Abstract
To quantify the spatial variation and spatial structure of nitrous oxide (N2O) and nitric oxide (NO) emission from forest soils, we measured N2O and NO emission rates from surface soil cores taken at 1 m intervals on a cross-line transect (65 m × 20 m) on a slope of Japanese cedar (Cryptomeria japonica) forest in a temperate region of central Japan and analyzed the spatial dependency of N oxide gas emissions using geostatistics. We divided N2O emission into N2O from denitrification and N2O from nitrification using the acetylene inhibition method. According to the geostatistical analysis, N2O emission rates on the slope had large spatial variation and weak spatial dependency. This weak spatial dependency was caused by the inordinately high N2O emissions on the slope, which were derived mainly from denitrification. In contrast, NO emission rate on the slope had large spatial variation, but strong spatial dependency and a distinct spatial distribution related to slope position, that is, high in the middle of the slope and low in the shoulder and the foot of the slope. The CN ratio and water-filled pore space were the dominant factors controlling NO emission rate on a slope. Our results suggest that spatial information about topographic factors helps to improve the estimation of both N2O emission and NO emission from forest soils.
INTRODUCTION
Nitrogen oxide gases play an important role in the nitrogen cycle, but also have significant environmental effects. Nitrous oxide (N2O) is known as a greenhouse gas, and is one of several gases responsible for global warming (CitationIntergovernmental Panel on Climate Change 2001). Nitric oxide (NO) is a precursor of acidic clouds and precipitation (CitationLogan 1983) and also acts as a catalyst in the synthesis of ozone in the troposphere (CitationHolland and Lamerqur 1997). Although these gases are present in the atmosphere in trace concentrations, they play an important role in atmospheric chemistry.
In soils, N2O and NO gases are produced by nitrification and denitrification (CitationDavidson et al. 2000). Nitrogen cycles in forest soils are known to have large spatial heterogeneity and strongly depend on topographic factors. In a coniferous forest, for example, N mineralization ranged from 22 to 209 mg N g dry weight−1 per 7 days along an 18 m line transect (CitationBrucker et al. 1999). CitationVentrea et al. (2003) reported that net nitrification ranged from –0.01 to 1.5 kg N ha−1 per day in a 3,160 ha northern hardwood conifer forest. Thus, spatial variation in soil N transformation is large at both plot and land scales. This type of variation is related, in part, to topography (CitationVentrea et al. 2003). In particular, slope position is important for N transformation (e.g. CitationGroffman and Tiedje 1989; CitationHirobe et al. 1998; CitationSchimel et al. 1985). Soil moisture (CitationMoore et al. 1993), C:N ratio (CitationHirobe et al. 1998) and the other elements involved in nutrient cycling (e.g. CitationHoneycutt et al. 1990) show a gradient along a slope. Consequently, N2O and NO emission rates could be considered to have specific spatial gradients on a slope. CitationDavidson and Swank (1986) and CitationOsaka et al. (2006) demonstrated that N2O emission at the foot of the slope was greater than that in the upper part of the slope. In contrast, hot spots of N2O emission were not related to topography (e.g. CitationIshizuka et al. 2005). N2O hot spots have made estimating N2O emission highly uncertain (CitationMosier 1998). Very little information on the spatial variation of NO in forest soils is available. More detailed information about the spatial structure of these gas emissions on a slope and the factors controlling their spatial variation are needed to improve the estimation of N oxide gas emissions from forest soils.
The present study aimed to quantify the spatial variability of N2O and NO emission potentials on a slope and to identify the main process for the emission of each gas. We measured N2O and NO emission rates from surface soil core samples obtained at 1 m intervals on a cross-line transect (65 m × 20 m) on a slope of Japanese cedar (Cryptomeria japonica) forest in a temperate region of central Japan and conducted a geostatistical analysis to identify the spatial dependency (the degree of spatial autocorrelation) of these N oxide gases on the slope.
MATERIALS AND METHODS
Site description
We set up an experimental site on a watershed in Nagoya University Forest (35°11′20″N, 137°33′40″E, altitude 1,010 m a.s.l.), Toyota, Aichi prefecture, Japan (), on a north-facing slope with an incline of 25°. The slope was planted with Japanese cedar (Cryptomeria japonica) in 1972. The tree density was approximately 2,000 individuals per hectare and the mean tree height was 20 m. The soil type is Inceptisols using US Department of Agriculture soil taxonomy (CitationUnited States Department of Agriculture–Natural Resource Conservation Service 1999). The mean annual temperature and precipitation at the University forest camp, 100 m from the site, from 2001 to 2004 were 10°C and 2,200 mm, respectively (CitationYoshida and Hijii 2006).
Figure 1 Location of the experimental site. Triangle indicates the location of the experimental site. (a) The geographical location of the X-transect and the Y-transect. X, the upper part (No. 1) of the X-transect. (b) The profile of longitudinal leveling of the X-transect. Circles indicate the sampling points and the solid square indicates soil core 35, at the intersect with the Y-transect. Numbers indicate the numbers of the soil cores.
