Abstract
Zinc (Zn) excess has significant toxicity to biological systems through metal-based cytotoxic reactions. Nicotianamine (NA) and deoxymugineic acid (DMA) are low-molecular-weight, high-affinity transition metal chelators. Studies have shown that NA may have a role in the tolerance of excess Zn. We show that a gene coding the iron (Fe)-regulated DNA-binding transcription factor (OsIRO2) and the downstream genes of OsIRO2, such as NA synthase, DMA synthase and the DMA-Fe3+ transporter, were induced in rice roots by excess Zn. Consistent with the expression of these genes, the amounts of endogenous NA, endogenous DMA and DMA secretion increased in the excess Zn roots. Although the Fe concentration in the excess Zn roots was much higher than that in the control, rice ferritin gene, OsFer1, was downregulated in Zn excess roots. OsIRT1, which is upregulated by Fe deficiency, was not induced in Zn excess roots, suggesting that OsIRO2 may not be induced simply by the Fe deficiency caused by excess Zn. The data indicate that the induction of OsIRO2 by excess Zn is responsible for the production of NA and DMA, which may play a role in maintaining cellular Zn availability.
INTRODUCTION
Zinc (Zn) is required for the activity of many enzymes, including alcohol dehydrogenase, Cu/Zn superoxide dismutase, carbonic anhydrase and many proteases (CitationVallee and Falchuk 1993). Although Zn is an essential nutrient, it can be toxic when accumulated at high levels in cells. Therefore, the uptake and transport of Zn must be strictly regulated to maintain an adequate intracellular Zn level. Intracellular Zn availability is achieved through the coordinated regulation of specific transporters engaged in Zn influx, efflux and intracellular compartmentalization.
Thlaspi caerulescens is a naturally selected Zn-tolerant Zn hyperaccumulator. In comparison with Arabidopsis thaliana, T. caerulescens exhibits transcript abundance of several Zn-related genes, such as a cellular Zn uptake system, a member of the Zn-regulated transporter and a Fe-regulated transporter-like protein (ZIP), a P-type metal ATPase (HMA), a cation diffusion facilitator (MTP), and nicotianamine synthase (NAS) genes. These genes may be related to the tolerance to Zn excess (Citationvan de Mortel et al. 2006; CitationTalke et al. 2006). Several ZIP transporters have been isolated from rice plants (CitationIshimaru et al. 2005, Citation2006, Citation2007; CitationRamesh et al. 2003). OsIRT1 is a functional Fe2+ transporter induced in Fe-deficient roots (CitationBughio et al. 2002). OsZIP1, OsZIP3 and OsZIP4 are transcriptionally upregulated in Zn-deficient roots and shoots, and these proteins take up Zn. However, information on the roles of HMA and Zn transporter of Arabidopsis thaliana in rice plants is still sparse.
The mugineic acid family phytosiderophores (MAs), a family of Fe chelators, are secreted from roots to solubilize sparingly soluble Fe in the rhizosphere, and play a major role in Fe acquisition. Rice plants synthesize and secrete 2′-deoxymugineic acid (DMA) among MAs. The biosynthesis of DMA and the corresponding genes have already been characterized. The DMA is synthesized from methionine (CitationMori and Nishizawa 1987), which is converted into S-adenosyl-L-methionine (SAM) by SAM synthase (SAMS). Subsequently, three molecules of SAM are combined to form one molecule of nicotianamine (NA) using NA synthase (NAS). The NA is then converted to a 3″-keto acid by NA aminotransferase (NAAT), and DMA is synthesized through DMA synthase (DMAS). The genes encoding the enzymes involved in DMA synthesis have been well characterized in Fe-deficient rice and barley. The expression of HvNAS1, HvNAAT-A, HvNAAT-B and HvDMAS1 is elevated in Fe-deficient barley roots (CitationBashir et al. 2006; CitationHiguchi et al. 1999; CitationTakahashi et al. 1999). In rice, OsNAS1, OsNAS2, OsNAAT1 and OsDMAS1 are induced in both roots and shoots with Fe deficiency (CitationBashir et al. 2006; CitationHiguchi et al. 2001a; CitationInoue et al. 2003; H. Inoue, unpubl. data, 2008).
