Abstract
Over the past several decades, the conversion of native forest to agricultural land use has accelerated and featured in the development of Philippine landscapes. This study evaluated the effect of land-use change on soil carbon, nitrogen, neutral sugar composition and other related soil chemical properties in a degraded soil (Typic Hapludult) in Leyte, Philippines. Using a space-for-time substitution (paired-area) approach, soil samples were collected and examined from secondary forest (SF), mahogany plantation (MP), rainforestation farming (RF), coffee plantation (CP) and grassland (GR) of comparable geology, parent material, soil type and climate. Soil pH, exchangeable Ca and Mg, and cation exchange capacity (CEC) tended to be higher after the conversion of deforested cultivated land into MP and RF land-use types. In contrast, land conversion decreased the soil carbon, nitrogen and carbohydrate-C in the order of SF, MP, CP, RF and GR, and the decrease was more marked in RF and GR sites that had been under intensive cultivation for a long period of time. Arabinose and xylose (mainly of plant origin) constituted the major non-cellulosic neutral sugar and represented 31–54% of the total soil carbohydrate-C. Soil carbohydrate-C content contributed 2–8% of the total soil organic carbon because of rapid decomposition of sugars. This suggests an adverse effect of land-use change leading to degradation of soil quality. The results of this study suggest that under the humid tropical climate of the Philippines, high temperature and favorable moisture enhanced the carbohydrate decomposition and, thus, affected the content and composition of neutral sugar in the soil.
INTRODUCTION
Over the past century (CitationGarrity et al. 1993), land-use change has featured in the development of Philippine landscapes, and has apparently contributed to the widespread occurrence of degraded land across the country (CitationAsio 1996). Prior to the 1900s, the country had an estimated 70% primary forest, but the acreage of primary forest had decreased markedly to 22% of the total country area towards the end of this last century. In Leyte Island, where our study was conducted, 45% of the primary forest was lost between 1969 and 1984 (CitationForest Management Bureau 1988), resulting in a considerable loss of biodiversity (CitationLangenberger et al. 2006).
Soil organic matter (SOM) is important in the maintenance of soil fertility, but its dynamics and composition are influenced by land-use changes and management practices. Despite a growing body of research on land use, the effect of land-use change on SOM dynamics appears fragmentary and sometimes contradictory. Although CitationNye and Greenland (1964) and CitationDetwiler (1986) reported substantial SOM depletion after deforestation, CitationSanchez (1976) argued that land-use change seldom resulted in SOM depletion. CitationMurty et al. (2002) reported that conversion of forest to cultivated land generally lead to the loss of soil carbon, although the magnitude of the changes depends on the confounding influence of bulk density change. The loss of SOM is also influenced by other factors, such as land-use type, climate and initial carbon content (CitationLugo and Brown 1993), management practices and time after land conversion (CitationNye and Greenland 1964). Depending on the usage and soil management, land-use change could have either a positive (e.g. increase in soil pH, exchangeable bases) or negative (e.g. loss of SOM by erosion) impact on the soil ecosystem and does not always result in a decline in soil fertility (CitationLugo and Brown 1993; CitationSanchez 1976; CitationStevenson 1994).
The pool of soil carbohydrate accounts for a small portion (5–25%) of the total SOM (CitationCheshire 1979; CitationStevenson 1994). Nevertheless, it is recognized that this macromolecule influences a number of biological, physical and chemical processes occurring in the soil (CitationCheshire 1979). Although they are less resistant to decomposition, CitationCheshire (1979) and CitationMurayama (1984) noted that some fractions that are associated with the soil mineral matrix are resistant to biodegradation (CitationTsutsuki and Kuwatsuka 1989) and, therefore, contribute to a recalcitrant organic carbon pool (CitationKiem and Kögel-Knabner 2003). CitationGuggenberger et al. (1994) reported that neutral sugar composition reflects SOM dynamics and can be considered to be a sensitive indicator to elucidate the effect of land-use change. Although several studies documented the impact of land-use change on soil carbohydrates (CitationGuggenberger et al. 1994; CitationNacro et al. 2005; CitationTrouve et al. 1996), very few studies reported on rainforestation using native tree species or native forest conversion to agriculture.
Whether or not changes in land use lead to SOM depletion and subsequently to soil degradation remain inconsistent or poorly understood. The aim of the present study was to elucidate changes in soil carbon, nitrogen, neutral sugar composition and other related soil chemical properties as influenced by land-use change in the humid tropical Leyte Island, Philippines. This paper reports for the first time the neutral sugar content of soils in the Philippines.
