Abstract
The host range of seven Frankia strains isolated from the root nodules of five actinorhizal plants in four genera growing in Japan and their relatedness based on 16S rDNA were examined. The Ceq1 strain isolated from Casuarina equisetifolia formed many nodules with high nitrogen fixation activity on the source host, C. equisetifolia. The Ceq1 strain formed nodules on Alnus sieboldiana, Alnus hirsta and Myrica rubra, but their nodulation ratio and nitrogen fixation activity were very low. The Asi1 strain isolated from A. sieboldiana, the Ahi1 strain isolated from A. hirsta, and the Mru1, Mru2 and Mru8 strains isolated from M. rubra formed many nodules on both Alnus species and M. rubra. The Ema2 strain isolated from Elaeagnus macrophylla formed nodules on both Elaeagnus species and M. rubra. Based on the aspect of nodulation and nitrogen fixation activity, Ceq1 strain is an effective strain only for Casuarina, five strains of Asi1, Ahi1, Mru1, Mru2 and Mru8 are effective strains for both Alnus and Myrica, and Ema2 strain is an effective strain for both Elaeagnus and Myrica. The results of phylogenetic analyses based on 16S rDNA sequences showed that seven strains were separated into two major clusters. Cluster 1 was further grouped into two subclusters, 1a and 1b. The Asi1, Ahi1, Mru1, Mru2 and Mru8 strains belonged to 1a and the Ceq1 strain belonged to cluster 1b. The Ema2 strain was classified into cluster 2. This phylogenetic classification of Frankia based on 16S rDNA sequences appears to be consistent with the host range determined using an inoculation test.
INTRODUCTION
Actinorhizal plants, in which nitrogen-fixing nodules can be detected on roots by infection with Frankia, are distributed in 24 genera in eight families (CitationSwensen and Mullin 1997). In Japan, species among Alnus, Myrica, Elaeagnus, Casuarina and Coriaria grow naturally. Most actinorhizal plants are capable of achieving high rates of nitrogen fixation (CitationDillon and Baker 1982; CitationFukumoto et al. 1992; CitationHiyoshi et al. 1988; CitationSasakawa 1995; CitationTani et al. 2003) and the estimated amount of nitrogen fixed by these plants annually per unit area can equal or surpass the amount fixed by Rhizobium–legume symbioses (CitationHibbs and Cromack 1990; CitationKondo and Yoneyama 1990; CitationTorrey 1978). Thus, the high nitrogen-fixing capability of these actinorhizal plants contributes to improve soil fertility for the recovery of vegetation in degraded land under severe environmental stress.
After CitationCallaham et al. (1978) succeeded in isolating and cultivating an actinomycete from the root nodules of Comptonia, hundreds of Frankia isolates have been obtained from various plant species growing in various geographical areas. The members of the genus Frankia can be clearly distinguished from other bacterial genera on the basis of their infectivity for actinorhizal plants, their morphology, their biochemistry, and their physiology (CitationLechevalier 1994). The host specificity of Frankia was comprehensively studied using a cross-inoculation test with pure-cultured Frankia strains and was classified into four host-specificity groups (CitationBaker 1987). In contrast, phylogenetic studies of effective (capable of forming nodules with nitrogen-fixing activity) Frankia strains based on 16S rDNA, nifH or glnA sequences generally show three major clades (CitationBenson and Clawson 2000; CitationClawson et al. 2004; CitationJeong et al. 1999; CitationNick et al. 1992; CitationNormand et al. 1996).
Table 1 Origins of the Frankia strains used in this study
We have tried to isolate Frankia strains from the root nodules of actinorhizal plants growing in Japan to establish useful actinorhizal plant–Frankia symbiotic systems for the recovery of vegetation in degraded areas. Seven Frankia strains have been isolated from the root nodules of five species in four genera, Myrica, Elaeagnus, Alnus and Casuarina (CitationHiyoshi et al. 1988; CitationSasakawa et al. 1998; CitationTani et al. 2003). Using these Frankia strains, estimation of the minimum amount of inoculant necessary for good nodulation and the salt tolerance of the Frankia strains and that of the host plants have been studied (CitationSasakawa 1995; CitationTani and Sasakawa 2000, Citation2006, CitationTani et al. 2003). However, the general characteristics and host range of these Frankia strains, including phylogenetic relationships, have not been examined systematically.