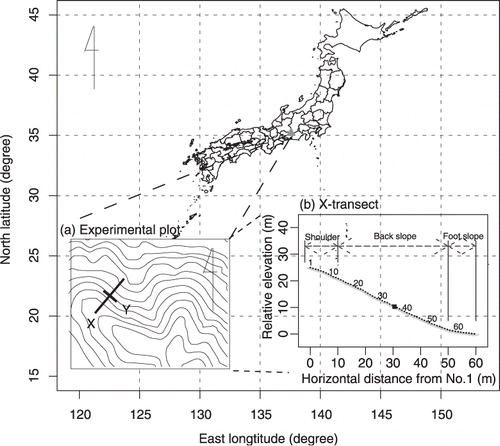
A 65 m line transect (X-transect) was established along the slope from the shoulder to the bottom of the slope (). The difference in elevation from the highest point to the lowest point on this transect was 25 m. Slope angle, point to point, ranged from 5° to 36° (24 ± 8°[mean ± standard deviation]). We classified the slope into three parts, shoulder, back slope and foot slope, for the X-transect, as described by CitationSchoeneberger et al. (1998) (). The horizontal lengths of the shoulder, back slope and foot slope were 10 m, 40 m and 10 m, respectively (). To check directional differences depending on the slope, a 20 m orthogonal transect to the X-transect (Y-transect) was also established that intersected at the center of the X-transect (35 m from the top of the slope).
Soil sampling and analysis
Soil core samples (5 cm internal diameter × 5.1 cm height) were taken from just below the organic (A0) layer at 1 m intervals along each transect in May 2006. The soil cores in the X-transect were numbered from 1 to 66 from top to bottom (), comprising 1–11, 12–56 and 57–66 from the shoulder, back slope and foot slope, respectively. In the Y-transect, the soil cores were numbered from 70 to 90 from the left part to the right part of the X-transect facing the top. All soil samples were stored at 4°C in a refrigerator until pre-incubation. The soil cores were incubated at 25°C for 24 h before incubation for gas emission measurement.
The NO emission rate was measured using the flow-through method. The soil cylinder was placed into a 500 mL glass container with a lid, which had an inlet and an outlet port. The inlet port was connected to a NOx scrubber (purafil and charcoal; Thermo Environmental Instruments, Massachusetts, MA, USA) to remove NO and NO2. The outlet port was connected to a chemiluminescence NOx analyzer (42C trace level; Thermo Environmental Instruments) with a flow pump. The NO emission rate (µg N m−2 h−1) was calculated using the following equation:
where Ce is the chamber effluent NO concentration (mg N m−3), Ci is the chamber influent NO concentration (mg N m−3; Ci was 0 by NOx scrubber absorption), q is the air flow rate (m3 min−1) (CitationVentrea and Rolston 2000), and A is soil core area (m2). Ce was determined using the average concentration for 10 min after the dynamic equilibrium of the NO concentration. The air flow rate was 0.0010 m3 min−1.
After measurement of NO emission rate, the same container was used to measure N2O emission rate. Immediately after the container was covered with a silicon rubber cap, a gas sample was withdrawn from the head space of the container using a 1 mL syringe. The concentration of N2O was determined using a gas chromatograph with a 63Ni electron capture detector (Shimadzu GC-8A; Shimadzu, Kyoto, Japan). Gas samples were also withdrawn 24 and 48 h after the container was covered (CitationKlemedtsson et al. 1988). The N2O gas emission rate was calculated from the slope of the gas concentrations with sampling time. After measurement of the N2O emission rate, the air in the container was exchanged to the atmosphere and the container was closed again. The head space was then adjusted to 10 Pa acetylene (using the acetylene inhibition method; CitationKlemedtsson et al. 1988) to inhibit N2O emission from nitrification. After pre-incubation for 24 h (CitationInubushi et al. 1996), the N2O emission rate was measured using the same procedure as that used to measure N2O emission rate, except for the addition of acetylene. We defined this emission as N2O emission from denitrification (DenitN2O). The N2O emission from nitrification (NitN2O) was calculated using the following equation:
When the DenitN2O emission rate exceeded the N2O emission rate, the NitN2O emission rate was defined as not detected (n.d.). The NitN2O emission rate using this method tends to be underestimated because of insufficient inhibition of nitrification when the soil moisture level is high (CitationKlemedtsson et al. 1988). Because inhibition of nitrification might have been insufficient, the NitN2O and DenitN2O data might not be statistically reliable; therefore, we did not analyze these data statistically. Incubation for the measurement of N2O and NO emissions was conducted at 25°C.
