Abstract
Lotus japonicus was used to study the distribution and interconnections of 15 elements in plant tissues, including essential and non-essential elements: boron (B), sodium (Na), magnesium (Mg), potassium (K), calcium (Ca), manganese (Mn), iron (Fe), nickel (Ni), copper (Cu), zinc (Zn), arsenic (As), strontium (Sr), molybdenum (Mo), cadmium (Cd) and cesium (Cs). Large amounts of B and Ca accumulated in plant leaves, while Fe, Na, Ni, As and Cd tended to mainly occur in the roots, and Mo was the only element to accumulate in the stems. The elemental compositions within plants were severely disturbed by treatment with toxic elements. Competition between element pairs in the same group (e.g. K and Cs; Ca and Sr) was not found. Iron, Cu and Zn accumulation were induced by Cd and Ni addition. When natural variants grew in a nutrition solution with subtoxic levels of As, Cd, Cs, Ni, Mo and Sr, intriguing relationships between the elements (such as Fe, As and K; Mg and Ni; Mn and Ca) were revealed using principal-component analysis. This study on the plant ionome offers detailed information of element interactions and indicates that chemically different elements might be closely linked in uptake or translocation systems.
INTRODUCTION
Seventeen elements are essential for plants to complete their life cycle, and more than five are considered to be beneficial for plant growth (CitationMarschner 1995). In addition, cadmium (Cd), chromium (Cr), lead (Pb), mercury (Hg), arsenic (As), cesium (Cs) and strontium (Sr) have been widely studied because anthropogenic or natural activities have introduced these toxic elements into the environment. Up to 42 elements have been investigated in field-collected plant leaves (CitationWatanabe et al. 2007); however, the relationships between elements in plant tissues have often been described from analyses of a very limited set of elements. An analysis of the distribution of a large number of elements under controlled conditions is still lacking. Although the behaviors of element uptake and distribution vary greatly between plant species and environments, most research has shown that elements belonging to the same group in the periodic table are not distinguishable by the transporters in plants (e.g. Zn and Cd; K and Cs; CitationClemens 2001; CitationWhite and Broadly 2000; CitationZhu and Smolders 2000) or distinguishable from ions with the same charge because of their chemical similarity and functional replaceability (CitationGuerinot 2000; CitationHolm et al. 1996; CitationRogers et al. 2000). There are also synergistic actions among carbon (C), nitrogen (N) and phosphorus (P) as a function of plant growth rate (CitationÅgren 2004; CitationSterner and Elser 2002) and between cations and anions to balance the ion charge in plant tissues (CitationHaynes 1990; CitationKirkby and Knight 1977).
The recent development of inductively coupled-plasma mass spectroscopy (ICP-MS) and inductively coupled-plasma atomic emission spectroscopy (ICP-AES) has allowed macroelements and microelements in plants to be detected simultaneously and rapidly. A new term “ionome” has been defined to describe all metals, metalloids and non-metals in an organism (CitationHirschi 2003; CitationRea 2003; CitationSalt 2004). Several studies have made great progress in studying plant and yeast ionomes. By analyzing the leaves of approximately 6,000 Arabidopsis mutants with ICP-MS, CitationLahner et al. (2003) constructed an ion-profile library with 18 elements to map the connection between plant ionome analysis and genomic information. The results indicated that one gene could control the homeostasis of more than one element in plant tissues, and approximately 2–4% of all genes contributed to ion uptake control. A clearer mapping of genes on elemental composition has been made using 4,385 yeast mutant strains from the Saccharomyces Genome Deletion Project (http://www-sequence.stanford.edu/group/yeast_deletion_project/deletions3.html). This project gave comparable results to CitationLahner et al. (2003) and highlighted previously unsuspected relationships between elements (CitationEide et al. 2005).
Ionomics analyses of Arabidopsis and yeast have expanded our knowledge of the connection between the ionome and the genome; however, the interconnection of multiple elements in plant tissue has only been partly revealed (CitationAzevedo et al. 2005; CitationBrune and Dietz 1995; CitationLarbi et al. 2002; CitationParida et al. 2004). The effects of the addition of an element on the plant ionome in different tissues and of ion-profile variations in wild accessions are not known. This information might reveal the relationships among elements in plants. The model legume Lotus japonicus (Regel) Larsen was selected for the present study because mineral nutrition in legumes has not been as extensively studied as it has in other important crops. A plant ionome of 15 elements (B, Na, Mg, K, Ca, Mn, Fe, Ni, Cu, Zn, As, Sr, Mo, Cd and Cs) was investigated after treatment with six elements (As, Cd, Cs, Ni, Mo and Sr), and the elemental composition in 45 natural accessions of L. japonicus was analyzed to depict changes to the plant ionome following natural plant genome variation with subtoxic levels of As, Cd, Cs, Ni, Mo and Sr.