MATERIALS AND METHODS
Site characteristics and environmental setting
The study was conducted at the reserved rainforest area on the lower western slope of Mt Pangasugan, approximately 8 km north of Baybay, Leyte, Philippines (10°46′N and 124°50′E) (). The study area is approximately 73–112 m a.s.l. on a <5% slope (). As a large part of the mountain in the area is extremely steep (CitationLangenberger et al. 2006), the lower part of the mountain is under increasing threat from farmers and illegal loggers. For this reason, the rich natural biodiversity in the area, which is habitat to some endangered floral and faunal species, is increasingly under threat (CitationLangenberger et al. 2006). The soil is classified as Typic Hapludult (Soil Taxonomy) or Haplic Alisol (Food and Agriculture Organization System) derived from andesitic pyroclastic materials of late Quaternary (probably Holocene to upper Pleistocene) origin (CitationAsio 1996). The climate is humid tropical monsoon with an annual rainfall of 2600 mm and an average annual air temperature of 27°C. The difference between the coldest (December) and the warmest month (April) is between 2 and 3°C (CitationJahn and Asio 1998). In a short period, from April to May, the precipitation drops below 100 mm per month, which corresponds to the average monthly potential evapotranspiration. Detailed information on the vegetation at the study site is given in CitationLangenberger et al. (2006).
Figure 1 Map of Leyte, Philippines, and the location of the study sites. CP, coffee plantation; GR, grassland; MP, mahogany plantation; RF, rainforestation farming; SF, secondary forest.
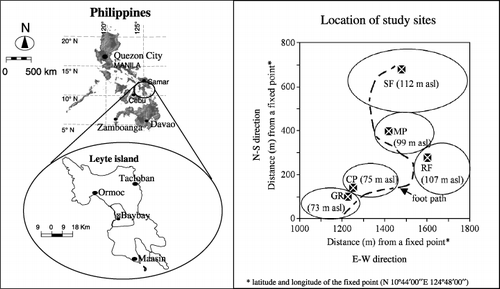
Table 1 Brief land-use history of the study sites
Research approach, site selection and sampling
The space-for-time substitution approach (e.g. CitationVeldkamp et al. 2003) or paired-area approach (e.g. CitationMurty et al. 2002) was used in this study. The approach works on the critical assumption that the soil properties were homogenous before the land-use change (e.g. similar with respect to soil physical and chemical properties) and that any change in the soil properties is caused by the land-use change (CitationVeldkamp et al. 2003). Although parameterization of these factors is difficult under field conditions, existing aerial photographs, vegetation maps and reconnaissance soil surveys (CitationBarrera et al. 1954), pedological (CitationAsio 1996; CitationJahn and Asio 1998) and vegetation (CitationLangenberger et al. 2006) studies of the area were reviewed and revealed that all sites were under forest until the 1950s. A brief land-use history of the study sites is presented in .
Five adjacent sites with comparable climate, parent material, geology and soil type, but with different land uses, were selected. The land-use types selected were: secondary forest (reference land use) (SF), mahogany plantation (MP), rainforestation farming (RF), coffee plantation (CP) and grassland (GR). Secondary forest was selected because it was difficult to find native forest at elevations below 250 m a.s.l. because of anthropogenic perturbation (CitationLangenberger et al. 2006). Soil sampling was carried out in August (summer season) 2004. To make a reliable assessment among the selected sites, uniform sampling depths of 0–20, 20–40 and 40–60 cm were adapted and a uniform depth increment of 20 cm was used to achieve higher resolution in the changes in soil properties among the study sites. From each site, 10–15 subsampling plots were selected randomly in the field and soil samples were collected from these plots using a soil auger and subsequently mixed. At each site, a representative soil profile was excavated manually and the characteristics of the soil horizons were described according to standard procedures (data not shown).