In the present study, we determined: (1) the morphological and physiological characteristics of seven Frankia strains isolated from the root nodules of actinorhizal plants growing in Japan, (2) the host range of the Frankia strains using an inoculation test, (3) the phylogenetic relationship of the Frankia strains based on 16S rDNA.
MATERIALS AND METHODS
Frankia strains
We used seven Frankia strains isolated from the root nodules of five actinorhizal plants in four genera growing in Japan (). Frankia strains have been subcultured at 28°C in the dark by transferring part of the Frankia cells to a new Qmod medium (CitationLalonde and Calvert 1979), primarily once per month for several years after isolation.
Characteristics of the Frankia strains
Frankia cells were cultured in Qmod medium at 28°C in the dark and collected by centrifugation at 1,700 g every 2 days for 2 weeks and the doubling time on the growth was estimated. Morphological characteristics were achieved by photomicroscopic observation. Frankia cells transferred to a glass slide using a Pasteur pipette were stained with 0.1% fuchsin (basic) solution as previously described (CitationSasakawa et al. 1998) and observed with a photomicroscope (BH2 Olympus, Tokyo, Japan).
Inoculation test of Frankia strains
Seven actinorhizal plants, Casuarina equisetifolia, Alnus sieboldiana, Alnus hirsta, Myrica rubra, Elaeagnus macrophylla, Elaeagnus umbellata and Coriaria japonica were used. Casuarina equisetifolia and M. rubra seeds were obtained from Ishigaki Island, Okinawa Prefecture, Japan, and from Tokushima Prefecture, Japan, respectively. Alnus hirsta seeds were obtained from Thuyama City in Okayama Prefecture, Japan. Elaeagnus macrophylla, E. umbellata and C. japonica seeds were collected from the Tsushima Campus of Okayama University and A. sieboldiana seeds were collected from the Tsudaka Livestock Farm of Okayama University.
The endocarp covering Elaeagnus seeds was removed. The seeds of E. umbellata and C. japonica were scarified with a razor blade until the seed coat was partially removed. Elaeagnus, Coriaria, Alnus and Casuarina seeds were surface sterilized for 15 min with sodium hypochlorite solution (available chlorine was approximately 2%) and then immersed in running tap water overnight. Seven Elaeagnus and Coriaria seeds, and approximately 50 Alnus and Casuarina seeds were sown in a plastic pot (12.5 cm in diameter, 10 cm in depth) containing autoclaved vermiculite soaked with distilled water. The seeds were then cultured in a growth chamber under a day length of 14 h, a light quantum of 100 µmol m−2 s−1, and a day/night temperature regime of 30/25°C. The seedlings were supplied with only distilled water until thinning. One month after germination, the seedlings were thinned to four actively growing seedlings in Elaeagnus and to approximately half of the seedlings in Alnus and Casuarina per pot and then they were inoculated with each Frankia strain. The Frankia cells, which were cultured for 1 month, were collected by centrifugation at 1,700 g and the packed cells were diluted 500-fold with N-free Arnon–Hoagland (CitationArnon and Hoagland 1940) solution and then 35 mL of a cell suspension was inoculated to each pot. Uninoculated seedlings were prepared as a control. The seedlings were cultured for 10 weeks in a growth chamber under the same conditions as those described above. N-free Arnon–Hoagland solution was supplied at 2-week intervals.
In M. rubra, seeds in which the endocarp had been removed were treated with gibberellin A3 at a concentration of 1 mmol L−1 and rinsed once with distilled water and then the seeds were allowed to germinate on agar plates as previously described (CitationSasakawa 1995). Seven seedlings were transplanted to a plastic pot and thinned to six actively growing seedlings per pot. The seedlings were then inoculated with each Frankia strain and cultivated for 10 weeks as described above.