MATERIALS AND METHODS
Pre-cultivation of plants
Lotus japonicus MG-20 seeds were soaked in 98% sulfuric acid for 20 min and then washed several times with tap water to remove the sulfuric acid. Other wild accession seeds were scarified with sand paper. These two scarification methods, which are used to break the hard seed coat, can effectively increase the germination rate of L. japonicus seeds. Soaking the seeds in sulfuric acid is good for bulk seed treatment, but the effectiveness of sulfuric acid scarification depends on the treatment time for various wild accession seeds, thus, an alternative method with sandpaper was used for scarification of wild accession seeds. The seeds were sterilized in 2% sodium hypochlorite containing 0.01% Tween 20 for 20 min, rinsed five times with deionized water and germinated in an incubator (MIR-553; Sanyo Electric, Gunma, Japan) at 25°C for 2 days. The seedlings were transplanted to hydroponic culture with continuous aeration. The standard nutrition solution (CR) contained 2.14 mmol L−1 N (NH4NO3), 0.30 mmol L−1 P (NaH2PO4·2H2O), 0.77 mmol L−1 K (K2SO4:KCl = 1:1), 1.25 mmol L−1 Ca (CaCl2·2H2O), 0.82 mmol L−1 Mg (MgSO4·7H2O), 35.8 µmol L−1 Fe (FeSO4·7H2O), 9.1 µmol L−1 Mn (MnSO4·4H2O), 46.3 µmol L−1 B (H3BO3), 3.1 µmol L−1 Zn (ZnSO4·7H2O), 0.16 µmol L−1 Cu (CuSO4·5H2O) and 0.05 µmol L−1 Mo ((NH4)6· Mo7O24·4H2O) and was replaced every 5 days. The pH was adjusted to 5.0 ± 0.1 with 0.05 mol L−1 HCl or 0.5 mol L−1 NaOH every day. After cultivation in hydroponics for 22 days in a temperature chamber (LPH-4P-NC, WT-0040; Nippon Medical and Chemical Instruments, Osaka, Japan) (23°C with 16 h daylight, 60% humidity, 140 µmol m−2 s−1) the plants were subjected to the experiments.
Element interaction experiments
A preliminary experiment showed that plant biomass did not decrease significantly when L. japonicus was grown in the standard nutrition solution with additions of 10 µmol L−1 As, 5 µmol L−1 Cd, 50 µmol L−1 Cs, 2,500 µmol L−1 Na, 10 µmol L−1 Ni, 5 µmol L−1 Mo and 250 µmol L−1 Sr compared with controls grown in the standard nutrition solution (data not shown). Taking this level as a basic level (L0), a series of concentrations of the six toxic elements (As, Cd, Cs, Ni, Mo and Sr, excluding Na) were set up to investigate the effects of these toxic elements on ion uptake and distribution in the plants (). After 22 days pre-cultivation in the standard nutrition solution, the plants were transplanted to another nutrient solution with different levels of the six toxic elements, with three replicates of each treatment. The seedlings were grown for 5 days. Before transplanting, the seedlings had any extra nutrient solution removed from their roots by blotting on tissue paper, and seedling fresh weight (FW0) was recorded. The fresh weights after treatment (FWT) were recorded and the element toxicity for plant growth was determined by a fresh weight percentage increase: [(FWT – FW0)/FW0] × 100%.
High-throughput cultivation for the natural variants assay
Forty-five L. japonicus accessions, including two experimental lines (MG-20 and Gifu B-129), were supplied by the National BioResource Project at Miyazaki University (details of the accessions can be seen at http://www.shigen.nig.ac.jp/bean/lotusjaponicus/wildStrainListAction.do.) An agar-supporting hydroponic cultivation system was used for high-throughput cultivation of L. japonicus. This method allows plants to grow naturally from seed to seedling without transplanting. Microcentrifuge test tubes (0.5 mL; Treff Lab, Degersheim, Switzerland) were filled with 0.6 mL of agar medium (0.9% agar containing half-strength standard nutrition solution) to support seed germination as shown in . After agar solidification, the tube conical bottoms (approximately 11 mm from the bottom) were cut to allow root elongation (Microtube cutter, Bel-art Products; Pequannock, NJ, USA). The scratched and sterilized seeds were sown onto the agar surface (one seed per tube). Water was sprayed to maintain humidity after settling the seeds, and the tubes with seeds were covered by wrap film and kept in an incubator at 25°C. The roots emerged and punctured through the agar within 3 days. The germinated lines were transferred to hydroponic devices in the temperature chamber. Three-week-old seedlings were treated with L0 level of toxic elements for a further 5 days.
Table 1 Element compositions of the treatments
Sample digestion and element analysis
Seedlings were separated into roots, stems and leaves using ceramic scissors (CS-250; Kenis, Osaka, Japan) in the element interaction experiment; in the natural variants assay experiment, only the plant shoots were harvested, and two plants were pooled as one replicate and there were three replicates for each treatment or each line. All samples were kept in an oven for 72 h at 60°C for dry weight determination. Dried samples were digested by 2 mL 61% HNO3 (EL grade, Kanto Chemical; Tokyo, Japan) at 110°C in a DigiPREP apparatus (SCP Science, Quebec, Canada) for 2 h, then 0.5 mL H2O2 (semiconductor grade; Santoku Chemical, Tokyo, Japan) was added and heated at 110°C for a further 20 min. After the solution was cooled to room temperature, it was diluted to 10 mL with 2% HNO3 and analyzed for 15 elements (B, Na, Mg, K, Ca, Mn, Fe, Ni, Cu, Zn, As, Sr, Mo, Cd and Cs) by ICP-MS (ELAN, DRC-e; Perkin Elmer, Waltham, MA, USA) according to the manufacturer's instructions.
