Abstract
Several researchers have hypothesized that organic nitrogen extracted using neutral phosphate buffer, termed phosphate buffer extractable organic nitrogen (PEON), is absorbed and utilized by some plant species as a nitrogen source without prior mineralization. Earlier reports have suggested that leaf lettuce (Lactuca sativa L.) depends mainly on inorganic nitrogen, whereas qing-geng-cai (Brassica chinensis L.) can absorb PEON. We tested leaf lettuce and qing-geng-cai growth under aseptic conditions using three nitrogen sources under different light conditions to investigate this hypothesis. Nitrate, amino acids or purified PEON were supplied as the sole nitrogen source. Five different levels of light intensity were used to detect the contribution of carbon to plant growth supplied from PEON. Leaf lettuce growth was promoted only by nitrate, whereas growth of qing-geng-cai was promoted by nitrate and amino acids. The positive effect of amino acids on qing-geng-cai was pronounced at higher light intensity. However, PEON imparted no significant effect on either of the plants under any of the light conditions. Our results do not support the hypothesis that PEON is used as a nitrogen source by plants.
INTRODUCTION
Soil organic nitrogen (N) has recently been widely recognized as a N source that can be absorbed directly and used by various plants (CitationSchimel and Bennett 2004). This recognition has spurred a re-evaluation of the traditional view of N dynamics in terrestrial ecosystems, which is based on the assumption that only inorganic N is available to plants (CitationLipson and Näsholm 2001; CitationSchimel and Bennett 2004). Among organic N types, low molecular weight constituents, mainly amino acids, have been studied intensively (CitationLipson and Näsholm 2001). The ability of plants to take up intact amino acids as a N source has been demonstrated in laboratory and field experiments.
Unlike low molecular weight organic N, high molecular organic N has attracted less attention as a N source because of a general understanding that plant roots are incapable of directly absorbing and using high molecular weight organic matter (CitationJones et al. 2005). However, the results of some studies have suggested that the soil organic N of high molecular weight matter might also be taken up directly by plant roots (CitationKoga et al. 2001; CitationMatsumoto et al. 1999, Citation2000c; CitationOkamoto and Okada 2004; CitationYamagata et al. 2001). These studies have specifically addressed soil organic N extracted by neutral phosphate buffer, which has been termed phosphate buffer extractable organic nitrogen (PEON) by CitationMatsumoto et al. (2000a). In fact, PEON has been described as “a protein-like N compound with a uniform molecular weight of 8,000–9,000” (CitationMatsumoto and Ae 2004). It is considered to originate from the cell walls of bacteria and can be extracted irrespective of soil type (CitationMatsumoto et al. 2000b).
The hypothesis of direct uptake of PEON was derived originally from the fact that the N uptake responses of plants to organic fertilization differed among species. Previous pot experiments have shown repeatedly that certain species assimilate more N in an organically fertilized treatment (low inorganic N and high PEON concentrations) than in an inorganically fertilized treatment (high inorganic N and low PEON concentrations) (CitationMatsumoto et al. 1999, Citation2000c; CitationOkamoto and Okada 2004; CitationYamagata et al. 2001). The difference in the ability of plants to uptake N under low inorganic N conditions has been found to coincide with the difference in the rate at which PEON decreased from the level in the culture solution over a 3-h culturing (CitationOkamoto and Okada 2004). A considerable amount of PEON was detected in plant roots after 6-h culturing with a solution containing biotin-labeled PEON (CitationKoga et al. 2001). The xylem sap of qing-geng-cai was analyzed using high-performance liquid chromatography with a size exclusion column. A peak similar to that of PEON was found in plants grown in a soil culture, but not from those plants grown in an inorganic solution culture (CitationMatsumoto et al. 2000c). The amount of N uptake by qing-geng-cai and carrot increased when PEON was supplied aseptically (CitationMatsumoto et al. 2000c).
If intact PEON were also an organic N source to be absorbed and used by plants, as hypothesized in the studies described above, the paradigm change in the terrestrial N cycle could be much more substantial than that of amino acids; the quantity of PEON in the soil is much higher than that of free amino acids (CitationLipson and Näsholm et al. 2001; CitationMatsumoto et al. 2000c). However, little evidence directly supports the contribution of PEON to plant growth; moreover, nothing is known about how efficiently plants can use PEON as a source of N.