After measurement of the N oxide gas emission rate, the soil air volume was measured using a gas pycnometer (DIK-1000; Daiki, Saitama, Japan). The soil water content was determined after oven-drying at 105°C. The percentage water-filled pore space (%WFPS) was calculated gravimetrically as the ratio of water volume to total pore volume (CitationDanielson and Sutherland 1986). The dried soil in a core was sieved through a 2 mm mesh. Total carbon (TC) and total nitrogen (TN) of the soil were then analyzed using a CN Corder (Yanaco MT-700; Yanaco, Kyoto, Japan). According to the laboratory tests, TC and TN determined using oven-dried soil were not significantly different from the values recorded using air-dried soil (Student's t-test, data not shown).
Statistical analysis
Using a χ2-test, each N oxide gas emission rate showed a log-normal distribution. Therefore, the parameters were logarithmically transformed for the following statistics. The Pearson product moment correlation coefficient between the gas emission rate and the soil properties was calculated for the X-transect and for the Y-transect data separately and for the overall data. In addition, we conducted multiple regression analysis to explain the emission rates of N oxide gases using WFPS, TC and TN. The parameters were selected using the stepwise removal method and Akaike's Information Criteria (AIC).
In addition, we conducted a geostatistical analysis to investigate the spatial dependence of N2O and NO emissions on soil properties in the X-transect. Values from the Y-transect were not used for geostatistical analysis because of insufficient sample size. Geostatistics is a useful tool for estimating values at unsampled places and for quantifying spatial structure in space (CitationOliver and Webster 1991). The formula of semivariance for estimating the semivariogram at the given lag, h (m), is:
where γ(h) is the semivariance, nc is the number of pairs separated by lag distance h, and Z(X α) and Z(X α + h) are the observation values at point X α and point X α + h, respectively. Log-transformed values of N2O and NO emission rates were used for the calculation. In the present study, we tested linear, exponential and spherical model fitting using the ordinary least square and we chose the model with the minimum sum of squares. All statistics were calculated using R (CitationIhaka and Gentleman 1996), which is an open source environment for statistical computing (CitationR Development Core Team 2006). The “geoR” package was used to calculate semivariance (CitationRibeiro and Diggle 2006).
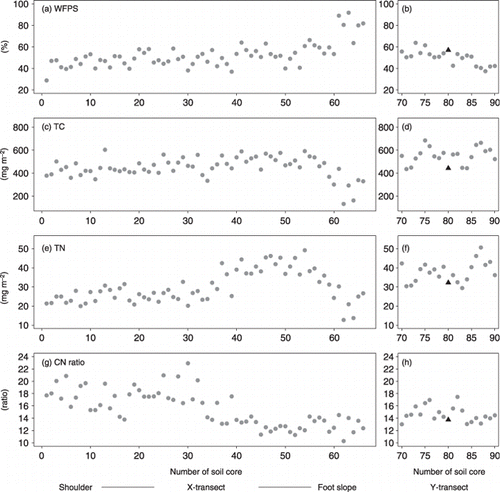
Figure 2 Spatial patterns of the soil properties in the X-transect and the Y-transect. (a) Water-filled pore space (WFPS) in the X-transect, (b) WFPS in the Y-transect, (c) total carbon (TC) in the X-transect, (d) TC in the Y-transect, (e) total nitrogen (TN) in the X-transect, (f) TN in the Y-transect, (g) C:N ratio in the X-transect, (h) C:N ratio in the Y-transect. In (b,d,f,h) the black solid triangles indicate soil core 35 of the X-transect.
RESULTS
Soil properties
The values of WFPS in the X-transect gradually increased with a decrease in elevation to the point of soil core 60 and dramatically increased from soil core 60 to soil core 66 (). The TC values in the X-transect showed no trend above soil core 55 and decreased below soil core 55 (). The TN values in the X-transect were relatively static from soil core 1 to soil core 36 and gradually increased from soil core 37 to soil core 55 in the X-transect (). The TN values decreased at the foot of the slope, similar to the values of TC, from the point of soil core 55 to soil core 66. The C:N ratio value in the X-transect gradually decreased down the slope ().
N2O and NO emission rates
The N2O and NO emission rates showed large spatial variation and a highly skewed distribution (; ). In the X-transect, N2O emission rate ranged from 0.10 to 224 µg N m−2 h−1 and the NO emission rate ranged from 0.93 to 260 µg N m−2 h−1. In the Y-transect, the N2O emission rate ranged from 9.84 to 20.7 µg N m−2 h−1 and the NO emission rate ranged from 7.19 to 90.4 µg N m−2 h−1. The range in emission rates of the two N oxide gases was similar, but the skewness of the N2O emission rate was greater than that of the NO emission rate (; ).
Figure 3 Spatial patterns of N2O and NO emission rates in the X-transect and the Y transect. N2O emission rate in the (a) X-transect and (b) Y-transect and (c) the frequency distribution of N2O emission including both transects; (d–f) NO emission rate, (g–i) NitN2O, (j–l) DenitN2O. In (b,e,h,k) the black solid triangles indicate soil core 35 of the X-transect. In (g) the open circles indicate NitN2O n.d. (not detected). In the histograms (c,f,i,l) the the grey bars indicate the X-transect and the white bars indicate the Y-transect.