Recently, OsIRO2, a basic helix–loop–helix (bHLH)-type transcriptional factor, was identified, and the OsIRO2-binding sequence 5’-CACGTGG-3′ was found to frequently occur in regions upstream of the Fe-deficiency inducible genes (CitationOgo et al. 2006). bHLH is characterized by two α helices connected by a loop and typically binds to a consensus sequence, CANNTG (CitationChaudhary and Skinner 1999). OsIRO2 has been shown to control the expression of OsNAS1, OsNAS2, OsNAAT1 and OsDMAS1, in addition to that of the DMA-Fe3+ transporter OsYSL15, but not OsIRT1 (CitationOgo et al. 2007). In A. thaliana, bHLH transcription factors homologous to OsIRO2 (AtbHLH38, AtbHLH39, AtbHLH100 and AtbHLH101) were reported to be induced by Fe deficiency, and experiments using mutants of these genes showed that none of the AtbHLH transcription factors regulate IRT1, FRO2 or FIT1 (CitationWang et al. 2007).
In the present study, we show that the expression of OsIRO2, OsNAS1, OsNAS2, OsNAAT1 and OsDMAS1 was highly induced and the amounts of endogenous NA, endogenous DMA and DMA secretion were increased in Zn excess roots, whereas OsIRT1 and OsFer1 were not induced in Zn excess roots. These results indicate that OsIRO2 induced by Zn excess may regulate the production of NA and DMA for excess Zn detoxification.
MATERIALS AND METHODS
Plant material
Oryza sativa L. cv. Nipponbare was used for the microarray, northern blot and metal concentration analyses. Seeds were germinated for 3 days at room temperature on paper soaked with distilled water. After germination, the seedlings were transferred to a Saran net floating on distilled water in a growth chamber (day: 25°C, 14 h of light at 320 µmol photons m−2 s−1; night: 10 h at 20°C). After 3 days, 45 seedlings were transferred to a 20-L plastic container containing a nutrient solution with the following composition: 0.7 mmol L−1 K2SO4, 0.1 mmol L−1 KCl, 0.1 mmol L−1 KH2PO4, 2.0 mmol L−1 Ca(NO3)2, 0.5 mmol L−1 MgSO4, 10 µmol L−1 H3BO3, 0.5 µmol L−1 MnSO4, 0.2 µmol L−1 CuSO4, 0.5 µmol L−1 ZnSO4, 0.05 µmol L−1 Na2MoO4, and 0.1 mmol L−1 Fe-ethylenediaminetetraacetic acid (Fe-EDTA). A further 100 µmol L−1 ZnSO4 was added to the solution to induce Zn excess. The pH of the nutrient solution was adjusted daily to 5.5 with 1 mol L−1 HCl, and the nutrient solution was renewed weekly. For the Zn excess treatment, 2-week-old plants were transferred to a nutrient solution with 200 µmol L−1 Zn and grown for two more weeks.
Oligo DNA microarray analysis
A rice 44K custom oligo DNA microarray kit (Agilent Technology, Tokyo, Japan), which contains 44,000 oligonucleotides based on the sequence data of the rice full-length cDNA project (http://cdna01.dna.affrc.go.jp/cDNA/), was used. Total RNA was extracted from shoots and roots using an RNeasy plant kit (Qiagen, Tokyo, Japan) according to the manufacturer's instructions. The yield and RNA purity were determined spectrophotometrically. The integrity of the RNA was checked using an Agilent 2100 Bioanalyzer. Total RNA (200 ng) was labeled with Cy-3 or Cy-5 using an Agilent Low RNA Input Fluorescent Linear Amplification kit. The fluorescently labeled targets were hybridized to Agilent rice 44K oligo DNA microarrays. The hybridization process was carried out according to the manufacturer's instructions, and the hybridized microarrays were scanned using an Agilent microarray scanner. Agilent's Feature Extraction software was used for the image analysis and the data extraction processes.
Measurement of NA and DMA
Nicotianamine and DMA secretion were measured as described in CitationSuzuki et al. (2006). To determine the level of endogenous DMA, 12 plant roots were rinsed with deionized water and then soaked in 600 mL of deionized water at sunrise. Root exudates were collected for 5 h. The deionized water was renewed 2 h after starting the collection. The antimicrobial agent Micropur (Katadyn, Wallisellen, Switzerland) was added to the water to prevent microbial degradation of the MAs after collection. After a second sampling, both root exudates were combined and filtered through filter paper (Advantec 5C; Toyo Roshi Kaisha, Tokyo, Japan). The condensed and microfiltered samples were subjected to high-performance liquid chromatography analysis as described previously (CitationMori et al. 1987; CitationShojima et al. 1989). The pH of the 0.15 mol L−1 Li-citrate buffer was decreased to 2.97 for better separation of DMA, and the pH of the 0.2 mol L−1 Li-citrate buffer was increased to 3.30 for better separation of NA.