Another issue exists concerning PEON utilization by plants. Both N and carbon (C) might contribute to plant growth when plants absorb organic matter. Experiments investigating the plant uptake of dual-labeled (13C and 15N) amino acids have shown that plants assimilate C as well as N (CitationInagaki and Kohzu 2005; CitationNäsholm et al. 2000). Plant dry weight increased by up to 30% when plant roots were supplied with dissolved inorganic C (CitationBialczyk et al. 1996; CitationVapaavuori and Pelkonen 1985). Root-absorbed inorganic C was transferred to shoots through the xylem sap in the form of organic compounds and constituted up to 10% of the CO2-assimilating photosynthetic activity of plants (CitationCramer and Richards 1999). PEON also contains carbon and its C:N ratio is approximately 8 to 15 (CitationAoyama 2006; CitationMatsumoto and Ae 2004). If PEON were absorbed directly, an appreciable amount of C would also be incorporated through the roots; can it also be a C source for plants?
We tested the growth of two vegetable species under aseptic conditions with three N sources to investigate whether plants use PEON as a source of N and C. We used leaf lettuce (Lactuca sativa L.) and qing-geng-cai (Brassica chinensis L.; “chingensai” in Japanese) because the results of previous experiments suggested that leaf lettuce depends mainly on inorganic N, whereas qing-geng-cai is capable of absorbing PEON (CitationMatsumoto et al. 1999, Citation2000c). The experiments were conducted under five different levels of light intensity to detect, indirectly, the contribution of C from the organic supplements to plant growth.
MATERIALS AND METHODS
Phosphate buffer extractable organic nitrogen purification
A soil sample was collected from a fallow field at the National Agricultural Research Center for Tohoku Region (Andosol, clay loam, pH 5.7, total C of 5.14%, total N of 0.37%). The air-dried soil (2.4 kg) was shaken for 1 h with 6 L phosphate buffer (1/15 mol L−1, pH 7.0). The suspension was centrifuged at 12,250 g for 10 min. The supernatant was passed through a glass-fiber filter (GA200; Advantec Toyo Kaisha; Tokyo, Japan) and concentrated approximately 10-fold using a rotary evaporator (N-1000; Tokyo RikaKikai; Tokyo, Japan). The concentrate was filtered through a cellulose acetate membrane filter (0.2 µm; Advantec Toyo Kaisha). The filtered sample was mounted (10 mL per loading) on a preparative size exclusion chromatography (PSEC) column (50 cm with 5 cm diameter, packed with Toyopearl HW-55F; Tosoh Corporation; Tokyo, Japan). Then PSEC was conducted with a mobile phase of 50 mmol L−1 phosphate buffer at a rate of 1.5 mL min−1. Using a fraction collector (DC1500; Tokyo RikaKikai), 12 fractions per cycle were collected with a fraction size of 18 mL. These fractions differed in their apparent molecular weight and hydrophobicity (CitationMiyazawa and Murayama 2007). Therefore, the fractions that had apparent molecular weights ranging from 6,000 to 11,000 Da based on the protein standard were retained for further purification. The PSEC procedure was repeated until a fraction of approximately 650 mL was obtained. The fraction was concentrated approximately threefold using the rotary evaporator. Approximately 100 mL of the concentrate was dialyzed using a cellulose membrane (3,500 MW CO, Cellu Sep; Membrane Filtration Products; Seguine, TX, USA) in a 5 L container with distilled water. The water was changed daily and dialysis was stopped when no inorganic N or phosphorus were detected in the dialysate water. The final concentration of PEON-N in the retentate was measured using an alkaline persulfate oxidation technique (CitationCabrera and Beare 1993). A persulfate reagent mixture for oxidation was added to 50-fold diluted samples and autoclaved at 120°C for 30 min. Then the nitrate concentration was measured using Cataldo's method (CitationCataldo et al. 1975). The residual phosphorus concentration was measured using the molybdenum blue method. The residual concentration of inorganic N was determined using an auto-analyzer (AAII, BL TEC K.K.; Tokyo, Japan). The dialysis procedure was conducted twice; consequently, two retentates that contained PEON at slightly different concentrations were obtained. The concentrations of PEON-N were 37.2 and 35.5 mg L−1, and those of phosphorous were 1.3 and 5.4 mg L−1. No inorganic N was detected. The former PEON solution was used for the light intensity treatments L1, L2 and L3, and the latter for L4 and L5. No degradation of PEON was observed during the purification procedures (CitationMiyazawa and Murayama 2007).