Soil physicochemical analysis
All analyses were conducted on air-dried samples passed through a 2-mm sieve. Particle size distribution was done by wet sieving and using pipette methods. The soil pH (H2O) was determined using a soil : solution suspension of 1:2.5. The total carbon and nitrogen contents were analyzed by dry combustion using a C/N analyzer (Vario-EL III; Elementar Analysensystem GmbH, Hanau, Germany). Available P was extracted using 0.03 mol L−1 NH4F in 0.1 mol L−1 HCl (Bray 2 method) with a soil/extraction ratio of 1:20 (CitationShoji et al. 1964) and using the method of Murphy and Riley for color development. Cation exchange capacity (CEC) was determined using the Schollenberger method. Five grams of air-dried soil was equilibrated using 1 mol L−1 NH4OAc at pH 7, washed with 80% ethanol, and extracted again with 10% KCl. The exchangeable bases (Ca, Mg, K and Na) were quantified using atomic absorption spectroscopy (Z-5010, Hitachi, Tokyo, Japan). The extracted NH4 + in the 1 mol L−1 KCl solution was determined using the steam distillation method and the CEC values were calculated.
Preparation of sugar derivatives and analytical methods by capillary gas chromatography
Prior to the gas chromatograph analysis, soil carbohydrates were hydrolyzed into their monomers. Neutral sugar was released using a two-step acid (H2SO4) hydrolysis according to the procedure described by CitationOades et al. (1970). One gram of finely ground soil (<50 µm) was placed in a 50-mL amber bottle with a Teflon-lined screw cap and 20 mL of 2.5 mol L−1 H2SO4 was added and hydrolyzed for 20 min at 100°C in a tightly closed bottle. The solution was centrifuged at 15,000 g and the supernatant solution was collected. The precipitate was washed with 20 mL of distilled water and was centrifuged again. The supernatant and the washed extracts were combined and designated as non-cellulosic neutral sugar hydrolysate. The residue of the non-cellulosic neutral sugar extraction was freeze-dried and 1 mL of 13 mol L−1 H2SO4 was added and left for 16 h at room temperature. Twenty-five milliliters of distilled water was added and heated for 5 h at 100°C. The supernatant was obtained by centrifugation and the residue was washed with 25 mL distilled water. The supernatant and the washed solutions were combined and were designated as cellulosic neutral sugar hydrolysate. One milliliter of 5 µg mL−1 myo-inositol was added to the hydrolysate as an internal standard. The hydrolysate solutions were passed through Amberlite XAD-7 HP (Organo, Tokyo, Japan) packed in a plastic column (10 mL) and were neutralized with Ba(OH)2, filtered (Advantec No. 131 filter paper) and evaporated to dryness by freeze-drying. Neutral sugar was dissolved in 0.1 mL of 1 mol L−1 ammonia and reduced with 1 mL of sodium borohydride (NaBH4) (2 g in 100 mL of dimethylsulphoxide) and left for 90 min at 40°C. After the reaction, the excess NaBH4 was decomposed by the addition of 18 mol L−1 acetic acid (0.1 mL). The reduced neutral sugar fraction was purified further by passing through a 10-mL plastic column containing Dowex (HCR-W2) cation exchange resin (Muromachi Technos, Tokyo, Japan) in H+ form, and was freeze-dried. The freeze-dried sample was treated with 10 mL of methanol and 1 mL of acetic acid and was evaporated by rotary evaporator to remove boric acid (repeated three times). For acetylation, 0.2 mL of 1-methylimidazole and 2 mL of acetic acid anhydride were added (CitationBlakeney et al. 1983) and the solution was mixed. After 20 min at room temperature, 5 mL of distilled water was added to decompose excess acetic anhydride. After cooling, 2 mL of dichloromethane was added and the mixture was agitated. After the two phases had separated, the lower phase was collected. This extraction was repeated three times. The combined dichloromethane extract was treated with anhydrous Na2SO4 (approximately 3 g) to remove the excess water. The alditol-acetate derivatives of hydrolyzed neutral sugar monomers were analyzed using a Shimadzu (GC-14B) capillary gas chromatograph (Shimadzu, Kyoto, Japan) equipped with a flame ionization detector. The used column was Chrompack CP-Sil 43CB (0.25 mm × 25 m; 0.20 µm film). The column temperature was raised from 195 to 225°C at a rate of 6°C min−1 then held at 225°C for 11 min; the temperatures at the injector and detector ports were 270 and 300°C, respectively. The alditol-acetate derivatives were identified based on their respective retention time by comparing the chromatogram of the standard mixture and the unknown, both containing the internal standard, myo-inositol. All analyses were run in duplicate and mean values are reported. The coefficient of variation among the replicates was <12% of the mean of the two experimental replicates.