Statistical analysis
Statistical differences in the ion concentrations in plant tissues were assessed using independent-sample t-tests or anovas with least square deviation (LSD) by SPSS 10.0 (SPSS, Chicago, IL, USA). Principal-component analysis (PCA) of the 15 elements in the natural variants was carried out using MiniTable 15 (MiniTable, State College, PA, USA) and a biplot was used to present the results (CitationGabriel 1971).
RESULTS
General aspects of the ion-profile responses to toxic elements
When L. japonicus was grown with subtoxic levels (L0) of As, Cd, Cs, Na, Ni, Mo and Sr in hydroculture, although there was no significant decrease in plant biomass, the ion profiles changed significantly (). Under L0 treatment, there were significant reductions in the concentrations of B, Mg and Mn in roots, Fe in shoots, and Ca and Zn in the whole plant. Copper accumulated in the roots and was reduced in the shoots, which indicated inactive Cu translocation from roots to shoots when these seven elements were added at a subtoxic level. Molybdenum concentration increased fourfold because the subtoxic level (L0) was 5 µmol L−1 in solution, which was 100-fold higher than the level in the standard nutrition solution. However, the addition of > 2,500 µmol L−1 Na to the L0 treatment did not increase the Na concentration in the plant roots, whereas Na increased in the shoots in the L0 treatment.
Figure 1 Seed sowing and germination in a bottomless tube with agar. The tube is filled with 0.6 mL 0.9% agar (I→II). After agar solidification, the tube bottom is cut (III.1) and a seed without a seedcoat is placed over the agar surface (III.2). The seed will germinate in 3 days (IV and far right).
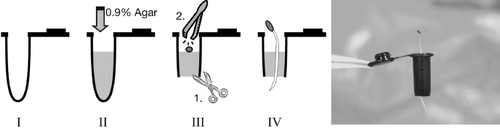
Table 2 Mineral element distributions under the control (CR) and L0 treatment (L0) among roots, stems and leaves
The percentage content distribution of the 15 elements in the plant tissues in the L0 treatment is shown in . In general, the amount of nutrition elements, such as B, Mg, K, Ca, Mn and Zn, significantly decreased under the L0 treatment with Ni, As, Sr, Cd, Sr, Cs, Mo and Na additions compared with that under the CR treatment. Large amounts of B and Ca accumulated in the leaves, while Fe, Na, Ni, As and Cd remained in the roots, particularly Fe and As (> 87% of the content was in the roots). Molybdenum was the only element to accumulate in the stems. The distribution patterns of Mg and Mn changed greatly in the L0 treatment compared with the control; their concentrations decreased severely in plant roots under L0 treatment (and were only 20% and 9% of the controls, respectively), but shoot concentrations were only slightly affected by the addition of toxic elements.
Variations in the ion profiles in response to toxic elements
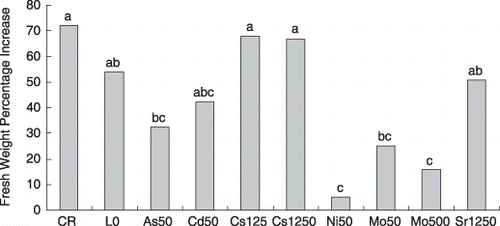
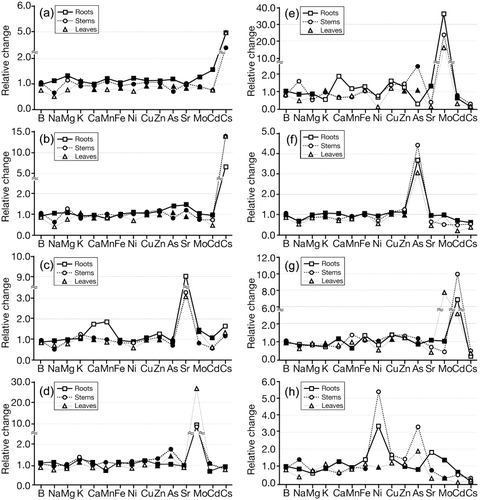
Based on the L0 treatment, we further investigated 15 elements in plant tissues under high levels of As, Cd, Cs, Mo, Ni and Sr. According to their effects on plant biomass and on the concentrations of other elements, As, Cd, Cs, Mo, Ni and Sr were divided into two groups. Plants could not survive 500 µmol L−1 As, Cd or Ni (data not shown), showed significant growth retardation with 500 µmol L−1 Mo, and were slightly affected by 1,250 µmol L−1 Cs or Sr (). Thus, Cs and Sr were categorized as low-toxicity elements, and concentrations of the other elements were slightly affected by the addition of Cs and Sr (). However, As, Cd, Ni and Mo were categorized as toxic elements and greatly disturbed the uptake of most other elements ().
The application of Cs or Sr decreased B, Na, Mg and Ni contents in the leaves and Cd in the shoots, and Sr application increased Ca, Mn and Cs in the roots (). The effects of the application of Cs or Sr were quite small for the other elements, and the expected decreases in K and Ca were not observed in the Cs and Sr treatments, respectively.