Aseptic culture experiment
Test tubes (25 mm × 120 mm) containing 3 g of vermiculite were sealed (MilliWrap; Millipore Corporation; Billeriea, MA, USA) and autoclaved at 120°C for 20 min. Autoclaved Hoagland solution without N was dispensed 4.5 mL per test tube. For N treatment, 4.5 mL of the following solutions were added to the test tubes: (1) control (distilled water), (2) nitrate, (3) amino acids (4) PEON. The N concentrations of the nitrate solution and the amino acid solution were adjusted to be equal to that of the PEON solution. The amino acid solution consisted of 15 amino acids based on the amino acid composition of PEON reported by CitationHiguchi (1982). The molecular weights of the amino acids ranged from 75 to 181. Because the dialyzed PEON solution still contained some phosphorus, the amount of phosphorus in the treatments was adjusted by adding phosphate buffer to the control, nitrate and amino acid solutions. For sterilization, the control and nitrate solutions were autoclaved, and the amino acid and PEON solutions were filtered through MILLEX-GS (0.22 µm; Millipore Corporation). Leaf lettuce (Lactuca sativa L. cv. Green) and qing-geng-cai (Brassica chinensis L. cv. Choyo) seeds were surface sterilized in 0.75% hypochlorous acid solution with a drop of TWEEN20 for 15 min and rinsed with sterilized water. One seed was sown in each test tube with four replicates per treatment. Subsets of these seeds were germinated on MS agar media to ensure the validity of sterilization. The experiment was conducted in growth chambers with five different light intensities: L1 (0 µmol m−2 s−1), L2 (16 µmol m−2 s−1), L3 (160 µmol m−2 s−1), L4 (250 µmol m−2 s−1) and L5 (500 µmol m−2 s−1). The growth chambers used were the NK system (LPH-1000MP; Nippon Medical and Chemical Instruments; Osaka, Japan) for L1, L2 and L3, and Koitotron (KG-106SLD/S; Koito Industries; Yokohama, Japan) for L4 and L5 with growth conditions of 25/15°C (day/night) with a 12-h photoperiod. The growth period was 14 days except for L1 (L1 was terminated at 10 days, which was before the plants reached the top of the test tube). The fresh biomass and height of each plant were measured. The root biomass was also measured in L4 and L5. Plant tissues were combined for each treatment and dried at 70°C for 24 h. The dry weight was measured per treatment, rather than per sample, to avoid artifacts caused by measuring ultra-light samples.
Statistical procedures
Statistical analyses were conducted using SAS 9.1 (SAS Institute, Cary, NC, USA). A one-way anova was conducted for the N source effect at each light intensity level, followed by one-tailed Dunnett's procedures to test the significance of differences between the control and each N treatment, or Tukey's procedures to test all pairwise comparisons (maintaining the α level at 0.05). The dry weight of the plants was not analyzed statistically because the values were obtained by dividing the weight of a composite sample by the number of replications.
RESULTS
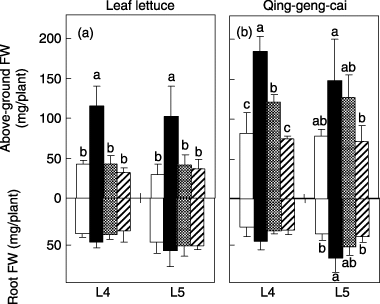
Total fresh biomass was greater in the nitrate treatment compared to that of the control, especially at higher light intensities, in both species (). Qing-geng-cai responded to the amino acid treatment as well. The biomass of both species under PEON treatment did not differ from the control at any light intensity. Total dry biomass also showed a similar pattern (). The height of the plants generally decreased as the light intensity increased (). The effect of the N source resembled that of the biomass: leaf lettuce responded to nitrate only; qing-geng-cai responded to both nitrate and amino acids. The positive effect of amino acids on qing-geng-cai was pronounced at higher light intensities (). The above-ground and root biomass were further investigated in L4 and L5 (). Both above-ground and root biomass showed a pattern similar to that of total biomass. The difference between the nitrate and amino acid treatments in the above-ground biomass of qing-geng-cai was not statistically significant in L5 ().