Determination of metal concentrations
Plants were dried for 1 week at 65°C. The dried plants (30–50 mg) were then wet ashed with 2 mL of 11 mol L−1 HNO3 for 5 h at 150°C. The metal concentrations were measured using inductively coupled plasma atomic emission spectrometry (SPS1200VR; Seiko, Tokyo, Japan) at wavelengths of 238.204 (Fe), 213.856 (Zn), 293.930 (Mn) and 324.754 (Cu) nm.
Northern blot analysis
Total RNA was extracted from roots and shoots, and 10 µg per lane was electrophoresed in 1.2% (w/v) agarose gels containing 0.66 mol L−1 formaldehyde and transferred to a Hybond-N+ membrane (Amersham, Piscataway, NJ, USA). The membranes were hybridized with 32P-labeled probes at 65°C following the method of CitationIshimaru et al. (2005). The amplified open reading frame (ORF) of OsIRT1 (CitationIshimaru et al. 2005) was used to prepare the probe. For OsNAS2 and OsFer1, specific probes were prepared using the following specific primers: OsNAS2, 5′-TGAGTGCGTGCATAGTAATCCTGGC-3′ and 5′-CAGACGGTCACAAACACCTCTTGC-3′; and OsFer1, 5′-AGGCTCCAGTCAATTGTCAC-3′ and 5′-CAACCTGCTCCTGAAGGAAT-3′.
RESULTS
Microarray analysis in excess Zn conditions
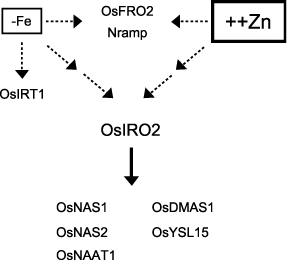
To examine the expression of genes induced by excess Zn in rice we carried out a 44K microarray analysis. In excess Zn conditions OsNAS1 and OsNAS2 were highly induced in roots, suggesting that NA synthesis was enhanced in Zn excess roots (). Interestingly, OsNAAT1 and OsDMAS1 were also upregulated in Zn excess roots, suggesting that DMA may also be synthesized in Zn excess roots. OsIRO2, OsYSL2 and OsYSL15 were also induced. In contrast, OsIRT1, which is not regulated by OsIRO2, was not induced in Zn excess roots. OsZIP4 induced by Zn deficiency was downregulated in Zn excess roots, consistent with the overexpression of OsZIP4 roots that accumulate Zn in roots (CitationIshimaru et al. 2007).
The expression pattern in Zn excess shoots was much different from that in Zn excess roots. OsIRO2, OsNAS1, OsNAS2, OsNAAT1 and OsDMAS1 were not induced in Zn excess shoots, suggesting that the synthesis of NA and DMA may not be enhanced in Zn excess shoots. Instead, the Fe3+-chelate reductase gene (OsFRO2) and an Nramp family gene were upregulated ().
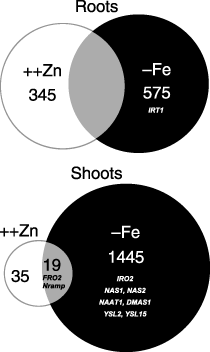
In Zn excess roots, 400 genes were upregulated, 55 of which are usually induced by Fe deficiency in wild-type (WT) plants (). In Zn excess shoots, 54 genes were upregulated, 19 of which were downregulated by Fe deficiency in WT plants ().
Amounts of endogenous NA, endogenous DMA and DMA secretion in Zn excess roots
The microarray analysis suggested the possibility that NA and DMA synthesis was enhanced in Zn excess roots. We, therefore, examined whether NA and DMA were synthesized and accumulated in the Zn excess rice roots, and whether DMA was secreted from the Zn excess rice roots. In Zn excess roots, the amount of endogenous NA was 2.3-fold higher, and the amount of endogenous DMA was 3.2-fold higher compared with the control condition (). In Zn excess shoots, the amount of endogenous NA did not change, and the amount of endogenous DMA decreased, consistent with the expression of OsNASs and OsNAAT1 in the microarray (). The amount of DMA secreted from Zn excess plants was approximately threefold that secreted from control plants ().