Measurement of nitrogen-fixing activity
Nitrogen-fixing activity was determined using acetylene reduction activity (ARA). The excised whole root was placed in a 110-mL Erlenmeyer flask and sealed tightly with a rubber serum stopper. Gas phases in the containers were adjusted to contain 10% (v/v) acetylene in air and incubated at 25°C. After a 1-h incubation, the amount of ethylene produced was measured as previously described (CitationSasakawa et al. 1986). Three or five average-sized seedlings per pot were selected and subjected to a determination of nitrogen-fixing activity.
Isolation of DNA from Frankia cells
Frankia cells were lysed for extraction of total DNA following the method described by CitationSimonet et al. (1984) with a slight modification. Approximately 200 µL of packed Frankia cells collected by centrifugation at 1,700 g were mixed thoroughly with 10 mg of lysozyme and 600 units of achromopeptidase dissolved in 400 µL of sterile distilled water and were incubated for 40 min at 37°C shaking at 100 strokes per minute. After three repeats of freeze–thaw with liquid nitrogen and 65°C hot water, 100 µL of the lysate was subjected to DNA extraction using a DNeasy Plant Mini Kit (Qiagen GmbH, Hilden, Germany). The extracted DNA was confirmed by agarose gel (1%, w/v) electrophoresis.
Polymerase chain reaction amplification
Amplification of the whole 16S rDNA was carried out using primers FGPS-1509′-153 (5′-AAGGAGGGGATCCAGCCGCA-3′), FGPS4–281bis (5′-ATGGA[G/A]AG[T/C]TTGATCCTGGCTCA-3′) (CitationHuguet et al. 2001) in a thermal cycler (iCycler, Bio-Rad Laboratories, Hercules, CA, USA) under the following conditions: initial denaturation for 4 min at 95°C, 35 cycles consisting of denaturation at 95°C for 1 min, annealing at 52°C for 1 min, and extension at 72°C for 2 min, and a final extension step at 72°C for 5 min. The reaction volume was 50 µL, containing approximately 200–300 ng DNA, 10× Ex Taq buffer (Takara Bio, Otsu, Japan), each deoxynucleoside triphosphate at a concentration of 200 µmol L−1, 0.5 µmol L−1 of each primer, and 2.5 U of TaKaRa Ex Taq polymerase (Takara Bio). The amplification of DNA was checked by agarose gel electrophoresis (1%, w/v) with 5 µL of polymerase chain reaction (PCR) product, and the gel was stained with ethidium bromide (0.5 µg mL−1) (Bio-Rad Laboratories). A single product of the expected size (approximately 1,500 bp) was purified using GenElute Agarose Spin Columns (Sigma–Aldrich Biotechnology, Saint Louis, MO, USA).
Sequencing of 16S rDNA
The amplified fragments were cloned into competent cells (JM109) using the pGEM-T Easy Vector (Promega, Madison, WI, USA), according to the protocol of the manufacturer. The plasmids were prepared in mini cultures and the correct size of the inserted fragments was inferred by restricted digestion with EcoRI. Amplification of the whole 16S rDNA was carried out using primers FGPS-1509′-153 and FGPS4-281bis. The internal primers designed to obtain the inner region were Fra16S1 (5′-GAAGCACCGGCCAACTACGT-3′) and Fra16S2 (5′-CTCACGACACGAGCTGACGA-3′). Sequencing was done with a DNA-1000L DNA Sequencer (Shimadzu, Kyoto, Japan) using 4.2% acrylamide gel.