Statistical analysis
Principal component analysis (PCA) was carried out using the JMP Program (Version 6, SAS Institute, Cary, NC, USA). Prior to PCA analysis, the non-cellulosic and cellulosic neutral sugars were expressed as a percentage to the summed total of the non-cellulosic and cellulosic neutral sugars, respectively. The obtained values followed a normal distribution, which was confirmed by the shape of the histogram plot and the normal quantile plot using the above statistical software. The first and second principal components accounted for 63% of the total variance and the relevant components reported were those whose eigenvalues were equal to or higher than 0.30.
RESULTS
Soil chemical properties
shows that all soils were moderately to strongly acidic with pH (H2O) values ranging from 4.96 to 5.65. Soil pH tended to be higher at the MP site compared with the SF site, whereas GR and RF land-use types had more or less similar soil pH to the SF land use. Available P was generally very low (1.8–3.7 mg kg−1) and showed no distinct measurable differences among the land-use types. The results further revealed that exchangeable Ca and Mg were higher at the MP and RF sites compared with the remaining land-use sites. The CEC of the soil ranged from 11 to 23 cmolc kg−1 and was slightly higher at the MP and RF sites. The clay content (356–829 g kg−1) was high at all sites, followed by sand (84–405 g kg−1) and silt (83–268 g kg−1). Except for the MP site, all sites had a clay soil texture and there was good comparability in the soil physical properties among the study sites.
shows that land use decreased the soil carbon and nitrogen contents and the extent of the decrease depended on the land-use type. Within land-use types, soil carbon content at SF and MP was more or less similar (1.2–2.4% and 0.9–2.2%, respectively) after a period of approximately 30 years. In contrast, RF and GR had very low soil carbon and nitrogen contents because of a long history of intensive cultivation (). The surface soil had higher soil C and N contents, whereas in the 20–40 and 40–60 cm soil depth increments, the C contents did not differ considerably. The C/N ratio ranged from 7 to 9, with no major differences among land-use types.
Neutral sugar content and composition
shows that the total carbohydrate-C content normalized to total soil carbon was lower at the RF, GR and CP sites and relatively higher at the SF and MP sites. Non-cellulosic neutral sugar was characterized by more pentoses (3.3–27.1 g C kg C−1), followed by hexoses (1.1–16.4 g C kg C−1) and deoxyhexoses (1.3–15.2 g C kg C−1), regardless of the land-use type (). Non-cellulosic neutral sugar decreased in the order of SF > MP > CP > GR > RF (), whereas for the cellulosic neutral sugar, it decreased in the order of GR > RF > SF > CP > MP (). The most abundant neutral sugar monomers in the non-cellulosic fractions were composed of arabinose (Ara) and xylose (Xyl) (1.28–14.42 g C kg C−1 and 0.99–9.80 g C kg C−1, respectively) (). Interestingly, the proportion of neutral sugar in the non-cellulosic neutral sugar () was lower than in the cellulosic neutral sugar () in the GR and RF land-use types.
Table 2 General soil properties of the study sites
Figure 2 (a) Total carbon, (b) total nitrogen and (c) C/N in soils under different land-use types. CP, coffee plantation; GR, grassland; MP, mahogany plantation; RF, rainforestation farming; SF, secondary forest.
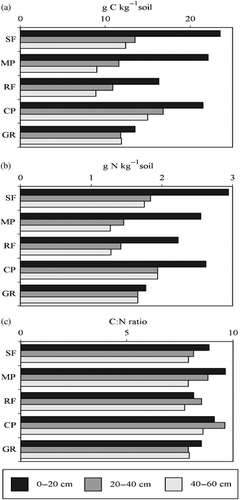
Figure 3 Principal component analysis of the neutral sugar in the non-cellulosic and cellulosic fractions. ▴, coffee plantation; •, grassland; ▀, mahogany plantation; ▵, rainforestation farming; ♦, secondary forest. The numbers 1, 2, and 3 indicate 0–20, 20–40, 40–60 cm soil depths, respectively. Ara, arabinose; Fuc, fucose; Gal, galactose; Glc, glucose; Man, mannose; Rib, ribose; Xyl, xylose.