DISCUSSION
Purified PEON showed no effect on the growth of leaf lettuce and qing-geng-cai under the aseptic conditions used in our experiment, which suggests that these plants did not use PEON. This result is contradictory to the results reported by CitationMatsumoto et al. (2000c); when PEON solution was added to N-free MS liquid medium qing-geng-cai grew better than the control. However, these researchers used N-free MS medium as the control, which should have had the highest osmotic pressure among all the treatments. We used N-free Hoagland solution (half-strength because treatment solutions of the same volume were added) for all treatments, including the control. An appropriate salt concentration may be necessary when evaluating plant growth to avoid the
Figure 1 Total fresh weight (FW) and dry weight (DW) of leaf lettuce (a,c) and qing-geng-cai (b,d) under four nitrogen treatments and at five light intensities (L1–L5). The total FW data represent the mean ± standard deviation (n = 4), and the total DW represent the mean values. An asterisk indicates that the value differs significantly from the control at each light intensity treatment (Dunnett's procedure). PEON, phosphate buffer extractable organic nitrogen.
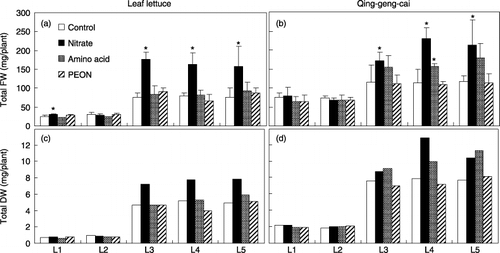
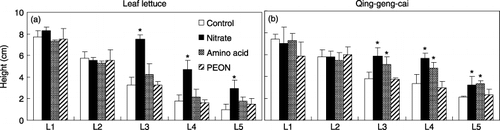
Figure 2 Height of (a) leaf lettuce and (b) qing-geng-cai under four nitrogen treatments and at five light intensities (L1–L5). The data represent the mean ± standard deviation (n = 4). An asterisk indicates a significant difference from the control at each light intensity treatment (Dunnett's procedure).
Assuming that PEON was not responsible for promoting plant N acquisition unless it was mineralized, can any other hypothesis explain the phenomenon of the different responses of plant species to organic fertilization shown in this series of pot experiments? In the previous pot experiments, carrot, qing-geng-cai and spinach assimilated more N when they were supplied with organic materials (a mixture of rice bran and rice straw, or rapeseed cake) than when supplied with
Figure 3 Above-ground and root fresh weights (FW) of (a) leaf lettuce and (b) qing-geng-cai under four nitrogen treatments and at two light intensities (L4–L5). The data represent the mean ± standard deviation (n = 4). Nitrogen treatments with different letters at each light intensity treatment indicate a significant difference (Tukey's procedure). The graph legend is the same as that used in Fig. 2.
One reason that might partly explain this phenomenon is the difference in the use of amino acids by these plants. In our experiment, leaf lettuce and qing-geng-cai differed in their responses to amino acids. Qing-geng-cai might have an advantage compared to leaf lettuce when amino acid N is supplied through organic materials. Amino acid utilization by rice as a N source is well known (CitationMori and Nishizawa 1979). Another possibility is the difference in the N mineralization rate. Both PEON and dissolved organic N are considered to be immobilized and stabilized by soil minerals (CitationIto and Ae 2000; CitationKalbitz et al. 2005). Some plant species are known to be able to release H+and OH− and/or excrete organic anions, which can drastically change the chemical conditions and nutrient availability in the rhizosphere (CitationBertrand et al. 1999; CitationJones 1998). The difference in the ability of desorption of organic N might have affected N mineralization, resulting in different responses to organic materials among species. However, these suppositions still do not explain why some plants grew better under organically fertilized treatments even if they had much less inorganic N available than their inorganically fertilized counterparts.
Another speculation worth exploring is the contribution of C supplied by organic materials. In fact, C supply through roots can contribute to C assimilation of plants, especially when plants are exposed to high temperature, high irradiation and salinity stress, which endanger low stomatal conductance (CitationCramer and Richards 1999). In our experiment, the above-ground biomass of qing-geng-cai under the amino acid treatment showed a tendency to increase as the light intensity increased, and the biomass approached that obtained under the nitrate treatment. Although our light intensity level was not extremely high, even at the highest level (500 µmol m−2 s−1), the amino acids might have contributed partially to plant C acquisition when C demand was high. If the organic materials supplied had significantly increased the C concentration in an artificial soil condition, it may have overridden the effect of the inorganic N availability. Dissolved inorganic C has also been reported to stimulate N uptake (CitationBialczyk 1994; CitationCramer and Lips 1995).