Phylogenetic analysis
All of the sequences obtained were compared with sequences in the National Center for Biotechnology Information (NCBI; http://www.ncbi.nlm.nih.gov/) and DNA Data Bank of Japan (DDBJ; http://www.ddbj.nig.ac.jp/index-j.html) databases using the BLAST program (CitationAltschul et al. 1990). The sequences were aligned using CLUSTAL W (CitationThompson et al. 1994) and phylogenetic trees were constructed using the PHYLIP package (CitationFelsenstein 1993). One thousand pseudo-alignments were generated with Seqbot. Evolutionary distances were calculated using DNA-DIST with a Kimura two-parameter model (transition/transversion ratio of two). A neighbor-joining tree was then constructed using the program Neighbor (CitationSaitou and Nei 1987). A consensus tree was generated with Consense and is illustrated as a cladogram using TreeView (CitationPage 1996).
RESULTS AND DISCUSSION
Characteristics of the Frankia strains
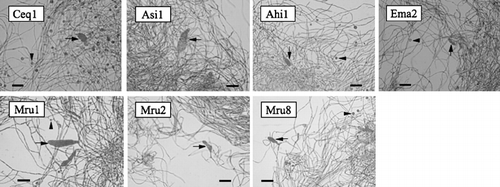
Morphological and physiological characteristics of seven Frankia strains isolated from the root nodules of five species in four genera, Casuarina, Alnus, Myrica and Elaeagnus are summarized in . Photomicrographs of seven Frankia strains are shown in . All strains had hyphae measuring 0.7 ± 0.2 µm in diameter and round, cylindrical or highly irregular sporangia usually observed in liquid culture. Apparent vesicles or vesicle-like structures, in which the enzyme nitrogenase was localized (CitationMeesters et al. 1987; CitationSasakawa et al. 1988), were observed in five strains (i.e. not in Asi1 and Mru2). These specialized structures were not observed in every strain and were often observed in nitrogen-free media or in some strains growing in media with a limited nitrogen source (CitationLechevalier and Lechevalier 1990). Ceq1 and Ahi1 strains grown in N-containing Qmod medium usually showed weak ARA in a free-living state (data not shown), indicating that the nitrogenase in the vesicles is functioning. When Ceq1 and Ema2 strains were cultured for several weeks in Qmod medium, the color of the medium changed to pale dark brown. Such a color change was not observed in the other strains. The relationship between the existence of vesicles or vesicle-like structures, color change of medium and ARA seems to be equivocal. Further examination will have to be done to clarify the relationships among vesicle formation and color change of medium and nitrogen-fixing activity. The doubling time of the seven strains subcultured just prior to the stationary phase was between 1 and 2 days.
Table 2 Characteristics of the Frankia strains
Inoculation tests
The host range of seven Frankia strains was determined by inoculation to six actinorhizal plants, including their source host and C. japonica. The nodulation ratio (nodulated plants/tested plants), the nodule fresh weight and the ARA of the nodulated plants are shown in . Ceq1 formed many nodules with high ARA on the host plant, C. equisetifolia. Ceq1 also formed nodules on M. rubra, A. sieboldiana and A. hirsta, but ARA was very low in each plant and, in particular, the nodulation ratio and nodule fresh weight were poor in Alnus species. Ceq1 did not form nodules on E. macrophylla. These results indicate that Ceq1 has high host specificity to the source host C. equisetifolia.
Table 3 Inoculation test of seven Frankia strains on actinorhizal plants
Asi1 and Ahi1 formed many nodules on their source hosts A. sieboldiana and A. hirsta and also nodulated well on M. rubra. Mru1, Mru2 and Mru8 isolated from M. rubra formed nodules very well on their source host and on Alnus species, and Asi1 and Ahi1 isolated from Alnus species nodulated well on M. rubra. Five strains isolated from Alnus and Myrica did not form nodules on C. equisetifolia. These results indicate that Frankia strains isolated from Alnus and Myrica have high host specificity to both Alnus and Myrica, although their microbiological characteristics are somewhat different. Mru1, Mru2 and Mru8 did not always nodulate on E. macrophylla. In this experiment only Mru1 in Mru strains formed nodules on E. macrophylla (), but in separate experiments either the Mru2 or Mru8 strain formed nodules on E. macrophylla or E. umbellata (data not shown). This suggests that three Mru strains have nodulation ability to Elaeagnaceae, although the frequency of nodulation was lower than that of Ema2.