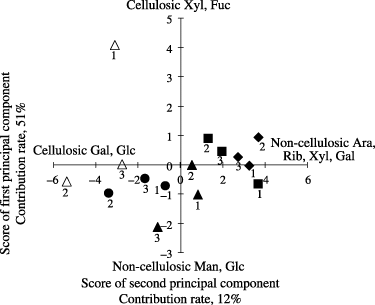
The two significant components of the PCA, accounting for 63% of the variance, were distinguished from the composition of non-cellulosic and cellulosic neutral sugar (). Principal component 1 (PC1) accounted for 51% of the total variance contribution. The main load variables were characterized by large positive (>0.3) eigenvectors of non-cellulosic rhamnose (Rha), galactose (Gal), Xyl and Ara, as well as by large negative (<0.3) eigenvectors of cellulosic (Gal) and glucose (Glc). Principal component 2 (PC2) accounted for 12% of the total variance. This was characterized by large positive (0.61 and 0.38) eigenvectors of cellulosic Xyl and fucose (Fuc), as well as by large negative (<–0.23) eigenvectors of non-cellulosic Glc and mannose (Man).
DISCUSSION
Changes in soil chemical properties
shows that the soil pH at the MP site was higher compared with the other land-use types. This is in agreement with the findings of CitationAsio (1996) who studied the chemical, mineralogical and weathering rates of soil in the area. CitationAsio (1996) hypothesized a possible role of soil rejuvenation through soil erosion (i.e. direct effect of land-use change) that eroded the base-poor surface soil and exposed the relatively less-weathered subsoil containing more bases (CitationJahn and Asio 1998). As indicated by CitationNye and Greenland (1964) and CitationSanchez (1976), the contribution of ash accumulated during vegetation burning may also contribute to higher soil pH at the SF site and the MP site, as well as high base status at the MP and RF sites (); however, 30 years seems too long to keep the basic elements derived from ash. Rather, the elements returned from the litter fall of secondary forest, mahogany plantation and rainforestation may have contributed to the higher pH and richer base status at these sites. As the phosphorous level in the examined soil was much lower than the favorable amount of available P in soils (8–15 mg kg−1; CitationLandon 1991), it appears to be the most limiting nutrient in degraded soils (CitationAsio 1996; CitationNavarrete et al. 2007). The low available P in the soil can be ascribed to the slightly high phosphate retention capacity of 60–82% and the low amount of total P in the andesite parent rocks (CitationAsio 1996).
Table 3 Pentoses, deoxyhexoses and hexoses of non-cellulosic saccharide and carbohydrate-C in total C in soils
Table 4 Extractable non-cellulosic neutral sugar content in soil (g C kg C−1) in the different land-use types
The soil carbon content varied depending on the type of land-use change (). The RF and GR sites had low soil C (0.89–1.63% and 1.19–1.35%, respectively) because they were subjected to long years of cultivation before they were turned to the present land-use. During the period of slash-and-burn cultivation, less organic material was returned to the soil (both above-ground and below-ground biomass), carbon was mineralized faster under humid tropical conditions, and erosion of the surface soil was assumed to take place at these sites. The periodic addition of organic fertilizer at the CP site might have caused the slightly higher soil carbon (1.5–2.1%). Interestingly, the soil carbon level under the MP site was approaching an equilibrium condition with reference to the soil carbon level at the SF site (), indicating that a period of approximately 30 years was enough to recover the organic carbon level in the soil. This conforms to the reports of CitationDetwiler (1986) and CitationBrown and Lugo (1990) who reported that at least 35 years was needed before the soil carbon level under secondary forest approached a steady state level. CitationBrown and Lugo (1990) cited studies in Puerto Rico that revealed that a 50-year-old mahogany plantation had high rates of litterfall and higher litter standing stock, which explained the high recovery of organic C in soil. Soil carbon content in the deeper soil was higher at CP compared with the other sites, probably because of the translocation of organic matter via bioturbation by earthworms and termites, which was observed during the soil profile description.
Table 5 Extractable cellulosic neutral sugar content in soil (g C kg C−1) in the different land-use types
Changes in neutral sugar content and composition
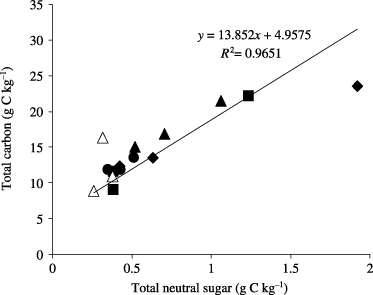
The soil carbohydrate-C content was affected by land-use change and ranged from 2 to 8% of the total soil organic carbon (). These values were much lower than the reported literature values of 5–20% for various soils in temperate zones (CitationAmelung et al. 1999; CitationCheshire 1979; CitationGuggenberger et al. 1994; CitationStevenson 1994) and in tropical zones (e.g. CitationNacro et al. 2005), suggesting the degraded nature of SOM as a result of land-use changes. Indeed, very low carbohydrate-C in soil is a typical characteristic of tropical soil because of high temperatures that enhance the rapid decomposition of SOM (CitationNacro et al. 2005). There was a positive correlation (r = 0.98; P < 0.001) between total soil carbon and total neutral sugar content (), which suggested that land use had a strong influence on the dynamics of carbon and neutral sugar in the soil (CitationAmelung et al. 1999; CitationGuggenberger et al. 1994).