Table 1 Summary of the microarray analysis of the genes related to Fe availability
Metal concentrations in Zn excess rice plants
We used 6-week-old rice seedlings that had received an excess Zn treatment for 2 weeks. There was no apparent difference in growth between Zn excess and control plants, except for a slight increase in the root weight induced by Zn excess (). We measured the concentrations of Zn, Mn, Fe and Cu in Zn excess rice plants to examine the nutritional status of these plants (). As expected, there was a significant increase in the Zn concentration in the roots and shoots. Furthermore, the Fe concentration in the Zn excess roots was fivefold higher than that found in the control roots and was 20% lower in shoots. Similarly, the Mn concentration increased in Zn excess roots and decreased in Zn excess shoots. Zinc excess in either roots or shoots did not affect the Cu concentration.
Confirmation of the gene expression patterns
We carried out a northern blot analysis using Zn excess plants (). Consistent with the results of the microarray analysis, OsNAS2 was induced in Zn excess roots, but not in Zn excess shoots. OsIRT1 was not induced in roots, contrary to the expression pattern of IRT1 in A. thaliana (Citationvan de Mortel et al. 2006; CitationRobinson et al. 1999; CitationTalke et al. 2006). We also carried out a northern blot analysis of the rice ferritin gene OsFer1 in excess Zn conditions. Plant ferritins are plastid proteins whose abundance is strictly controlled at the transcriptional level by the Fe status of the cells. The transcripts of OsFer1 decreased in Zn excess shoots and roots, suggesting that Zn excess did not cause Fe excess in root cells, despite the high accumulation of Fe in Zn excess roots.
Figure 2 Amounts of endogenous nicotianamine (NA) and deoxymugineic acid (DMA) secretion in Zn excess plants. (a) The amount of endogenous NA in Zn excess (++Zn) and control (C) conditions. (b) The amount of endogenous DMA in Zn excess (++Zn) and control (C) conditions. (c) The amount of DMA secreted from rice roots in Zn excess (++Zn) and control (C) conditions. Values followed by different letters are statistically different according to a t-test (**P < 0.01). D.W., dry weight; F.W., fresh weight.
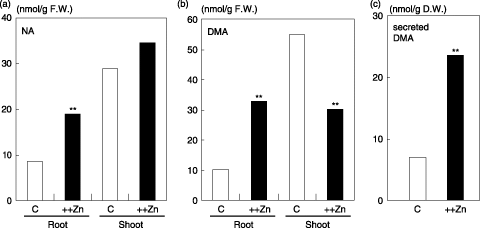
Figure 3 Plant weight and metal concentrations of Zn excess plants. The weight of (a) roots and (b) shoots of plants grown under Zn excess (++Zn) or control (C) conditions. The concentrations of (c) Fe, (d) Zn, (e) Mn and (f) Cu in the roots and shoots are expressed as milligrams per gram dry weight. The values followed by different letters are statistically different according to T test (*P < 0.05; **P < 0.01).
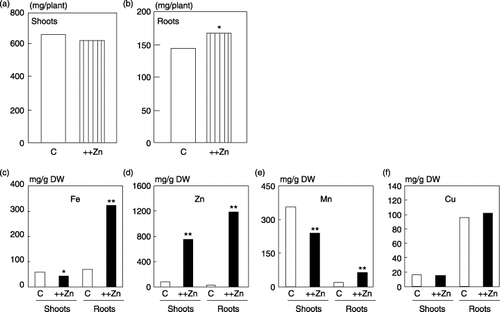
Figure 4 Northern blot analysis of OsNAS1, OsIRT1 and OsFer1 mRNA in the roots and leaves of rice plants grown under excess Zn conditions. Total RNA (10 µg) was extracted from plants grown in normal nutrient solution (control [C]) or under conditions of excess Zn (++Zn). Ethidium-bromide-stained rRNA is shown as a control for loading.