Our results did not support the hypothesis that PEON is used directly by plants as a N source. However, this is not to deny the possibility of PEON uptake by plant roots. Macromolecules have long been known to be absorbed directly by roots (CitationMcLaren et al. 1960; CitationNishizawa and Mori 1977). Thus, PEON might have been taken up in our experiment, but did not affect plant growth. Furthermore, our results with young seedlings under aseptic condition provide only a limited indication of PEON utilization by plants in situ. As plants develop and encounter stress events, plant roots might respond by activating strategies for nutrient acquisition, which our experiment might not have been able to detect. For example, invagination of cortex cells of plant roots, which incorporated polysaccharides into root cells, was stimulated by desiccation stress (CitationNishizawa and Mori 1984, Citation1986).
Recent studies have indicated that PEON is not a homogeneous compound, but rather a humic substance (CitationAoyama 2006; CitationMiyazawa and Murayama 2007). Numerous technical difficulties arise in demonstrating organic N utilization by plants in situ, even in studies using simple amino acids (CitationJones et al. 2005). Soil organic matter, such as PEON, whose composition is yet to be identified, requires further studies before any conclusion can be reached as to its actual contribution to plant nutrient acquisition.
We would like to thank Dr Noriharu Ae, Dr Nobuhisa Koga, Dr Miwa Okamoto and Dr Kensuke Okada for kindly providing advice on the experimental procedures, and Toshiyuki Kimura and Masahiro Suzuki for valuable technical advice on the PEON purification procedures.
REFERENCES
- Aoyama , M . 2006 . Properties of neutral phosphate buffer extractable organic matter in soils revealed using size exclusion chromatography and fractionation with polyvinylpyrrolidone . Soil Sci. Plant Nutr , 52 : 378 – 386 .
- Bertrand , I , Hinsinger , P , Jaillard , B and Arvieu , JC . 1999 . Dynamics of phosphorus in the rhizosphere of maize and rape grown on synthetic, phosphated calcite and goethite . Plant Soil , 211 : 111 – 119 .
- Bialczyk , J . 1994 . Growth of tomato seedlings under different HCO3-concentration in the medium . J. Plant Nutr , 17 : 801 – 816 .
- Bialczyk , J , Lechowski , Z and Libik , A . 1996 . Fruiting of tomato cultivated on medium enriched with bicarbonate . J Plant Nutr , 19 : 305 – 321 .
- Cabrera , ML and Beare , MH . 1993 . Alkaline persulfate oxidation for determining total nitrogen in microbial biomass extracts . Soil Sci. Soc. Am. J , 57 : 1007 – 1012 .
- Cataldo , DA , Haroon , M , Schrader , LE and Youngs , VL . 1975 . Rapid colorimetric determination of nitrate in plant tissue by nitration of salycylic acid . Commun. Soil Sci. Plant Anal , 6 : 71 – 80 .
- Cramer , MD and Lips , SH . 1995 . The influence of enriched root-zone CO2concentrations on growth, nitrogen metabolism and root HCO3-incorporation in salinity stressed Lycopersicon esculentum . Physiol. Plantarum , 94 : 425 – 432 .
- Cramer , MD and Richards , MB . 1999 . The effect of rhizosphere dissolved inorganic carbon on gas exchange characteristics and growth rates of tomato seedlings . J. Exp. Bot , 50 : 79 – 87 .
- Higuchi , M . 1982 . Characterization of soil organic nitrogen extracted with phosphate buffer . Jpn. J. Soil Sci. Plant Nutr , 53 : 1 – 5 . (in Japanese)
- Inagaki , Y and Kohzu , A . 2005 . Microbial immobilization and plant uptake of different N forms in three forest types in Shikoku district, southern Japan . Soil Sci. Plant Nutr , 51 : 667 – 670 .
- Ito , C and Ae , N . 2000 . Inference of form of available nitrogen in soils extracted by chemical solutions . Jpn. J. Soil Sci. Plant Nutr , 71 : 777 – 785 . (in Japanese with English summary)
- Jones , DL . 1998 . Organic acids in the rhizosphere – a critical review . Plant Soil , 205 : 25 – 44 .