Figure 4 Relationship between total C and sugar content in the different land-use types. ▴, coffee plantation; •, grassland; ▀, mahogany plantation; ▵, rainforestation farming; ♦, secondary forest.
The neutral sugars Xyl and Ara were higher in the surface soils of SF and MP land-use types. This may be attributed to the fact that polysaccharides of vascular plant origin are mostly composed of Xyl and Ara (CitationCheshire 1979). CitationSpiteller (1980) and CitationBochter (1984) reported that Xyl and Ara were the dominant neutral sugars, particularly in the O-horizon in soil. CitationTrouve et al. (1996) suggested that Xyl is a good marker in elucidating vegetation inheritance. The high Xyl and Ara contents are considered to be derived directly from the litters of secondary forests and mahogany trees, respectively. The small contribution of non-cellulosic neutral sugar content in GR land use can be ascribed to the fast decomposition of grasses and low net primary production of grassland. In contrast, the influence of rainforestation in RF land use was smaller because of the shorter time (10–14 years) since the trees has been established compared with the length of time under SF and MP land-use types. The higher cellulosic neutral sugar in the RF and GF land-use types relative to the non-cellulosic neutral sugar (compare and ) indicates that cellulosic sugar was more refractory than non-cellulosic neutral sugar, although it appears to be site specific to these areas. CitationTsutsuki and Kuwatsuka (1989) and CitationKiem and Kögel-Knabner (2003) reported that some fraction of the carbohydrates is subjected to long-term stabilization by interaction with soil minerals and hence very stable in the soil.
The influence of land use on non-cellulosic and cellulosic neutral sugar composition was clear from the PCA data (). To summarize the tendency, PC1 increased with the contribution of non-cellulosic neutral sugar (Ara, ribose [Rib], Xyl and Gal) and decreased with the contribution of cellulosic neutral sugar (Gal and Glc). In contrast, PC2 seemed to increase with the contribution of relatively stable neutral sugars derived from wood (cellulosic xylose) and decrease with the contribution of microbial neutral sugar (non-cellulosic Glc and Man).
The SF and MP land uses showed a similar trend with higher PC1 values with larger non-cellulosic neutral sugar (Ara, Rib, Xyl and Gal) reflecting a large input of plant residue into the soil. The depletion of non-cellulosic neutral sugar and the enrichment of more stable cellulosic sugar (Gal and Glc) in the RF and GR land uses may still inherit the adverse effect of land-use change leading to the deterioration of soil quality, which occurred during the period of shifting cultivation. In the case of the RF site, PC2 values were high in the surface soil because of high cellulosic Xyl and Fuc coming from the present tree vegetation and low PC1 values with a larger contribution of cellulosic Gal and Glc in the deeper horizon. In contrast, the effect of land-use change at the CP site was intermediate because the area had not been used intensively for cultivation previously (). In general, the PC1–PC2 plots showed that neutral sugar compositions tended to shift from right to (lower) left, indicating that non-cellulosic, plant-derived neutral sugars degrade preferentially and/or are enriched with newly formed microbe-derived, non-cellulosic sugars.