![Figure 4 Northern blot analysis of OsNAS1, OsIRT1 and OsFer1 mRNA in the roots and leaves of rice plants grown under excess Zn conditions. Total RNA (10 µg) was extracted from plants grown in normal nutrient solution (control [C]) or under conditions of excess Zn (++Zn). Ethidium-bromide-stained rRNA is shown as a control for loading.](/cms/asset/9677eb4e-db15-45e1-a3b6-88ee92b6d104/tssp_a_10382602_o_f0004g.gif)
DISCUSSION
In Zn excess rice plants, the expression patterns of the genes related to Fe availability indicated that OsIRO2 could be one of the transcriptional factors for gene regulation in response to Zn excess. We previously reported that OsIRO2 regulates the expression of genes, such as OsNAS1, OsNAS2, OsDMAS1, OsNAAT1 and OsYSL15, but not OsIRT1, in Fe-deficient rice plants (CitationOgo et al. 2007). In the present study, a microarray analysis of Zn excess rice plants revealed that OsIRO2, OsNAS1, OsNAS2, OsNAAT1, OsDMAS1 and OsYSL15 were induced in roots, but not in shoots (), suggesting that OsIRO2 may control these genes and may be involved in Zn availability in Zn excess plants.
In A. thaliana, excess Zn causes an increase in the transcript levels of AtNAS1, IRT1 and FRO2 in roots (CitationBecher et al. 2004; Citationvan de Mortel et al. 2006, CitationRobinson et al. 1999; CitationTalke et al. 2006). There were, however, no significant differences in the Fe concentration in the shoots and roots of A. thaliana exposed to excess Zn (CitationBecher et al. 2004), suggesting that Zn excess does not cause Fe deficiency. In rice, the Fe concentration was much higher in the roots and slightly lower in the shoots of Zn excess plants (). Despite the high Fe concentration in the roots, OsFer1 was slightly downregulated in both the roots and shoots (), suggesting that the excess Fe was not located within the cytoplasm but rather was highly accumulated in the root apoplasm, and that excess Zn in the roots and shoots might cause Fe deficiency owing to the low amount of available Fe. OsNAS1, OsNAS2, OsNAAT1, OsDMAS1 and OsYSL15 were induced, but OsIRT1 was not induced in Zn excess roots. These data suggest that Zn excess would affect only the genes regulated by OsIRO2 in roots, and that Zn excess would play a part in the Fe deficiency response ().
In Zn excess shoots, the Fe concentration decreased, OsFer1 was downregulated and OsFRO2 and an Nramp family gene, which are induced by Fe deficiency, were upregulated. These data suggest that Zn excess might cause Fe deficiency in shoots. However, OsIRO2 and the genes involved in NA and DMA synthesis were not induced, the amount of endogenous NA did not change, and endogenous DMA decreased (). Therefore, the induction of these genes may not be attributable simply to Fe deficiency originating from Zn excess in the shoots.
Zinc deficiency increases the secretion of MAs from wheat and barley roots into the rhizosphere (CitationCakmak et al. 1994; CitationWalter et al. 1994; CitationZhang et al. 1989), and it has been hypothesized that Zn deficiency causes Fe deficiency, resulting in increased MA secretion (CitationWalter et al. 1994). However, CitationSuzuki et al. (2006) demonstrated that the increased biosynthesis and secretion of MAs in barley arising from a shortage of Zn is not because of an induced Fe deficiency, and that secreted MAs are effective in absorbing Zn from the soil. In contrast, Zn-deficient rice secretes decreased amounts of DMA and Zn deficiency did not induce OsIRO2 (CitationSuzuki et al. in press).
Nicotianamine is also a strong chelator of Zn in vitro, in addition to Fe, Mn, Cu and Ni (CitationBenes et al. 1983; CitationStephan and Scholz 1993; CitationStephan et al. 1996; CitationVacchina et al. 2003). A recent study showed that the overexpression of HvNAS in A. thaliana and tobacco transgenic plants conferred Zn resistance (CitationKim et al. 2005), strengthening the idea that NA could play a role in Zn tolerance. In Fe-deficient roots, endogenous NA is decreased because NA is consumed for DMA synthesis, and DMA secretion is increased (CitationHiguchi et al. 1999). In Zn excess roots, endogenous NA, endogenous DMA and DMA secretion increased, suggesting that NA is responsible for the tolerance of excess Zn, in addition to the being the precursor of DMA. Although the function of DMA is not known in excess Zn conditions, the synthesis of DMA in Zn excess roots may also be involved in maintaining Zn availability in rice plants.