- Jones , DL , Healey , JR , Willett , VB , Farrar , JF and Hodge , A . 2005 . Dissolved organic nitrogen uptake by plants – an important N uptake pathway? . Soil Biol. Biochem , 37 : 413 – 423 .
- Kalbitz , K , Schwesig , D , Rethemeyer , J and Matzner , E . 2005 . Stabilization of dissolved organic matter by sorption to the mineral soil . Soil Biol. Biochem , 37 : 1319 – 1331 .
- Koga , N , Yamagata , M , Matsumoto , S and Ae , N . 2001 . “ Evidence for direct organic nitrogen uptake by plants using specific tracer proteins ” . In Book Series Developments in Plant and Soil Sciences 92, Plant Nutrition , Edited by: Horst , WJ , Schenk , MK Bürkert , A . 212 – 213 . Dordrecht, , Netherlands : Springer .
- Lipson , D and Näsholm , T . 2001 . The unexpected versatility of plants: organic nitrogen use and availability in terrestrial ecosystems . Oecologia , 128 : 305 – 316 .
- Matsumoto , S and Ae , N . 2004 . Characteristics of extractable soil organic nitrogen determined by using various chemical solutions and its significance for nitrogen uptake by crops . Soil Sci. Plant Nutr , 50 : 1 – 9 .
- Matsumoto , S , Ae , N and Yamagata , M . 1999 . Nitrogen uptake response of vegetable crops to organic materials . Soil Sci. Plant Nutr , 45 : 269 – 278 .
- Matsumoto , S , Ae , N and Yamagata , M . 2000a . Extraction of mineralizable organic nitrogen from soils by a neutral phosphate buffer solution . Soil Biol. Biochem , 32 : 1293 – 1299 .
- Matsumoto , S , Ae , N and Yamagata , M . 2000b . The status and origin of available nitrogen in soils . Soil Sci. Plant Nutr , 46 : 139 – 149 .
- Matsumoto , S , Ae , N and Yamagata , M . 2000c . Possible direct uptake of organic nitrogen from soil by chingensai (Brassica campestris L.) and carrot (Daucus carota L.) . Soil Biol. Biochem , 32 : 1301 – 1310 .
- McLaren , AD , Jensen , WA and Jacobson , L . 1960 . Absorption of enzymes and other proteins by barley roots . Plant Physiol , 35 : 549 – 556 .
- Miyazawa , K and Murayama , T . 2007 . Heterogeneity of neutral phosphate buffer extractable soil organic matter . Soil Sci. Plant Nutr , 53 : 1 – 6 .
- Mori , S and Nishizawa , N . 1979 . Nitrogen absorption by plant root from the culture medium where organic and inorganic nitrogen coexist . Soil Sci. Plant Nutr , 25 : 51 – 58 .
- Näsholm , T , Huss-Danell , K and Högberg , P . 2000 . Uptake of organic nitrogen in the field by four agriculturally important plant species . Ecology , 81 : 1155 – 1161 .
- Nishizawa , N and Mori , S . 1977 . Invagination of plasmalemma: Its role in the absorption of macromolecules in rice roots . Plant Cell Physiol , 18 : 767 – 782 .
- Nishizawa , N and Mori , S . 1984 . Desiccation-induced heterophagy in corn root cells . J. Electron. Microsc , 33 : 230 – 235 .
- Nishizawa , N and Mori , S . 1986 . Occurrence of stress grains in the root cells of broad bean and barley plants under desiccation conditions . Soil Sci. Plant Nutr , 32 : 599 – 608 .
- Okamoto , M and Okada , K . 2004 . Differential responses of growth and nitrogen uptake to organic nitrogen in four gramineous crops . J. Exp. Bot , 55 : 1577 – 1585 .
- Schimel , JP and Bennett , J . 2004 . Nitrogen mineralization: challenges of a changing paradigm . Ecology , 85 : 591 – 602 .
- Vapaavuori , EM and Pelkonen , P . 1985 . HCO3-uptake through the roots and its effect on the productivity of willow cuttings . Plant Cell Environ , 8 : 531 – 534 .
- Yamagata , M , Matsumoto , S and Ae , N . 2001 . “ Possibility of direct acquisition of organic nitrogen by crops ” . In Plant Nutrient Acquisition, New Perspective , NIAES Series 4 Edited by: Ae , N , Arihara , J , Okada , K and Srinirasan , S . 399 – 420 . Tokyo : Springer .