Lotus japonicus endured 500 µmol L−1 Mo for 5 days (), and Mo toxicity was reflected in the plant ion profiles under the toxic element treatments. At a low Mo level (50 µmol L−1), the ion profile of the plant was similar to the Cs or Sr treatment (), while the effects of Mo on the other elements were greater when the Mo concentration was increased up to 500 µmol L−1 (). Under Mo, As, Cd and Ni stress, the concentrations of most essential elements decreased, except for Fe, Cu and Zn. This phenomenon was more pronounced in the leaves. In particular, the application of Mo increased Ca in the roots and Na in the stems; Cd application increased Mo in the leaves and Mn in the stems; and Ni application increased As in the shoots and Sr in the roots (). Among all of the elements analyzed, the concentrations of Cs and Cd dropped markedly when toxic elements were added into the solution, indicating the quick release and high mobility of Cs and Cd in plant tissues under stress conditions.
Table 3 Sum of the mineral elements in the control (CR) and L0 treatment (L0) and their distribution among roots, stems and leaves
Figure 2 Effects of toxic elements on plant fresh weights. Data in columns with the same letters are not significantly different (P < 0.05). L0 represents the nutrition solution with additions of 10 µmol L−1 As, 5 µmol L−1 Cd, 50 µmol L−1 Cs, 2,500 µmol L−1 Na, 10 µmol L−1 Ni, 5 µmol L−1 Mo and 250 µmol L−1 Sr. As50 represents the nutrition with 50 µmol L−1 As and other elements at the same levels as L0. Cd50, Cs250, Cs1,250, Ni50, Mo50, Mo500, Sr1,250 are similar to As50.
Ionome survey of wild lines of Lotus japonicus
Element accumulations in 45 lines of L. japonicus Miyakojima wild accessions, including two experimental lines (MG-20 and Gifu B-129), were investigated using nutrient solutions with subtoxic levels of non-essential elements, and the dataset of the 15 elements in all wild lines was analyzed by PCA. The mean (± standard deviation) concentrations of the 15 elements in the shoots of all wild lines were 55.7 ± 1.4 for B; 787 ± 37 for Na; 2,271 ± 40 for Mg; 16,433 ± 227 for K; 4,279 ± 99 for Ca; 91.0 ± 2.5 for Mn; 111 ± 8 for Fe; 8.9 ± 0.2 for Ni; 9.0 ± 0.2 for Cu; 70.1 ± 2.4 for Zn; 1.5 ± 0.1 for As; 468 ± 15 for Sr; 342 ± 9 for Mo, 6.0 ± 0.2 for Cd; 1,172 ± 35 for Cs (µg g−1 dry weight, three replicates per line, two plants per replicate). No element hyperaccumulators were found in the present study. The average element concentrations in each line are listed in Table S1 (http://www.agr.hokudai.ac.jp/botagr/pln/ChenSSPN2008/TableS1.pdf).
Figure 3 Profile variations of 15 elements in the roots, stems and leaves under Cs, Sr, Mo, As, Cd and Ni treatment. The relative change in the element concentration is indicated as: element concentration in the treatment of element addition/element concentration in the L0 treatment. (a) Cs 250, (b) Cs 1,250, (c) Sr 1250, (d) Mo 50, (e) Mo 500, (f) As 50, (g) Cd 50 and (h) Ni 50. L0 represents the nutrition solution with additions of 10 µmol L−1 As, 5 µmol L−1 Cd, 50 µmol L−1 Cs, 2,500 µmol L−1 Na, 10 µmol L−1 Ni, 5 µmol L−1 Mo and 250 µmol L−1 Sr. Cs250 represents the nutrition with 250 µmol L−1 Cs and other elements at the same levels as L0. Cs250, Cs1,250, Sr1,250, Mo50, Mo500, As50, Cd50, Ni50 are similar to Cs250. Open symbols indicate values significantly different from the L0 treatment (P < 0.05).
DISCUSSION
Toxicity-induced ion-profile variation and growth retardation
We investigated changes in the ionome of L. japonicus under low-toxic and high-toxic element additions. It was not surprising that toxic element stress severely disturbed plant nutritional status (CitationBrune and Dietz 1995) because of the specific and complex interactions with metabolic reactions, such as translocation, compartmentation and circulation of elements within the plants. At the same time, a significant decrease in biomass was also found in plants under As, Cd, Ni and Mo treatment, particularly Ni and Mo. Excess trace elements cause a number of toxic symptoms in plants, for example, growth retardation, nutritional imbalance, inhibition of photosynthesis, induction and inhibition of enzymes, altered stomatal action, water relations and generation of free radicals (CitationMeharg and Hartley-Whitaker 2002; CitationPrasad 1995; CitationSeregin and Kozhevnikova 2006). Growth retardation and ionome disturbance occurred in our study. On the one hand, the depletion of nutrient elements might be deleterious to the plants in addition to other primary damage and might restrict plant growth (CitationBrune and Dietz 1995); On the another hand, the reduced viability of plant roots under toxic element stress might affect normal uptake by membrane transporters (CitationHall 2002). In our study, the addition of 50 µmol L−1 Mo greatly decreased plant biomass and slightly disturbed the ion profile (,); however, the addition of 50 µmol L−1 As and Cd affected the plant biomass at a similar level to that of 50 µmol L−1 Mo (,), but led to severe variation in the plant ionome. Obviously ionome disturbance and growth retardation caused by excess trace element toxicity are related, but their interaction depends on the toxicity of the particular element in the plant tissues.