ACKNOWLEDGMENTS
We thank Dr T. Kobayashi and Dr K. Bashir for carefully reading the manuscript, and Dr Nagamura and the Rice Genome Project (National Institute of Agrobiological Sciences, Tsukuba, Japan) for help with the microarray analysis. This work was supported by a grant from the Ministry of Agriculture, Forestry and Fisheries of Japan (Green Technology Project IP-5003) and the Program for the Promotion of Basic Research Activities for Innovative Biosciences. We thank Ms R. N. Itai for valuable discussions.
REFERENCES
- Bashir , K , Inoue , H Nagasaka , S . 2006 . Cloning and characterization of deoxymugineic acid synthase genes from graminaceous plants . J. Biol. Chem , 43 : 32395 – 32402 .
- Becher , M , Talke , IN , Krall , L and Krämer , U . 2004 . Cross-species microarray transcript profiling reveals high constitutive expression of metal homeostasis genes in shoots of the zinc hyperaccumulator Arabidopsis halleri . Plant J , 37 : 251 – 268 .
- Benes , I , Schreiber , K , Ripperger , H and Kirsceiss , A . 1983 . Metal complex formation of nicotianamine, a possible phytosiderophore . Experientia , 39 : 261 – 262 .
- Bughio , N , Yamaguchi , H , Nishizawa , NK , Nakanishi , H and Mori , S . 2002 . Cloning an iron-regulated metal transporter from rice . J. Exp. Bot , 53 : 1677 – 1682 .
- Cakmak , I , Gülüt , KY , Marschner , H and Graham , RD . 1994 . Effect of zinc and iron deficiency on phytosiderophore release in wheat genotypes differing in zinc deficiency . J. Plant Nutr , 17 : 1 – 17 .
- Chaudhary , J and Skinner , MK . 1999 . Basic helix-loop-helix proteins can act at the E-Box within the serum response element of the c-fos promoter to influence hormone-induced promoter activation in sertoli cells . Mol. Endocrinol , 12 : 774 – 786 .
- Higuchi , K , Suzuki , K , Nakanishi , H , Yamaguchi , H , Nishizawa , NK and Mori , S . 1999 . Cloning of nicotianamine synthase genes, novel genes involved in the biosynthesis of phytosiderophores . Plant Physiol , 119 : 471 – 479 .
- Higuchi , K , Takahashi , M , Nakanishi , H , Kawasaki , S , Nishizawa , NK and Mori , S . 2001a . Analysis of transgenic rice containing barley nicotianamine synthase gene . Soil Sci. Plant Nutr , 47 : 315 – 322 .
- Higuchi , K , Watanabe , S Takahashi , M . 2001b . Nicotianamine synthase gene expression differs in barley and rice under Fe-deficient conditions . Plant J , 25 : 159 – 167 .
- Inoue , H , Higuchi , K , Takahashi , M , Nakanishi , H , Mori , S and Nishizawa , NK . 2003 . Three rice nicotianamine synthase genes, OsNAS1, OsNAS2, and OsNAS3 are expressed in cells involved in long-distance transport of iron and differentially regulated by iron . Plant J , 36 : 366 – 381 .
- Ishimaru , Y , Masuda , H Suzuki , M . 2007 . Overexpression of the OsZIP4 zinc transporter confers disarrangement of zinc distribution in rice plants . J. Exp. Bot , 58 : 2909 – 2915 .
- Ishimaru , Y , Suzuki , M Kobayashi , T . 2005 . OsZIP4, a novel zinc-regulated zinc transporter in rice . J. Exp. Bot , 56 : 3207 – 3214 .
- Ishimaru , Y , Suzuki , M Tsukamoto , T . 2006 . Rice plants take up iron as an Fe3+-phytosiderophore and as Fe2+ . Plant J , 45 : 335 – 346 .
- Kim , S , Takahashi , M Higuchi , K . 2005 . Increased nicotianamine biosynthesis confers enhanced tolerance of high levels of metals, in particular nickel, to plants . Plant Cell Physiol , 46 : 1809 – 1818 .
- Mori , S , Nishizawa , N , Kawai , S , Sato , Y and Takagi , S . 1987 . Dynamic state of mugineic acid and analogous phytosiderophores in Fe-deficient barley . J. Plant Nutr , 10 : 1013 – 1020 .
