment (). To keep these amino acid concentrations, aspartic acid might be used under Cd-treated conditions. Further research is necessary to elucidate how these amino acids function in the xylem vessels of Cd-treated plants.
Inorganic cation concentration and anion concentration did not change drastically (). In tobacco seedlings, treatment with 100 µmol L−1 of Cd for 2 weeks induced Fe deficiency responses (CitationYoshihara et al. 2006). We could not investigate these symptoms in our experimental conditions. With regard to these points, we can confirm that Cd exposure in our experiments did not have serious problems for oilseed rape plants.
Protein assay analysis demonstrated that Cd did not have an effect on XEP concentration (). The results suggest that XEP might mainly play a housekeeping function. Two proteins (36 kDa and 45 kDa) increased in the xylem exudate with Cd treatment (). Two 20 kDa proteins (pI 4.35 and 4.40) were detected using 2D-PAGE analysis of Cd-induced protein in Datura innoxia (Mill.) cells (CitationUrwin et al. 1996). However, these proteins were not identified. If the Cd-induced XEP are identified, the mechanism of Cd transport or detoxification might be elucidated. Proteins with molecular weights of 13 kDa, 25 kDa and 45 kDa were characteristic of the exudates (). These differences in the protein composition reflect the physiological functions of xylem vessels, for example, long-distance transport of materials and signal transport. Identification of these proteins leads to elucidating the functions of xylem vessels.
We have confirmed that the exudates from the cut end of the stem are derived from xylem vessels and we have characterized the effect of Cd treatment on the amino acid, organic acid and protein composition of the xylem exudate of oilseed rape plants. Further research to characterize these compounds may provide clues to elucidate the mechanism of Cd transport in the xylem vessels. The application of this information is a step toward the practical use of oilseed rape plants in a phytoremediation scheme.
ACKNOWLEDGMENTS
This study was supported by the Research and Development Program for New Bio-industry Initiatives of the Bio-oriented Technology Research Advancement Institution.
REFERENCES
- Bradford , MM . 1976 . A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding . Anal. Biochem , 72 : 248 – 254 .
- Buhtz , A , Kolasa , A , Arlt , K , Walz , C and Kehr , J . 2004 . Xylem sap protein composition is conserved among different plant species . Planta , 219 : 610 – 618 .
- Canny , MJ and McCully , ME . 1988 . The xylem sap of maize roots: its collection, composition and formation . Aust. J. Plant Physiol , 15 : 557 – 566 .
- Cataldo , DA , Garland , TR and Wildung , RE . 1983 . Cadmium uptake kinetics in intact soybean plants . Plant Physiol , 73 : 844 – 848 .
- Cavell , AC , Lydiate , DJ , Parkin , IAP , Dean , C and Trick , M . 1998 . Collinearity between a 30-centimorgan segment of Arabidopsis thaliana chromosome 4 and duplicated regions within the Brassica napus genome . Genome , 41 : 62 – 69 .
- Chaney , RL , Malik , M Li , YM . 1997 . Phytoremediation of soil metals . Curr. Opin. Biotechnol , 8 : 279 – 284 .
- Clemens , S , Palmgren , MG and Krämer , U . 2002 . A long way ahead: understanding and engineering plant metal accumulation . Trends Plant Sci , 7 : 309 – 315 .
- Florijin , PJ , Knecht , JA and Van Beusichem , ML . 1993 . Phytochelatin concentration and binding state of Cd in roots of maize genotypes differing in shoot/root Cd partitioning . J. Plant Physiol , 142 : 537 – 542 .
- Florijin , PJ and Van Beusichem , ML . 1993 . Uptake and distribution of cadmium in maize inbred lines . Plant Soil , 150 : 25 – 32 .
- Hernández , LE , Carpena-Ruiz , R and Gárate , A . 1996 . Alterations in the mineral nutrition of pea seedlings exposed to cadmium . J. Plant Nutri , 19 : 1581 – 1598 .
- Hocking , PJ . 1980 . The composition of phloem exudates and xylem sap from tree tobacco (Nicotiana glauca Grah.) . Ann. Bot , 45 : 633 – 643 .
- Krämer , U , Cotter-Howells , JD , Charnock , JM , Baker , AJM and Smith , JAC . 1996 . Free histidine as a metal chelator in plants that accumulate nickel . Nature , 379 : 635 – 638 .
- Larsson , EH , Bornman , JF and Asp , H . 1998 . Influence of UV-B radiation and Cd2+on chlorophyll fluorescence, growth and nutrient content in Brassica napus . J. Exp. Bot , 49 : 1031 – 1039 .
- Lohaus , G and Moellers , C . 2000 . Phloem transport of amino acids in two Brassica napusL. genotypes and one B. carinatagenotype in relation to their seed protein content . Planta , 211 : 833 – 840 .
- López-Millán , AF , Morales , F , Abadía , A and Abadía , J . 2000 . Effects of iron deficiency on the composition of the leaf apoplastic fluid and xylem sap in sugar beet. Implication for iron and carbon transport . Plant Physiol , 124 : 873 – 884 .