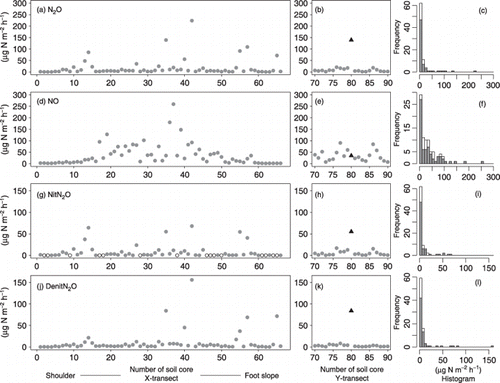
The N2O emission rates for soil cores 1 to 11 were relatively low (1.48–8.09 µg N m−2 h−1; ), whereas high N2O emissions appeared irregularly from soil cores 12 to 15 and from soil cores 35 to 66 (). The NO emission rates from soil cores 1 to 12 and from soil cores 59 to 66 were relatively low (1.48–8.09 and 0.93–4.33 µg N m−2 h−1, respectively, ); however, high NO emission rates were observed from soil cores 11 to 50 and from soil cores 70 to 90 ().
NitN2O and DenitN2O emission rates in the 10 Pa acetylene addition experiment
According to the acetylene inhibition method, NitN2O and DenitN2O emission rates ranged from n.d. to 68.0 µg N m−2 h−1, and from 0.09 to 156 µg N m−2 h−1, respectively (). In the X-transect, the NitN2O emission rate ranged from 0.16 to 16.6 µg N m−2 h−1 and the DenitN2O emission rate ranged from 0.83 to 8.13 µg N m−2 h−1 (). The skewness and kurtosis of the DenitN2O emission rate were greater than the skewness and kurtosis of NitN2O.
High NitN2O emission rates occasionally appeared on the slope between soil cores 10 and 60 (). High DenitN2O emission rates appeared at points lower than soil core 35 ().
Table 1 Summary statistics of N oxide gases and soil properties for the X-transect (n = 66), the Y-transect (n = 20) and the overall dataset (n = 86)
Table 2 Correlation coefficient between N oxide gases and soil properties for the X-transect (n = 66), the Y-transect (n = 20) and the overall dataset (n = 86)
Relationship between N2O and NO emission rates
The ratio of NO emission rate to N2O emission rate (NO/N2O) varied from 0.02 to 41.5 (). The NO/N2O ratio showed no marked trend in relation to slope position (data not shown). Furthermore, no correlation was observed between the NO and N2O emission rates (R = 0.101, P = 0.356).
Correlation coefficient between N oxide gas emission rates and soil properties
No significant correlation was observed between log-transformed N2O emission rates and WFPS for the overall data or for either transect (; ); however, log-transformed NO emission rates were significantly negatively correlated with WFPS for the overall data and for the X-transect data (R = 0.366 and R = –0.375, respectively). For the overall data, the log-transformed N2O and NO emission rates were positively correlated with TC and TN (; ). However, the log-transformed NO emission rate was not significantly correlated with TC or TN for the Y-transect data.
Figure 4 Relationship between N oxide gases and soil properties. (a) N2O emission rate versus water-filled pore space (WFPS), (b) N2O emission rate versus total carbon (TC), (c) N2O emission rate versus total nitrogen (TN), (d) N2O emission rate versus C:N ratio, (e) NO emission rate versus WFPS, (f) NO emission rate versus TC, (g) NO emission rate versus TN and (h) NO emission rate versus C:N ratio. Solid circles indicate samples from the X-transect and open circles indicate samples from the Y-transect.
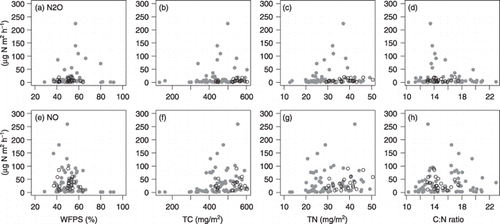
Table 3 Geostatistical parameters of N oxide gases
Multiple regression analysis
The multiple regression for N2O emission rate showed that log-transformed N2O was explained only by TC according to the equation:
The NO emission rate was explained using TC and WFPS according to the equation:
These two models were statistically significant, but could only explain < 30% of the variation in the gas emission rates.