The relative contribution of microbial-derived and plant-derived carbohydrates in soil can be estimated by calculating the mass ratios of neutral sugar monomers, except for Glc, which originates from both microbial and plant products. Pentoses such as Xyl and Ara are mainly of plant origin, whereas hexoses such as Man, Gal, and to a lesser extent Rha and Fuc, are partially derived from microbial origin (CitationCheshire 1979; CitationOades 1984). Remarkably, Rib is the least abundant neutral sugar and its presence reflects microbial rather than plant origin. Therefore, the molar ratios of [(Man + Gal)/(Ara + Xyl)] (CitationOades 1984) and [(Fuc + Rham)/(Ara + Xyl)] (CitationMurayama 1984) serve as an indicator of the source of sugars. According to CitationOades (1984), the [(Man + Gal)/(Ara + Xyl)] ratio is > 2 for microbe-derived neutral sugar and < 0.5 for plant-derived neutral sugar. shows a higher [(Man + Gal)/(Ara + Xyl)] ratio (0.19–0.91) in the non-cellulosic neutral sugar, suggesting enrichment of microbe-derived neutral sugar. Under silvicultural and agricultural land use, the ratio of [(Man + Gal)/(Ara + Xyl)] tended to be higher in the surface horizon at the MP and CP sites, suggesting a larger contribution of microbial sugar. The [(Rha + Fuc)/(Ara + Xyl)] values were also high and suggested that the neutral sugar was of microbial origin (CitationMurayama 1984). These results were consistent with the data from the PCA (). This implies that under humid tropical conditions, high microbial activities in soil coupled with high temperatures and favorable moisture conditions resulted in the rapid decomposition of neutral sugars.
Conclusion
From the results of the present study, forest conversion into secondary land uses decreased the carbon, nitrogen and neutral sugar contents in the soil. These parameters showed a tendency to recover at the SF and MP sites, but the decrease was more marked at the RF and GR sites, which had been used for intensive cultivation for a longer period. Within land-use type, the differences in soil carbon, nitrogen and neutral sugar composition could be attributed to differences in the vegetation cover, the amount of organic matter added to the soil, differences in past land use and the succeeding soil management after land use. Soil carbohydrate-C content as a proportion of the total soil organic carbon was very low (2–8%) and indicated rapid decomposition of neutral sugar. The results of this study suggested that both land-use change and the humid tropical conditions in the Philippines influenced the content and composition of neutral sugar and accelerated any changes. Additional studies are necessary to understand the effect of land-use change on the neutral sugar composition in various degraded soils in the Philippines.
Table 6 Comparative ratios of non-cellulosic neutral sugar in the different land-use types
ACKNOWLEDGMENTS
The senior author thanks the Ministry of Education, Science, Sports and Culture of Japan for scholarship support. Professor Victor Asio, Professor Renzo Kondo and Mr Rey Navarrete are thanked for their assistance with the soil sampling and Dr Masayuki Tani and Ms Chihiro Mizota are thanked for their analytical support.
REFERENCES
- Amelung , W , Flach , KW and Zech , W . 1999 . Neutral and acidic sugars in particle-size fractions as influenced by climate . Soil Sci. Soc. Am. J , 63 : 865 – 873 .
- Asio , VB . 1996 . “ Characteristics, weathering, formation, and degradation of soils from volcanic rocks in Leyte, Philippines ” . In Hohenheimer Bodenkundliche Hefte , vol. 33 , Germany : Stuttgart .
- Barrera , A , Aristorenas , I and Tingzon , J . 1954 . Soil Survey of Leyte Province, Philippines , Manila : Department of Agriculture and Natural Resources .
- Blakeney , AB , Harris , PJ , Henry , RJ and Stone , BA . 1983 . A simple and rapid preparation of alditol acetates for monosaccharide analysis . Carbohydr. Res , 113 : 291 – 299 .
- Bochter , R . 1984 . Determination of cellulosic and non-cellulosic neutral sugars in soil hydrolysates by the help of high performance thin-layer chromatography . Z Pflanszenernähr. Bodenk , 147 : 203 – 209 . (in German)
- Brown , S and Lugo , AE . 1990 . Effects of forest clearing and succession on the carbon and nitrogen content of soils in Puerto Rico and US Virgin Islands . Plant Soil , 124 : 53 – 64 .
- Cheshire , MV . 1979 . Nature and Origin of Carbohydrates in Soils , London : Academic Press .
- Detwiler , RP . 1986 . Land use change and the global carbon cycle: the role of tropical soils . Biogeochem , 2 : 67 – 93 .
- Forest Management Bureau . 1988 . Natural Forest Resources of the Philippines , Manila : Philippine–German Forest Resources Inventory Project .
- Garrity , DP , Kummer , DM and Guiang , ES . 1993 . “ The Philippines ” . In Sustainable Agriculture and the Environment in the Humid Tropics , Edited by: Committee on Sustainable Agriculture and the Environment in the Humid Tropics, National Research Council . 549 – 624 . Washington : National Academy Press .