Effects of similar metals
Of the 15 elements we analyzed in L. japonicus, Sr and Ca are considered to act in chemically similar ways, the distribution pattern of Sr (root : stem : leaf = 11:23:66) was similar to Ca (6:16:78); however, Sr was not as effectively translocated to the shoots as Ca. Furthermore, although K, Na and Cs are alkali metals and are assumed to act similarly, the distribution pattern of K (root : stem : leaf = 15:26:59) was different to that of Na (53:23:24) and Cs (33:15:52). In addition, K concentration was almost equal among roots, stems and leaves, while the concentrations of Na and Cs in the roots were much higher than in the shoots. This result is consistent with CitationSmith et al. (1980), who reported that unlike the Brassica spp., legumes had relatively high K:Na ratios in their tissues and the ratios followed a sequence of root < stem < leaf, which indicated that in the Leguminosae, K was more effectively transported to the shoots than Na, and probably Cs.
It has been extensively reported that the addition of K can reduce Cs uptake by plants because of their chemical similarities (CitationBange and Overstreet 1960; CitationHampton et al. 2004; CitationHandley and Overstreet 1961; CitationIsaure et al. 2006; CitationShaw and Bell 1991), and that K and Cs enter root cells by the same molecular mechanism(s) (reviewed by CitationWhite and Broadly 2000); while decreased K concentration in Arabidopsis was not observed until the Cs external concentration reached a toxic level (CitationHampton et al. 2004). Similar results were found in the present study, that is, Cs did not significantly decrease plant growth and did not affect K concentration in the plant tissues (,). One possible reason for these phenomena is that at low external K concentrations (often < 0.3 mmol L−1) the K transporter shows little discrimination against Cs, while the K channel may be dominant at high external K concentration and show high discrimination against Cs (CitationSacchi et al. 1997; CitationZhu and Smolders 2000). Sodium, another alkali metal ion, competed with Cs in the shoots when additional Cs was introduced to the solution () and showed similar Na:Cs ratios in the roots and stems at L0 (approximately four, calculated from ). The K channel KAT1 (a voltage-dependent inward rectifying K channel) cloned from Arabidopsis has a selectivity of K > Na > Cs (CitationSchachtman et al. 1992), and it is suggested that LCT1 (a low-affinity cation transporter) also transports Cs and Na (CitationSchachtman et al. 1997; CitationWhite and Broadly 2000). Thus, it is possible that Cs can share, at least in part, the Na translocation system in L. japonicus.
In contrast, selectivity for Sr uptake was higher than that of Ca when both elements were present (CitationRoca and Vallejo 1995). CitationShaw (1993) and CitationTyson et al. (1999) both concluded that the less Ca in the soil, the more Sr accumulated; however, CitationSoudek et al. (2006) found a contradictory phenomenon under hydroponic conditions; Sr accumulation by sunflower was enhanced by Ca addition when the Ca concentration was < 8 mmol L−1. Furthermore, there seemed to be no competition or interaction between Ca and Sr during their transport to the shoot in Arabidopsis (CitationWhite 2001). Given the current lack of information concerning Sr and Ca uptake mechanisms and transport systems, we can only hypothesize that in L. japonicus, Sr may enter the plant system through transport pathways for Mg or Na, which showed competitive uptake with Sr ().
It is interesting that increased Fe, Cu and Zn concentrations only occurred in the Cd and Ni treatments. This finding was not expected because many studies have shown that Cd enters plants via transport processes that normally function in Zn and Fe uptake (reviewed by CitationClemens 2001; CitationGuerinot 2000; CitationMäser et al. 2001), and Ni is possibly transported by similar ligands to Zn (CitationAssunção 2003) or via Zn transporters (CitationTaylor and Macnair 2006). However, other studies have found no interaction or even synergism between Zn and Cd (CitationBrune and Dietz 1995; CitationPapoyan et al. 2007; CitationWhite and Chaney 1980). There is no adequate theory clarifying the mechanisms of the synergism between Zn and Cd in plants. Circumstantial evidence indicates that Fe, Cu and Zn accumulation induced by the addition of Cd and Ni is highly likely to result from a shared detoxification mechanism using homophytochelatin in L. japonicus (CitationLoscos et al. 2006). CitationLoscos et al. (2006) studied the substrate specificity of phytochelatin synthases and found no L. japonicus phytochelatin synthase 1 (LjPCS1) activity when Mn(II), Mo(VI) and B(III) were included in the assay medium. In contrast, the activity was significant with Cu(II), Fe(II), Cd(II) and Zn(II). We speculate that phytochelatin synthases activated by Cd and Ni might be a key reason for Fe, Cu and Zn accumulation under the Cd and Ni treatments.
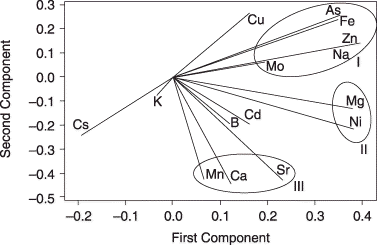
Figure 4 Biplot representation of the principal-component analysis results from the ionomes of 45 accessions. The length of each eigenvector is proportional to the variance in the data for that element. The angle between the eigenvectors represents the correlations among the different elements. Three groups of elements (circled and denoted I, II and III) show strong positive correlations.