Ema2 isolated from E. macrophylla formed nodules on the source host E. macrophylla and M. rubra. Consequently, Ema2 is classified as a strain with host specificity to Myrica and Elaeagnus. None of the seven strains used in this study formed nodules on C. japonica.
CitationBaker (1987) classified pure-cultured Frankia strains into four host specificity groups (HSG) according to nodulation: HSG1, Alnus (Betulaceae) and Myrica (Myricaceae); HSG2, Casuarina (Casuarinaceae) and Myrica; HSG3, Myrica and Elaeagnus (Elaeagnaceae); and HSG4, only members of Elaeagnaceae (Elaeagnus, Hippophae, Shepherdia). The magnitude of host specificity of the seven Frankia strains was classified into four degrees by comprehensively estimating the nodulation ratio, nodule fresh weight and ARA (). Asi1 and Ahi1 strains isolated from Alnus were definitely classified into Baker's HSG1 and Mru1, Mru2 and Mru8 strains isolated from Myrica were also primarily classified into HSG1. However, because Mru strains occasionally nodulate on a few plants of Elaeagnus, they might be classified into another group, Alnus–Myrica–Elaeagnus. The Ceq1 strain can be primarily classified into HSG2. However, because the Ceq1 strain formed a small number of nodules with low ARA on Alnus species in a manner similar to that reported previously (CitationTani et al. 2003), the host range of Ceq1 strain might be Casuarina–Myrica–Alnus. Ema2 strain is definitely classified into HSG3, which nodulate on Myrica and Elaeagnus.
The present results suggest the existence of diverse Frankia strains that have diverse host ranges. Thus, the classification of the host range of Frankia is not simple, although Baker's grouping of Frankia strains based on nodulation is primarily useful and acceptable. We have no information about the host specificity of Frankia on Coriaria because we have not yet succeeded in isolating Frankia from the root nodules of C. japonica. Isolation of Frankia from the root nodules of Coriaria may be difficult because only Frankia-like actinomycetes have been isolated from Coriaria nepalensis, and these failed to induce nodules on Coriaria seedlings (CitationMirza et al. 1992). We will continue isolating Frankia from the root nodules of C. japonica and will clarify the characteristics and the host range of the isolates.
Table 4 Host specificity of the seven Frankia strains
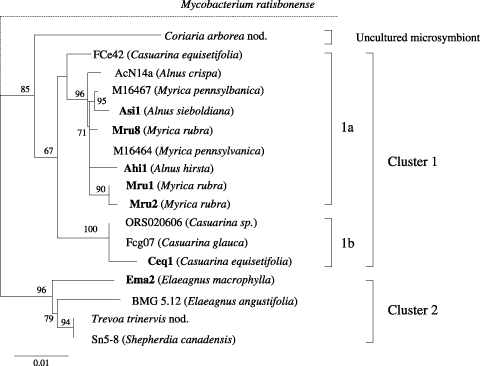
Figure 2 Phylogeny of Frankia shown using a neighbor-joining tree of 16S rDNA sequences. Bootstrap values are based on 1,000 samplings; those above 50% are shown. The position of the outgroup species (Mycobacterium ratisbonense) is indicated by dashed lines. Host plants are shown in parentheses. The seven Frankia strains characterized in the present study are shown in bold.
Sequencing and phylogenetic analysis
We determined the complete 16S rDNA sequences of the expected size (approximately 1,500 bp) in seven Frankia strains and drew a phylogenetic tree (). The sequences of 16S rDNA in some Frankia strains published in the database are presented together in with those of the seven strains used in this study. In the phylogenetic tree of the 16S rRNA gene, the seven strains were roughly distinguished into two major clusters, Cluster 1 and 2. Five strains, Ahi1, Asi1, Mru1, Mru2 and Mru8, isolated from Alnus and Myrica and the Ceq1 strain isolated from Casuarina belonged to Cluster 1. The Ema2 strain isolated from Elaeagnus was classified into Cluster 2. Cluster 1 can be subclassified into two subclusters, 1a and 1b, although the bootstrap value between subcluster 1a comprised of five strains and subcluster 1b comprised of Ceq1 strain may not be high enough to confirm the branching.