- Guggenberger , G , Christensen , BT and Zech , W . 1994 . Land use effects on the composition of organic matter in particle-size separates of soils: I. Lignin and carbohydrate signature . Eur. J. Soil Sci , 45 : 449 – 458 .
- Jahn , R and Asio , VB . 1998 . “ Soils of the tropical forests of Leyte, Philippines-I. Weathering, soil characteristics, classification, and site qualities ” . In Soils of Tropical Forest Ecosystems , Edited by: Schulte , A and Ruhiyat , D . 29 – 36 . Berlin : Springer-Verlag .
- Kiem , R and Kögel-Knabner , I . 2003 . Contribution of lignin and polysaccharides to the refractory carbon pool in C-depleted arable soils . Soil Biol. Biochem , 35 : 101 – 118 .
- Langenberger , G , Martin , K and Sauerborn , J . 2006 . Vascular plant species inventory of a Philippine lowland rain forest and its conservation value. . Biodivers. Conserv , 15 : 1271 – 1301 .
- Lugo , AE and Brown , S . 1993 . Management of tropical soils as sinks or sources of atmospheric carbon . Plant Soil , 149 : 27 – 41 .
- Murayama , S . 1984 . Changes in monosaccharide composition during the decomposition of straws under field conditions . Soil Sci. Plant Nutr , 30 : 367 – 381 .
- Murty , D , Kirschbaum , MUF , Mcmurtie , RS and Mcgilvray , H . 2002 . Does conversion of forest to agricultural land change soil carbon and nitrogen? A review of the literature . Global Change Biol , 8 : 105 – 123 .
- Nacro , HB , Larré-Larrouy , MC , Feller , C and Abbadie , L . 2005 . Hydrolysable carbohydrate in tropical soils under adjacent forest and savanna vegetation in Lamto, Côte d’Ivoire . Aust. J. Soil Res , 43 : 705 – 711 .
- Navarrete , IA , Asio , VB , Jahn , R and Tsutsuki , K . 2007 . Characteristics and genesis of two highly weathered soils in Samar, Philippines . Aust. J. Soil Res , 45 : 153 – 163 .
- Landon , JR . 1991 . Booker Tropical Soil Manual , London : Longman Scientific and Technical .
- Food and Agriculture Organization . 2006 . Guidelines for Soil Description , 3rd edn , Rome : Food and Agriculture Organization .
- Nye , PH and Greenland , DJ . 1964 . Changes in the soil after clearing tropical forest . Plant Soil , 21 : 101 – 112 .
- Oades , JM . 1984 . Soil organic matter and structural stability: Mechanisms and implications for management . Plant Soil , 76 : 319 – 337 .
- Oades , JM , Kirkman , MA and Wagner , GH . 1970 . The use of gas–liquid chromatography for the determination of sugars extracted from soils by sulfuric acid . Soil Sci. Soc. Am. Proc , 34 : 230 – 235 .
- Sanchez , PA . 1976 . Properties and Management of Soils in the Tropics , New York : John Wiley & Sons .
- Shoji , S , Miyake , M and Takeuchi , Y . 1964 . Comparisons of various determination methods of available phosphate (Part 2) . Bull. Hokkaido Agric. Exp. Stn , 84 : 32 – 39 . (in Japanese)
- Spiteller , M . 1980 . Capillary gas chromatographic determination of sugars from different soils . Z. Pflanszenernähr. Bodenk , 143 : 720 – 729 . (in German)
- Stevenson , FJ . 1994 . Humus Chemistry: Genesis, Compositions, Reactions , 2nd edn , New York : John Wiley & Sons .
- Trouve , C , Disnar , JR , Mariotti , A and Guillet , B . 1996 . Changes in the amount and distribution of neutral monosaccharides of savanna soils under plantation of Pinus and Eucalyptus in the Congo . Eur. J. Soil Sci , 47 : 51 – 59 .
- Tsutsuki , K and Kuwatsuka , S . 1989 . Degradation and stabilization of humus on buried volcanic ash soils. I. Humus composition, molecular size distribution of humic acids, and sugars composition of soils . Soil Sci. Plant Nutr , 35 : 207 – 216 .
- Veldkamp , E , Becker , A , Schwendenmann , L , Clark , DA and Schulte-Bisping , H . 2003 . Substantial labile carbon stocks and microbial activity in deeply weathered soils below a tropical wet forest . Global Change Biol , 9 : 1171 – 1184 .