Biological interconnectedness among elements
The levels of different elements were interconnected in element-accumulated or deficient Arabidopsis mutants (CitationLahner et al. 2003) or yeast (CitationEide et al. 2005). Unlike in mutants, more than one unrelated pathway for the uptake of elements might co-exist in an accession. Statistical analyses of data from a great number of samples make it possible to reduce the random connections of elements in an accession and emphasize the relationships among those elements strongly connected through metal-homeostasis genes. Thus, we considered that the ion profile of different lines revealed the connections between the elements in the plants. The PCA results as a biplot graph (CitationGabriel 1971) are in . The length of each eigenvector is proportional to the standard deviation of the data for each element. Potassium, B, Cd and Mo had small variances compared with the other elements, which had large variances (). The biplot representation also displays the relationships among the elements. The acute angles between the eigenvectors represent positive correlations, while the obtuse ones show negative correlations. Elements with no correlation have 90° eigenvector angles. The “distance” between two elements measures their dissimilarity in L. japonicus lines. Significantly, elements were clustered into three positively correlated groups.
Group I includes As, Fe, Na, Zn and Mo and can be divided into two subgroups: one is As and Fe, and the other Na, Zn and Mo. There are few studies that have examined the interactions among Na, Zn and Mo; however, As toxicity inducing Fe deficiency has been reported in rice (CitationShaibur et al. 2006). More circumstantial evidence comes from studies on Fe and P, which are highly connected with As because of chemical similarity; Fe accumulation and distribution in Arabidopsis were affected by phosphate supply (CitationHirsch et al. 2006; CitationMisson et al. 2005). In addition, the elements in group I showed a strong negative correlation with Cs and K. Potassium is involved in increased adsorption and translocation of Fe in both monocotyledons and dicotyledons (CitationAlam et al. 2002; CitationBarak and Chen 1984; CitationBolle-Jones 1955; CitationHughes et al. 1992; CitationJolley et al. 1988; CitationOertli and Opoku 1974). Many genes also respond to K, Fe and P deficiencies in tomato roots, suggesting cross-talk of K, Fe and P at the molecular level in the plant system (CitationWang et al. 2002).
In group II, Mg and Ni had a strong positive correlation with each other. Magnesium-alleviated Ni toxicity has been found in oats, maize and a fungus (CitationBabich and Stotzky 1982; CitationProctor and McGowan 1976; CitationRobertson 1985) and Ni-reduced Mg uptake has been found in oat and birch (CitationCrooke and Inkson 1955; CitationJones and Hutchinson 1988). Furthermore, all prokaryotic Mg transporter systems have been shown to transport Ni with high affinity or to be inhibited by Ni (reviewed by CitationMaguire 2006), and an Arabidopsis gene family (AtMGT) encoding putative Mg transport protein is capable of transporting Ni (CitationLi et al. 2001). A potential significant link between Mg and Ni in plants is emphasized in the present study by the element behaviors in plants and the molecular mechanisms of elemental uptake.
Group III included Ca and Mn, and a positive correlation between Ca and Mn is supported by the existence of some known plant genes, such as calcium exchange 2 (CAX2) and endoplasmic reticulum-type Ca-ATPase 1 (ECA1). Expression of Arabidopsis CAX2 in tobacco increased Mn tolerance and made plants accumulate more Mn (CitationHirschi et al. 2000). ECA1 has been shown to play a dual role in both Ca and Mn homeostasis (CitationWu et al. 2002). In yeast, a high correlation between Ca and Mn was found in transport (CitationRudolph et al. 1989) and cell-cycle progression (CitationLoukin and Kung 1995). Interestingly, a PCA of screened yeast mutants showed altered ionome profiles, and gave similar positive correlations between Mg and Ni, Mn and Ca, and a negative correlation between Fe and K (CitationEide et al. 2005).
A complex gene net in plants controls element uptake, translocation, detoxification, storage and metabolism. Approximately 5% of the Arabidopsis genome appears to encode membrane transport proteins (CitationMäser et al. 2001), and 2–4% of the Arabidopsis genome is involved in regulating plant nutrition and trace element content (CitationLahner et al. 2003). Most genes with functions in plant nutrition tend to affect more than one element in plants; a change in three elements was most frequent in a study of Arabidopsis (CitationLahner et al. 2003), indicating that ion-homeostasis networks in plants are closely linked. Our results from 45 L. japonicus natural variants make a rough map of the relationships of 15 elements (), the closer the “distance” in the map, the more genes the elements should share. Some of our results are contradictory and not easy to explain, for example, we suggest that Na and Cs use a similar translocation mechanism in plants (positive relationship); however, the element survey showed a negative relationship between these elements. Possibly, a sample of 45 groups was too limited to map all the connections between the plant ionome and genome.
In summary, our study provides detailed information of element interactions and an outline of their interconnection in L. japonicus. Severely toxic elements resulted in great disturbance to the plant ionome; however, chemically similar elements might not show corresponding interconnection inside plant tissues. The interactions among Fe, Cu, Zn, Cd and Ni suggest a common detoxification mechanism for heavy metals in L. japonicus. Based on the element analyses of 45 natural variants, we speculate that there are some unclear genetic links between Fe, As and K; Mg and Ni; and Mn and Ca.