Phylogenetic relationships among Frankia genomic species have been determined using amplified 16S rDNA sequences (CitationClawson et al. 2004; CitationNazaret et al. 1991; CitationNormand et al. 1996). The phylogenetic tree showed that strains belonging to the Alnus infectivity group are closely related to strains belonging to the Casuarina infectivity group and that strains of the Elaeagnus infectivity group are well separated from Alnus and Casuarina infectivity groups (CitationNazaret et al. 1991). CitationNormand et al. (1996) showed that the genus Frankia can be separated into four main clusters by phylogenetic analysis based on complete 16S rDNA sequences: (1) a very large group including Alnus-infective, Casuarina-infective and Myrica-infective strains, (2) unisolated mirosymbionts of Dryas, Coriaria and Datisca species, (3) Elaeagnus-infective strains, (4) “atypical” strains like an Alnus-infective, non-nitrogen-fixing strain. Furthermore, CitationClawson et al. (2004) showed that infective Frankia strains can be separated into three major clades (I–III) and Clade I consists of unisolated symbionts from Coriariaceae, Datiscaceae, Rosaceae, and Ceanothus of the Rhamnaceae, Clade II consists of Alnus-, Myrica- and Casuarina-infective strains, and Clade III consists of mainly Elaeagnus-infective strains. Frankia strains based on 16S rDNA, nifH or glnA sequences have generally revealed three major clades. The phylogenetic analysis based on 16S rDNA in the present study indicated that the seven Frankia strains isolated from five actinorhizal plants in four genera growing in Japan can be classified into three clusters: Cluster 1a consists of Alnus–Myrica and Alnus–Myrica–Elaeagnus infective strains, Cluster 1b consists of Casuarina–Alnus–Myrica infective strains, and Cluster 2 consists of Elaeagnus–Myrica infective strains (). The seven Frankia strains in this study were classified into three groups by host range using inoculation tests: (1) primarily Alnus–Myrica infectivity group, (2) primarily Casuarina-infectivity group, (3) Elaeagnus–Myrica infectivity group (,). The classification of Frankia strains by host range and phylogenetic analysis based on 16S rDNA appears to be consistent. The homology of 16S rDNA sequences among the seven strains was high and the similarity was in the range of 97–99%. Phylogenetic analysis based on 16S rDNA is useful in the classification of Frankia strains, which are genetically closely related, together with their biological functions, for example, nodulation. CitationClawson et al. (2004) examined correspondence between Frankia clades and acrtinorhizal plant clades established using analyses of rbcL gene sequences (cf. CitationSwensen and Mullin 1997) and showed that current associations of actinorhizal plants and Frankia strains indicate non-congruent phylogenies. Coevolutional relationships between Frankia and actinorhizal plants will be further clarified by the advance of molecular phylogenetic analysis concerning both Frankia and actinorhizal plants. We will continue our studies to further clarify the host range and phylogenetic relationships of Frankia strains by using new strains that were not used in the present study and Frankia strains that may be isolated from the root nodules of C. japonica.
ACKNOWLEDGMENTS
This work was supported in part by a Grant-in-Aid for Scientific Research (B) (16380051) from the Japan Society for the Promotion of Science.
Notes
Present address: Research Faculty of Agriculture, Hokkaido University, N9W9, Kita-ku, Sapporo 060-8589, Japan.
REFERENCES
- Altschul , SF , Gish , W , Miller , W , Myers , EW and Lipman , DJ . 1990 . Basic local alignment search tool . J. Mol. Biol , 215 : 403 – 410 .
- Arnon , DI and Hoagland , DR . 1940 . Crop production in artificial solutions and soils with special reference to factors influencing yield and absorption of inorganic nutrients . Soil Sci , 50 : 463 – 471 .