- van de Mortel , JE , Almar Villanueva , L Schat , H . 2006 . Large expression differences in genes for iron and zinc homeostasis, stress response, and lignin biosynthesis distinguish roots of Arabidopsis thaliana and the related metal hyperaccumulator Thlaspi caerulescens . Plant Physiol , 142 : 1127 – 1147 .
- Ogo , Y , Itai , RN Nakanishi , H . 2006 . Isolation and characterization of IRO2, a novel iron-regulated bHLH transcription factor in graminaceous plants . J. Exp. Bot , 57 : 2867 – 2878 .
- Ogo , Y , Itai , RN Nakanishi , H . 2007 . The rice bHLH protein OsIRO2 is an essential regulator of the genes involved in Fe uptake under Fe-deficient conditions . Plant J , 51 : 366 – 377 .
- Ramesh , SA , Shin , R , Eide , DJ and Schachtman , DP . 2003 . Differential metal selectivity and gene expression of two zinc transporters from rice . Plant Physiol , 133 : 126 – 134 .
- Suzuki , M , Tsukamoto , T Inoue , H . “ Synthesis of deoxymugineic acid for Zn translocation in Zn-deficient rice plants ” . In Plant Mol Biol in press
- Mori , S and Nishizawa , N . 1987 . Methionine as a dominant precursor of phytosiderophores in Graminaceae plants . Plant Cell Physiol , 28 : 1081 – 1092 .
- Robinson , NJ , Procter , CM , Connolly , EL and Guerinot , ML . 1999 . A ferric-chelate reductase for iron uptake from soils . Nature , 397 : 694 – 697 .
- Stephan , UW and Scholz , G . 1993 . Nicotianamine: mediator of transport of iron and heavy metals in the phloem? . Physiol. Plantarum , 88 : 522 – 529 .
- Stephan , UW , Schmidke , I , Stephan , VW and Scholz , G . 1996 . The nicotianamine molecule is made-to-measure for complexation of metal micronutrients in plants . Biometals , 9 : 84 – 90 .
- Shojima , S , Nishizawa , NK Fushiya , S . 1989 . Biosynthesis of nicotianamine in the suspension-cultured cells of tobacco (Nicotiana megalosiphon . Biol. Metals , 2 : 142 – 145 .
- Suzuki , M , Takahashi , M Tsukamoto , T . 2006 . Biosynthesis and secretion of mugineic acid family phytosiderophores in zinc-deficient barley . Plant J , 48 : 85 – 97 .
- Takahashi , M , Yamaguchi , H , Nakanishi , H , Shioiri , T , Nishizawa , NK and Mori , S . 1999 . Cloning two genes for nicotianamine aminotransferase, a criticalenzyme in iron acquisition (strategy II) in graminaceous plants . Plant Physiol , 121 : 947 – 956 .
- Talke , IN , Hanikenne , M and Kramer , U . 2006 . Zinc-dependent global transcriptional control, transcriptional deregulation, and higher gene copy number for genes in metal homeostasis of the hyperaccumulator Arabidopsis halleri . Plant Physiol , 142 : 148 – 167 .
- Vacchina , V , Mari , S Czernic , P . 2003 . Speciation of nickel in a hyperaccumulating plant by high-performance liquid chromatography-inductively coupled plasma mass spectrometry and electrospray MS/MS assisted by cloning using yeast complementation . Anal. Chem , 75 : 2740 – 2745 .
- Vallee , BL and Falchuk , KH . 1993 . The biochemical basis of zinc physiology . Physiol. Rev , 73 : 79 – 118 .
- Walter , A , Römheld , V , Marschner , H and Mori , S . 1994 . Is the release of phytosiderophores in zinc-deficient wheat plants a response to impaired iron utilization? . Physiol. Plantarum , 92 : 493 – 500 .
- Wang , HY , Klatte , M , Jakoby , M , Baumlein , H , Weisshaar , B and Bauer , P . 2007 . Iron deficiency-mediated stress regulation of four subgroup Ib BHLH genes in Arabidopsis thaliana . Planta , 226 : 897 – 908 .
- Zhang , F , Römheld , V and Marschner , H . 1989 . Effect of zinc deficiency in wheat on the release of zinc and iron mobilizing root exudates . Z. Pflanzenernhr. Bodenkd , 152 : 205 – 210 .