ACKNOWLEDGMENTS
This study was financially supported by a Grant-in-Aid for Scientific Research (No. 16208008) (Plant Nutrition and Transport) from the Japan Society for the Promotion of Science. Seeds of L. japonicus natural accessions were provided by the National BioResource Project, Miyazaki University, Japan. We are grateful to You-Bo Su, Dai Tokuhisa and Ke-Qin Zhou for their laboratory assistance.
REFERENCES
- Ågren , GI . 2004 . The C:N:P stoichiometry of autotrophs – theory and observations . Ecol. Lett , 7 : 185 – 191 .
- Alam , S , Rahman , MH , Kamei , S and Kawaii , S . 2002 . Alleviation of manganese toxicity and manganese-induced iron deficiency in barley by additional potassium supply in nutrition solution . Soil Sci. Plant Nutr , 48 : 387 – 392 .
- Assunção , AGL . 2003 . “ Exploring intraspecific variability in metal accumulation and tolerance traits in the heavy metal hyperaccumulator ” . In Thlaspi caerulescens , Amsterdam, , the Netherlands : Vrije University of Amsterdam . PhD thesis
- Babich , H and Stotzky , G . 1982 . Nickel toxicity to fungi: influence of environmental factors . Ecotox. Environ. Safe , 6 : 577 – 589 .
- Bange , GG and Overstreet , R . 1960 . Some observations on absorption of cesium by excised barley roots . Plant Physiol , 35 : 605 – 608 .
- Barak , P and Chen , Y . 1984 . The effect of potassium on iron chlorosis in calcareous soils . J. Plant Nutr , 7 : 125 – 133 .
- Bolle-Jones , EW . 1955 . The interrelationships of iron and potassium in the potato plant . Plant Soil , 6 : 129 – 173 .
- Brune , A and Dietz , KJ . 1995 . A comparative analysis of element composition of roots and leaves of barley seedlings grown in the presence of toxic cadmium, molybdenum, nickel, and zinc concentrations . J. Plant Nutr , 18 : 853 – 868 .
- Clemens , S . 2001 . Molecular mechanisms of plant metal tolerance and homeostasis . Planta , 212 : 475 – 486 .
- Crooke , WM and Inkson , RHE . 1955 . The relationship between nickel toxicity and major nutrient supply . Plant Soil , 6 : 1 – 15 .
- Eide , DJ , Clark , S Nair , TM . 2005 . Characterization of the yeast ionome: a genome-wide analysis of nutrient mineral and trace element homeostasis in Saccharomyces cerevisiae . Genome Biol , 6 : R77
- Gabriel , KR . 1971 . The biplot graphics display of matrices with application to principal component analysis . Biometrika , 58 : 453 – 467 .
- Guerinot , ML . 2000 . The ZIP family of metal transporters . Biochim. Biophys. Acta , 1465 : 190 – 198 .
- Hall , JL . 2002 . Cellular mechanisms for heavy metal detoxification and tolerance . J. Exp. Bot , 53 : 1 – 11 .
- Hampton , CR , Bowen , HC Broadley , MR . 2004 . Cesium toxicity in Arabidopsis . Plant Physiol , 136 : 3824 – 3837 .
- Handley , R and Overstreet , R . 1961 . Effect of various cations upon adsorption of carrier free cesium . Plant Physiol , 36 : 66 – 69 .
- Haynes , RJ . 1990 . Active ion uptake and maintenance of cation–anion balance: A critical examination of their role in regulating rhizosphere pH . Plant Soil , 126 : 247 – 264 .
- Hirsch , J , Marin , E Floriani , M . 2006 . Phosphate deficiency promotes modification of iron distribution in Arabidopsis plants . Biochimie , 88 : 1767 – 1771 .
- Hirschi , KD . 2003 . Strike while the ionome is hot: making the most of plant genomic advances . Trends Biotechnol , 21 : 520 – 521 .
- Hirschi , KD , Korenkov , VD , Wilganowski , NL and Wagner , GJ . 2000 . Expression of Arabidopsis CAX2 in tobacco: Altered metal accumulation and increased manganese tolerance . Plant Physiol , 124 : 125 – 133 .
- Holm , RH , Kennepohl , P and Solomon , EI . 1996 . Structural and functional aspects of metal sites in biology . Chem. Rev , 96 : 2239 – 2314 .
- Hughes , DF , Jolley , VD and Brown , JC . 1992 . Role of potassium in the iron-stress response mechanism of iron-efficient oat . Soil Sci. Soc. Am. J , 56 : 830 – 835 .
- Isaure , MP , Fraysse , A Devès , G . 2006 . Micro-chemical imaging of cesium distribution in Arabidopsis thaliana plant and its interaction with potassium and essential trace elements . Biochimie , 88 : 1583 – 1590 .
- Jolley , VD , Brown , JC , Blaylock , MJ and Camp , SD . 1988 . A role for potassium in the use of iron by plants . J. Plant Nutr , 11 : 1159 – 1175 .
- Jones , MD and Hutchinson , TC . 1988 . Nickel toxicity in mycorrhizal birch seedlings infected with Lactarius rufus or Scleroderma flavidum II. Uptake of nickel, calcium, magnesium phosphorus and iron . New Phytol , 108 : 461 – 470 .