- Baker , DD . 1987 . Relationships among pure cultured strains of Frankia based on host specificity . Physiol. Plant , 70 : 245 – 248 .
- Benson , DR and Clawson , ML . 2000 . “ Evolution of the actinorhizal plant symbioses ” . In Prokaryotic Nitrogen Fixation: A Model System for Analysis of Biological Process , Edited by: Triplett , EW . 207 – 224 . Wymondham : Horizon Scientific Press .
- Callaham , D , Del Tredici , P and Torrey , JG . 1978 . Isolation and cultivation in vitro of the actinomycete causing root nodulation in Comptonia . Science , 199 : 899 – 902 .
- Clawson , ML , Bourret , A and Benson , DR . 2004 . Assessing the phylogeny of Frankia-actinorhizal plant nitrogen-fixing root nodule symbioses with Frankia 16S rRNA and glutamine synthetase gene sequences . Mol. Phylogenet. Evol , 31 : 131 – 138 .
- Dillon , JT and Baker , D . 1982 . Variations in nitrogenase activity among pure-cultured Frankia strains tested on actinorhizal plants as an induction of symbiotic compatibility . New Phytol , 92 : 215 – 219 .
- Felsenstein , J . 1993 . PHYLIP (Phylogeny Inference Package) , Seattle : University of Washington Press . Version 3.67
- Fukumoto , T , Ishizawa , K and Muto , N . 1992 . A tube culture of Frankia plants for studying Frankia–root interactions . Jpn. J. Soil Sci. Plant Nutr , 63 : 325 – 331 . (in Japanese with English summary)
- Hibbs , DE and Cromack , K Jr. 1990 . “ Actinorhizal plants in Pacific Northwest forests ” . In The Biology of Frankiaand Actinorhizal Plants , Edited by: Schwintzer , CR and Tjepkema , JD . 343 – 363 . London : Academic Press .
- Hiyoshi , T , Sasakawa , H and Yatazawa , M . 1988 . Isolation of Frankia strains from root nodule of Myrica rubra . Soil Sci. Plant Nutr , 34 : 107 – 116 .
- Huguet , V , Batzli , JM , Zimpfer , JF , Normand , P , Dawson , JO and Fernandez , MP . 2001 . Diversity and specificity of Frankia strains in nodules of sympatric Myrica gale, Alnus incana, and Shepherdia canadensis determined by rrs gene polymorphism . Appl. Environ. Microbiol , 67 : 2116 – 2122 .
- Jeong , SC , Ritchie , NJ and Myrold , DD . 1999 . Molecular phylogenies of plants and Frankia support multiple origins of actinorhizal symbioses . Mol. Phylogen. Evol , 13 : 493 – 503 .
- Kondo , M and Yoneyama , T . 1990 . Recent research progress in biological nitrogen fixation (26). Nitrogen fixation of field crops cultivated in various areas of the world (2) . Agric. Hortic , 65 : 863 – 870 . (in Japanese)
- Lalonde , M and Calvert , HE . 1979 . “ Production of Frankia hyphae and spores as an infective inoculant for Alnus species ” . In Symbiotic Nitrogen Fixation in the Management of Temperate Forests , Edited by: Gordon , JC , Wheeler , CT and Perry , DA . 95 – 110 . Corvallis : Forest Research Laboratory, Oregon State University .
- Lechevalier , MP . 1994 . Taxonomy of the genus Frankia (Actinomycetales) . Int. J. Syst. Bacteriol , 44 : 1 – 8 .
- Lechevalier , MP and Lechevalier , HA . 1990 . “ Systematics, isolation and culture of Frankia ” . In The Biology of Frankiaand Actinorhizal Plants , Edited by: Schwintzer , CR and Tjepkema , JD . 35 – 60 . London : Academic Press .
- Meesters , TM , Van Vliet , WM and Akkermans , ADL . 1987 . Nitrogenase is restricted to the vesicles in Frankia strain EAN1pec . Physiol. Plant. , 70 : 267 – 271 .