Spatial dependency of N2O and NO emission rates and other soil properties
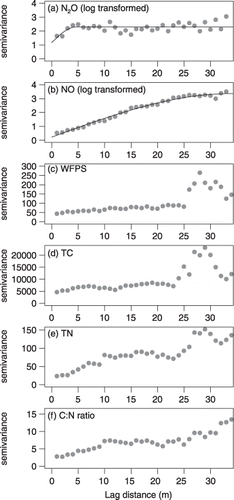
The spatial dependencies of N oxide gases and soil variables in the X-transect were analyzed using a semivariogram (). The semivariograms for N oxide gases fitted a spherical model best. Log-transformed N2O emission rates showed weak spatial dependency with a range of 5.32 m (). In contrast, the log-transformed NO emission rate showed strong spatial dependency with a range of over 33 m (). The Q value for NO emission was 0.92 (close to 1), indicating that most of the variation could be explained by spatial dependency.
For the WFPS, TC, TN and C:N ratios, the parametric models did not fit the semivariogram (). The semivariance values of these soil properties increased at a distance of more than 25 m.
Figure 5 Semivariograms of N oxide gases versus soil properties in the X-transect. (a) N2O emission rate, (b) NO emission rate, (c) water-filled pore space (WFPS), (d) total carbone (TC), (e) total nitrogen (TN) and (f) C:N ratio. The solid line was fitted with the spherical model using least square (see Table 3 for the model parameters).
DISCUSSION
Spatial structure of N2O and NO gas emission rates on a slope
We found that the N2O emission rate from forest soils on a slope showed large spatial variation and weak spatial dependency (,). The NO emission rate on a slope also showed large spatial variation, but strong spatial dependency (,). The spatial dependencies of these two N oxide gases were quite different, although both were produced mainly by nitrification and denitrification (CitationDavidson et al. 2000).
A number of studies have reported large spatial variation in N2O emission and small-scale autocorrelation using field measurements in various soils. Values calculated using a semivariogram have ranged from 0.8 to 6 m (CitationAmbus and Christensen 1994; CitationVelthof et al. 1996; both in grassland). CitationClemens et al. (1999) did not find spatial autocorrelation of N2O flux in arable land. The N2O emission rate measured by laboratory incubation also showed large spatial variation, but no spatial dependency according to geostatistics using 3 m grid sampling in a cultivated field (CitationMathieu et al. 2006). Although several studies have reported large-scale autocorrelation of N2O emission rate using field measurements (ranges over 30 m; CitationVan den Pol-van Dasselaar et al. 1998, in grassland; CitationYanai et al. 2003, in arable land; CitationIshizuka et al. 2005, in tropical rainforest), the spatial structures of N2O emission were weak in these reports. This weak spatial structure of N2O emission was considered to result from the contribution of denitrification, which occurs at randomly existing anaerobic microsites, such as particle organic material in soils (CitationParkin 1987). Our results showed that high N2O emission rates derived from denitrification randomly occurred on the middle to lower slope. Therefore, our results could support the hypothesis that the large spatial variation and weak spatial structure of the N2O emission rate are caused by heterogeneity in N2O emission derived from denitrification.
In contrast, NO emission rates showed strong spatial structure. NO emission from soils is derived mainly from nitrification (e.g. CitationGödde and Conrad 1998, Citation2000; CitationRussow et al. 2000; CitationSkiba et al. 1993). As nitrification calculated using semivariograms has ranged from 10 to 100 m for various fields (CitationRobertson et al. 1988, Citation1993, Citation1997, in arable land; CitationGonzalez et al. 1994, in tropical rainforest; CitationGross et al. 1995, in arable land and temperate forest), the relatively large range of NO emission rates recorded in our study (over 33 m) might result from the relatively large range of nitrification processes.
Spatial distribution of N2O and NO gas emission rates in relation to slope position
Variability in both N oxide gases was greater in the X-transect than in the Y-transect, indicating that slope position is important for controlling spatial variability.
Both N oxide gas emission rates were low on the shoulder (soil cores 1–10), where soil WFPS was low and C:N ratio high (). Many studies have reported that N mineralization and nitrification rate are low on the shoulder where the C:N ratio is high and the water content is low (e.g. CitationHirobe et al. 1998; CitationSchimel et al. 1985; CitationTokuchi et al. 1999). Therefore, low N oxide gas emission rates on the shoulder might reflect a low rate of N cycling.
On the back slope, a transition area between the shoulder and the foot slope, WFPS gradually increased and the C:N ratio gradually decreased with decreasing elevation. These gradients were comparable with those previously reported in studies along slopes (e.g. CitationHirobe et al. 1998; CitationTokuchi et al. 1999), which indicate that nitrification rate on the mid slope (back slope) increases with decreasing elevation. This trend might be responsible for the patchy high N2O emissions observed on the lower part of the back slope because high nitrification rate might contribute not only to NitN2O emissions, but also to DenitN2O emissions through the substrate (nitrate) supply for denitrification. High DenitN2O emissions appeared in positions lower than soil core 35 (i.e. soil cores 35, 40, 42, 54, 55 and 57) with high WFPS (53.5–66.3%). This is consistent with the observation that N2O emission from denitrification depends strongly on WFPS (e.g. CitationSmith et al. 2003). Therefore, it is possible that denitrification is primarily responsible for the high N2O emission in the lower part of the back slope. As N2O from nitrification is less sensitive to WFPS (CitationDavidson and Swank 1986), the high NitN2O emissions over a broad range of the back slope () might depend on the relatively high nitrification rate compared with that on the shoulder. Thus, the ratio of NitN2O and DenitN2O contributions to the high N2O emissions might be spatially different according to the location of the back slope. The NO emission rate on the back slope was higher than the N2O emission rate (). Because the conditions favorable for NO emission were drier than for N2O emission (e.g. CitationDavidson et al. 2000; CitationDrury et al. 1992), and NO might be emitted by nitrification, NO emission depended strongly on the position of the slope: it was low on the foot slope and shoulder and high on the back slope.