- Ma , JF and Hiradate , S . 2000 . Form of aluminium for uptake and translocation in buckwheat (Fagopyrum esculentum Moench) . Planta , 211 : 355 – 360 .
- McLaughlin , MJ , Parker , DR and Clarke , JM . 1999 . Metals and micronutrients – food safety issues . Field Crops Research , 60 : 143 – 163 .
- Nakamura , S , Watanabe , A Chongpraditnun , P . 2004 . Analysis of phloem exudates collected from fruit-bearing stems of coconut palm: palm trees as a source of molecules circulating in sieve tubes . Soil Sci. Plant Nutr , 50 : 739 – 745 .
- O’Halloran , TV and Culotta , VC . 2000 . Metallochaperones, an intracellular shuttle service for metal ions . J. Biol. Chem , 275 : 25057 – 25060 .
- Obata , H and Umebayashi , M . 1997 . Effects of cadmium on mineral nutrient concentration in plants differing in tolerance for cadmium . J. Plant Nutr , 20 : 97 – 105 .
- Palmer , C , Warwick , S and Keller , W . 2001 . Brassicaceae (Cruciferae) family, plant biotechnology and phytoremediation . Int. J. Phytoremediation , 3 : 245 – 287 .
- Raskin , I , Smith , RD and Salt , DE . 1994 . Phytoremediation of metals: using plants to remove pollutants from the environment . Curr. Opin. Biotecnol , 8 : 221 – 226 .
- Rauser , WE . 1986 . The amount of cadmium associated with Cd-binding protein in root of Agrostis gigantean maize and tomato . Plant Sci , 43 : 85 – 91 .
- Rauser , WE , Schupp , R and Rennenberg , H . 1991 . Cysteine, γ-glutamylcysteine, and glutathione levels in maize seedlings: distribution and translocation in normal and cadmium-exposed plants . Plant Physiol , 97 : 128 – 138 .
- Salt , DE , Prince , RC , Pickering , IJ and Raskin , I . 1995 . Mechanisms of cadmium mobility and accumulation in Indian mustard . Plant Physiol , 109 : 1427 – 1433 .
- Satoh , S , Iizuka , C , Kikuchi , A , Nakamura , N and Fujii , T . 1992 . Proteins and carbohydrates in xylem sap from squash root . Plant Cell Physiol , 33 : 841 – 847 .
- Schurr , U and Schulze , ED . 1995 . The concentration of xylem sap constituents in root exudates, and in sap from intact, transpiring castor bean plants (Ricinus communis L.) . Plant Cell Environ , 18 : 409 – 420 .
- Senden , MHMN , Van Paassen , FJM , Van der Meer , AJGM and Wolterbeek , HT . 1992 . Cadmium–citric acid–xylem cell wall interactions in tomato plants . Plant Cell Environ , 15 : 71 – 79 .
- Shah , K and Dubey , RS . 1995 . Effect of cadmium on RNA level as well as activity and molecular forms of ribonuclease in growing rice seedlings . Plant Physiol. Biochem , 33 : 577 – 584 .
- Shinmachi , F , Kumanda , Y , Noguchi , A and Hasegawa , I . 2003 . Translocation and accumulation of cadmium in cadmium-tolerant Polygonum thunbergii . Soil Sci. Plant Nutr , 49 : 355 – 361 .
- Siripornadulsil , S , Traina , S , Verma , DPS and Sayre , RT . 2002 . Molecular mechanisms of proline-mediated tolerance to toxic heavy metals in transgenic microalgae . Plant Cell , 14 : 2837 – 2847 .
- Tanaka , K , Fujimaki , S , Fujiwara , T , Yoneyama , T and Hayashi , H . 2003 . Cadmium concentrations in the phloem sap of rice plants (Oryza sativa L.) treated with a nutrient solution containing cadmium . Soil Sci. Plant Nutr , 49 : 311 – 313 .
- Tanaka , K , Fujimaki , S , Fujiwara , T , Yoneyama , T and Hayashi , H . 2007 . Quantitative estimation of the contribution of the phloem in cadmium transport to grains in rice plants (Oryza sativa L.) . Soil Sci. Plant Nutr , 53 : 72 – 77 .
- Tsugita , A , Kamo , M , Kawakami , T and Ohki , Y . 1996 . Two-dimensional electrophoresis of plant proteins and standardization of gel patterns . Electrophoresis , 17 : 855 – 865 .
- Urwin , PE , Groom , QJ and Robinson , NJ . 1996 . Characterization of two cDNAs and identification of two proteins that accumulate in response to cadmium in cadmium-tolerant Datura innoxia (Mill.) cells . J. Exp. Bot , 47 : 1019 – 1024 .
- Yoshihara , T , Hodoshima , H , Miyano , Y , Shoji , K , Shimada , H and Goto , F . 2006 . Cadmium inducible Fe deficiency responses observed from macro and molecular views in tobacco plants . Plant Cell Rep , 28 : 1361 – 1367 .