- Mirza , MS , Hahn , D and Akkermans , ADL . 1992 . Isolation and characterization of Frankia strains from Coriaria nepalensis . System. Appl. Microbiol , 15 : 289 – 295 .
- Nazaret , S , Cournoyer , B , Normand , P and Simonet , P . 1991 . Phylogenetic relationships among Frankia genomic species determined by use of amplified 16S rDNA sequences . J. Bacteriol , 173 : 4072 – 4078 .
- Nick , G , Paget , E , Simonet , P , Moiroud , A and Normand , P . 1992 . The nodular endophytes of Coriaria spp. form a distinct lineage within the genus Frankia . Mol. Ecol , 1 : 175 – 181 .
- Normand , P , Orso , S Cournoyer , B . 1996 . Molecular phylogeny of the genus Frankia and related genera and emendation of family Frankiaceae . Int. J. Syst. Bacteriol , 46 : 1 – 9 .
- Page , RD . 1996 . TreeView: an application to display phylogenetic trees on personal computers . Comput. Appl. Biosci , 12 : 357 – 358 .
- Saitou , N and Nei , M . 1987 . The neighbor-joining method: a new method for reconstructing phylogenetic trees . Mol. Biol. Evol , 4 : 406 – 425 .
- Sasakawa , H . 1995 . Effect of Frankia inoculation on growth and nitrogen-fixing activity of Myrica rubra seedlings prepared aseptically . Soil Sci. Plant Nutr , 41 : 691 – 698 .
- Sasakawa , H , Hiyoshi , T and Sugiyama , T . 1988 . Immuno-gold localization of nitrogenase in root nodules of Elaeagnus pungens Thunb . Plant Cell Physiol , 29 : 1147 – 1152 .
- Sasakawa , H , Kawai , H , Takahashi , N and Tanigawa , S . 1998 . Isolation of Frankia from the root nodules of Elaeagnus macrophylla and Alnus sieboldiana and their infectivities to the host plant . Microbes and Environ , 13 : 143 – 148 .
- Sasakawa , H , Trung , BC and Yoshida , S . 1986 . Stem nodulation on Aeschynomene indica plants by isolated rhizobia . Jpn. J. Soil Sci. Plant Nutr , 32 : 145 – 149 .
- Simonet , P , Capellano , A , Navarro , E , Bardin , R and Moiroud , A . 1984 . An improved method for lysis of Frankia with achromopeptidase allows detection of new plasmids . Can. J. Microbiol , 30 : 1292 – 1295 .
- Swensen , SM and Mullin , BC . 1997 . Phylogenetic relationships among actinorhizal plants. The impact of molecular systematics and implications for the evolution of actinorhizal symbioses . Physiol. Plant , 99 : 565 – 573 .
- Tani , C and Sasakawa , H . 2000 . Salt tolerance of Elaeagnus macrophylla and Frankia Ema1 strain isolated from the root nodules of E. macrophylla . Soil Sci. Plant Nutr , 46 : 927 – 937 .
- Tani , C and Sasakawa , H . 2006 . Proline accumulates in Casuarina equisetifolia seedlings under salt stress . Soil Sci. Plant Nutr , 52 : 21 – 25 .
- Tani , C , Sasakawa , H Takenouchi , K . 2003 . Isolation of endophytic Frankia from root nodules of Casuarina equisetifolia and infectivity of the isolate to host plants . Soil Sci. Plant Nutr , 49 : 137 – 142 .
- Thompson , JD , Higgins , DG and Gibson , TJ . 1994 . CLUSTAL W: improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice . Nucleic Acids Res , 22 : 4673 – 4680 .
- Torrey , JG . 1978 . Nitrogen fixation by actinomycete-nodulated angiosperms . BioScience , 28 : 586 – 592 .
- Present address: Research Faculty of Agriculture, Hokkaido University, N9W9, Kita-ku, Sapporo 060-8589, Japan.