On the foot slope, WFPS was high and the C:N ratio was low. Values for TC and TN on the foot slope were lower than those on the upper slope, indicating high microbial decomposition of organic material. Many laboratory and field experiments have reported higher denitrification rates and N2O emission rates on the foot slope compared with the upper slope (e.g. CitationDavidson and Swank 1986; CitationMcSwiney et al. 2001; CitationOsaka et al. 2006; CitationSilver et al. 1999) because of the high nitrification rate and N mineralization induced by high water content and low C:N ratio (CitationTokuchi et al. 1999). In addition, in our study, high DenitN2O emissions (soil cores 57 and 65) were observed on the foot slope, whereas high N2O emission rates were patchy, even on the foot slope. In contrast, NO emissions were uniformly low on the foot slope. The correlation analysis suggests that these low NO emissions might depend on a high WFPS, which promotes the subsequent reduction of NO to N2 through denitrification (e.g. CitationDrury et al. 1992).
In our study, the coefficients of determination of the multiple regression models for N2O and NO emission rates were low. Therefore, we have not emphasized the results of the linear model for either N oxide gas in the present study. CitationLark et al. (2004) indicated that the complex scale-dependent covariation of N2O emission rate and soil properties masked the effect of each soil property for the spatial dataset. In the present study, a relatively large nugget () observed in the semivariogram for N2O emission rate suggests a structure of spatial variation at a scale smaller than 1 m or high measurement error. Therefore, our sampling distance could be too large for the statistical analysis to reveal the regulating factors of N2O emission rate. Although the nugget of NO emission rate was relatively small, the low coefficient of determination of the multiple regression model, which was only 31.1% of total NO variance, indicates that complex scale-dependent covariation could also mask the effect of each of the soil properties on NO emission. To avoid the scale-dependent correlation problem, CitationLark et al. (2004) conducted adapted maximal overlap discrete wavelet transform analysis, which can analyze scale-specific correlation by spectrum analysis. As this analysis needs more repeated samples than our dataset to clarify the scale-dependent correlation in the slope, we could not conduct this analysis in the present study, but it should be done in future studies.
Although our results were obtained from a single sampling on one slope, we found specific spatial distribution of N oxide gases in relation to slope position. Measuring seasonal change in the spatial distribution and spatial structure on a slope is important to help identify the stability of these spatial structures. To the best of our knowledge, this is the first report to consider spatial variation in NO gas emission. Therefore, further studies comparing different sites will contribute significantly to our understanding of this aspect of NO emission.
In conclusion, specific spatial distributions of NO and N2O emissions on a line-transect along a slope were found in a Japanese cedar forest. Large spatial variation and weak spatial dependency were observed in the N2O emission rate. A patchy N2O emission derived from denitrification was observed on the lower part of the slope with relatively high WFPS. The NO emission rate on the slope also had large spatial variation, but in contrast to the N2O emission rate it had a strong spatial dependency, which was high on the middle back slope and low on the shoulder and the foot slope. Our results showed that WFPS and C:N ratio controlled the spatial distribution of NO emission on the slope. Although it has been reported that N2O emission rates are controlled by topographic factors, there are few reports showing that the slope of a location affects NO emission rate. Our results indicate that estimations of NO emission over a watershed scale should be improved by taking into account this topographic factor.
REFERENCES
- Ambus , P and Christensen , S . 1994 . Measurement of N2O emission from a fertilized grassland: An analysis of spatial variability . J. Geophys. Res , 99 : 16549 – 16555 .
- Brucker , A , Kandeler , E and Kampichler , C . 1999 . Plot-scale spatial patterns of soil water content, pH, substrate-induced respiration and N mineralization in a temperate coniferous forest . Geoderma , 93 : 207 – 223 .
- Clemens , J , Schillinger , MP , Goldbach , H and Huwe , B . 1999 . Spatial variability of N2O emissions and soil parameters of an arable silt loam – a field study . Biol. Fert. Soils , 28 : 403 – 406 .
- Danielson , RE and Sutherland , PL . 1986 . “ Porosity ” . In Methods of Soil Analysis, Part I – Physical and Mineralogical Methods , 2nd edn. , Edited by: Kulute , A . 443 – 461 . Madison : Soil Science Society of America .
- Davidson , EA , Keller , M , Ericson , HE and Verchot , LV . 2000 . Testing a conceptual model of soil emission of nitrous and nitric oxides . Bioscience , 50 : 667 – 680 .
- Davidson , EA and Swank , WT . 1986 . Environmental parameters regulating gaseous nitrogen losses from two forested ecosystems via nitrification and denitrification . Appl. Environ. Microb , 52 : 1287 – 1292 .
- Drury , CF , McKenney , DJ and Findlay , WI . 1992 . Nitric oxide and nitrous oxide production from soil: water and oxygen effects . Soil Sci. Soc. Am. J , 56 : 766 – 770 .
- Gödde , M and Conrad , R . 1998 . Simulation measurement of nitric oxide production and consumption in soil using a simple static incubation system, and the effect on soil water content of the contribution of nitrification . Soil Biol. Biochem , 30 : 433 – 442 .
- Gödde , M and Conrad , R . 2000 . Influence of soil properties on the turnover of nitric oxide and nitrous oxide by nitrification and denitrification at constant temperature and moisture . Biol. Fert. Soils , 32 : 120 – 128 .
- Groffman , PM and Tiedje , JM . 1989 . Denitrification in north temperate forest soils: spatial and temporal patterns at the landscape and seasonal scales . Soil Biol. Biochem , 21 : 613 – 620 .
- Hirobe , M , Tokuchi , N and Iwatubo , G . 1998 . Spatial variability of soil nitrogen transformation patterns along a forest slope in Cryptomeria japonica D. Don plantation . Eur. J. Soil Biol , 34 : 123 – 131 .
- Holland , EA and Lamerqur , J-F . 1997 . Modeling bio-atmospheric coupling of the nitrogen cycle though NOx emission and NOy deposition . Nutr. Cycl. Agroecosys , 48 : 7 – 24 .
- Honeycutt , CW , Heil , RD and Cole , CV . 1990 . Climatic and topographic relations of three Great Plains soils: II. Carbon, nitrogen and phosphorus . Soil Sci. Am. J , 54 : 476 – 483 .
- Ihaka , R and Gentleman , R . 1996 . R; a language for data analysis and graphics . J. Comput. Graph. Stat , 5 : 299 – 314 .
- Ishizuka , S , Iswandi , A Nakajima , Y . 2005 . Spatial patterns of greenhouse gas emission in a tropical rainforest in Indonesia . Nutr. Cycl. Agroecosys , 71 : 55 – 62 .
- Klemedtsson , L , Svensson , BK and Rosswall , T . 1988 . A method of selective inhibition to distinguish between nitrification and denitrification as source of nitrous oxide in soil . Biol. Fert. Soils , 6 : 112 – 119 .
- Logan , JA . 1983 . Nitrogen oxides in the troposphere – Global and regional budgets . J. Geophys. Res , 88 : 10785 – 10807 .
- McSwiney , CP , McDowell , WH and Keller , M . 2001 . Distribution of nitrous oxide and regulators of its production across a tropical rain forest catena in the Luquillo experimental forest, Puerto Rico . Biogeochem , 56 : 265 – 286 .
- Moore , ID , Gessler , PE , Nielsen , GA and Petrson , GA . 1993 . Soil attribute prediction using terrain analysis . Soil Sci. Soc. Am. J , 57 : 443 – 452 .
- Mosier , AR . 1998 . Soil processes and global change . Biol. Fert. Soils , 27 : 221 – 229 .
- Oliver , MA and Webster , R . 1991 . How geostatistics can help you . Soil Use Manage , 7 : 206 – 217 .
- Gonzalez , OJ and Zak , DR . 1994 . Geostatistical analysis of soil properties in a secondary tropical dry forest, St. Lucia, West Indies . Plant Soil , 163 : 45 – 54 .
- Osaka , K , Ohte , N , Koba , K , Katuyama , M and Nakajima , T . 2006 . Hydrogic controls on nitrous oxide production and consumption in a forested headwater catchment in central Japan . J. Geophys. Res , 111 : G01013 doi:10.1029/2005JG000026
- Parkin , TB . 1987 . Soil microsites as a source of denitrification variability . Soil Sci. Soc. Am. J , 51 : 1194 – 1199 .
- R Development Core Team . 2006 . R: A language and environment for statistical computing , Vienna : R Foundation for Statistical Computing . ISBN 3-900051-07-0. Available from URL: http://www.R-project.org [cited July 2008]
- Ribeiro , PJ Jr and Diggle , PJ . 2006 . The geoR package . A package for geostatistical analysis , Available from URL: http://www.est.ufpr.br/geoR [cited July 2008]
- Russow , R , Sich , I and Neue , H-U . 2000 . The formation of the trace gases NO and N2O in soils by the coupled processes of nitrification and denitrification: result of kinetics 15N tracer investigation . Chemosphere , 2 : 359 – 366 .
- Schimel , D , Stillwell , MA and Woodmansee , RG . 1985 . Biogeochemistry of C, N and P in soil cetena of the shortgrass steppe . Ecology , 66 : 276 – 282 .
- Schoeneberger , PJ , Wysocki , DA , Benham , EC and Broderson , WD . 1998 . Field Book for Describing and Sampling Soils , Lincoln : US Department of Agriculture, Natural Resources Conservation Service, National Soil Survey Center .
- Silver , WL , Lugo , AE and Keller , M . 1999 . Soil oxygen availability and biogeochemistry along rainfall and topographic gradients in upland tropical forest soil . Biogeochem , 44 : 301 – 328 .
- Skiba , U , Smith , KA and Fowler , D . 1993 . Nitrification and denitrification as sources of nitric oxide and nitrous oxide in a sandy loam soil . Soil Biol. Biochem , 25 : 1527 – 1536 .
- Tokuchi , N , Takeda , H , Yoshida , K and Iwatsubo , G . 1999 . Topographical variations in a plant–soil system along a slope on Mt Ryuoh, Japan . Ecol. Res , 14 : 361 – 369 .
- Van den Pol-van Dasselaar , A , Corre , WJ Prieme , A . 1998 . Spatial variability of methane, nitrous oxide and carbon dioxide emissions from drained grasslands . Soil Sci. Soc. Am. J , 62 : 810 – 817 .
- Velthof , GL , Jarvis , SC , Stein , A , Allen , AG and Oenema , O . 1996 . Spatial variability of nitrous oxide fluxes in mown and grazed grasslands on a poorly drained clay soil . Soil Biol. Biochem , 28 : 1215 – 1225 .
- Ventrea , RT , Lovett , GM , Groffman , PM and Schwarz , PA . 2003 . Landscape patterns of net nitrification in a northern hardwood-conifer forest . Soil Sci. Soc. Am. J , 67 : 527 – 539 .
- Ventrea , RT and Rolston , DE . 2000 . Mechanism and kinetics of nitric and nitrous oxide production during nitrification in agricultural soil . Glob. Change Biol , 6 : 303 – 316 .
- Yanai , J , Sawamoto , T Oe , T . 2003 . Spatial variability of nitrous oxide emissions and their soil-related determining factors in an agricultural field . J. Environ. Qual , 32 : 1965 – 1977 .
- Yoshida , T and Hijii , N . 2006 . Spatiotemporal distribution of aboveground litter in a Cryptomeria japonica plantation . J. For. Res , 11 : 419 – 426 .
- Gross , KL , Pregitzer , KS and Burton , AJ . 1995 . Spatial variation in nitrogen availability in three successional plant communities . J. Ecol , 83 : 357 – 367 .
- Intergovernmental Panel on Climatic Change . 2001 . Climate Change 2001 – The scientific basis , London : Cambridge University Press .
- Inubushi , K , Naganima , H and Kitahara , S . 1996 . Contribution of denitrification and autotrophic and heterotrophic nitrification to nitrous oxide production in andosols . Biol. Fertil. Soils , 23 : 292 – 298 .
- Lark , RM , Milne , AE , Addiscott , TM , Goulding , KWT , Webster , CP and Flahertry , SO . 2004 . Scale- and location-dependent correlation of nitrous oxide emissions with soil properties: an analysis using wavelets . Eur. J. Soil Biol , 55 : 611 – 627 .
- Mathieu , O , Lévêque , J , Hénault , C , Milloux , M-J , Bizouard , F and Andreux , F . 2006 . Emissions and spatial variability of N2O, N2and nitrous oxide mole fraction at the field scale, revealed with 15N isotopic techniques . Soil Biol. Biochem , 38 : 941 – 951 .
- Robertson , GP , Hutson , MA , Evans , FC and Tiedje , JM . 1988 . Spatial variability in a successional plant community: patterns of nitrogen availability . Ecology , 69 : 1517 – 1524 .
- Robertson , GP , Crum , JR and Ellis , BG . 1993 . The spatial variability of soil resources following long-term disturbance . Oecologia , 96 : 451 – 456 .
- Robertson , GP , Klingensmith , KM , Klug , MJ , Paul , EA and Crum , JR . 1997 . Soil resources, microbial activity and primary production across an agricultural ecosystems . Ecol. Appl , 7 : 158 – 170 .
- Smith , KA , Ball , T , Conen , F , Dobbie , KE , Massheder , J and Rey , A . 2003 . Exchange of greenhouse gases between soil and atmosphere: interactions of soil physical factors and biological processes . Eur. J. Soil Sci , 54 : 779 – 791 .
- United States Department of Agriculture–Natural Resource Conservation Service . 1999 . Soil Taxonomy, a Basic System of Soil Classification for Making and Interpreting Soil Surveys , 2nd edn , Washington : United States Department of Agriculture, Soil Conservation Service .