- Kirkby , EA and Knight , AH . 1977 . Influence of the level of nitrate nutrition on ion uptake and assimilation, organic acid accumulation and cation–anion balance in whole tomato plants . Plant Physiol , 60 : 349 – 353 .
- Lahner , B , Gong , J Mahmoudian , M . 2003 . Genomic scale profiling of nutrient and trace elements in Arabidopsis thaliana . Nat. Biotechnol , 21 : 1215 – 1221 .
- Larbi , A , Morales , F , Abadia , A , Gogorcena , Y , Lucena , JJ and Abadia , J . 2002 . Effects of Cd and Pb in sugar beet plants grown in nutrient solution: induced Fe deficiency and growth inhibition . Funct. Plant Biol , 29 ( 12 ) : 1453 – 1464 .
- Li , L , Tutone , AF , Drummond , SM , Gardner , RC and Luan , S . 2001 . A novel family of magnesium transport genes in Arabidopsis . Plant Cell , 13 : 2761 – 2775 .
- Loscos , J , Naya , L , Ramos , J , Clemente , MR , Matamoros , MA and Becana , M . 2006 . A reassessment of substrate specificity and activation of phytochelatin synthases from model plants by physiologically relevant metals . Plant Physiol , 140 : 1213 – 1221 .
- Loukin , S and Kung , C . 1995 . Manganese effectively supports yeast cell-cycle progression in place of calcium . J. Cell Biol , 131 : 1025 – 1037 .
- Maguire , ME . 2006 . Magnesium transporters: properties, regulation and structure . Front. Biosci , 11 : 3149 – 3163 .
- Marschner , H . 1995 . Mineral Nutrition of Higher Plants , London : Academic Press .
- Mäser , P , Thomine , S Schroeder , JI . 2001 . Phylogenetic relationships within cation transporter families of Arabidopsis . Plant Physiol , 126 : 1646 – 1667 .
- Meharg , AA and Hartley-Whitaker , J . 2002 . Arsenic uptake and metabolism in arsenic resistant and nonresistant plant species . New Phytol , 154 : 29 – 43 .
- Misson , J , Raghothama , KG Jain , A . 2005 . A genome-wide transcriptional analysis using Arabidopsis thaliana Affymetrix gene chips determined plant responses to phosphate deprivation . Proc. Natl Acad. Sci , 102 : 11934 – 11939 .
- Oertli , JJ and Opoku , AA . 1974 . Interaction of potassium in the availability and uptake of iron from ferric hydroxide . Soil Sci. Soc. Am. J , 38 : 451 – 454 .
- Papoyan , A , Piñeros , M and Kochian , LV . 2007 . Plant Cd2+and Zn2+status effects on root and shoot heavy metal accumulation in Thlaspi caerulescens. . New Phytol , 175 : 51 – 58 .
- Parida , AK , Das , AB and Mittra , B . 2004 . Effects of salt on growth, ion accumulation, photosynthesis and leaf anatomy of the mangrove, Bruguiera parviflora . Trees , 18 : 167 – 174 .
- Prasad , MNV . 1995 . Cadmium toxicity and tolerance in vascular plants . Environ. Exp. Bot , 35 : 525 – 545 .
- Proctor , J and Mcgowan , ID . 1976 . Influence of magnesium on nickel toxicity . Nature , 260 : 134
- Rea , PA . 2003 . Ion genomics . Nat. Biotechnol , 21 : 1149 – 1151 .
- Robertson , AI . 1985 . The poisoning of roots of Zea mays by nickel ions, and the protection afforded by magnesium and calcium . New Phytol , 100 : 173 – 189 .
- Roca , MC and Vallejo , VR . 1995 . Effect of soil potassium and calcium on caesium and strontium uptake by plant roots . J. Environ. Radioactiv , 28 : 141 – 159 .
- Rogers , EE , Eide , DJ and Guerinot , ML . 2000 . Altered selectivity in an Arabidopsis metal transporter . Proc. Natl Acad. Sci , 97 : 12356 – 12360 .
- Rudolph , HK , Antebi , A Fink , GR . 1989 . The yeast secretory pathway is perturbed by mutations in PMR1, a member of a Ca2+ATPase family . Cell , 58 : 133 – 145 .
- Sacchi , GA , Espen , L , Nocito , F and Cocucci , M . 1997 . Cs+uptake in subapical maize root segments: mechanism and effects on H+release, transmembrane electric potential and cell pH . Plant Cell Physiol , 38 : 282 – 289 .
- Salt , DE . 2004 . Update on plant ionomics . Plant Physiol , 136 : 2451 – 2456 .
- Schachtman , DP , Kumar , R , Schroeder , JI and Marsh , EL . 1997 . Molecular and functional characterization of a novel low-affinity cation transporter (LCT1) in higher plants . Proc. Natl Acad. Sci , 94 : 11079 – 11084 .
- Schachtman , DP , Schroeder , JI , Lucas , WJ , Anderson , JA and Gaber , RF . 1992 . Expression of an inward-rectifying potassium channel by the Arabidopsis KAT1 cDNA . Science , 258 : 1654 – 1658 .
- Seregin , IV and Kozhevnikova , AD . 2006 . Physiological role of nickel and its toxic effects on higher plants . Russ. J. Plant Physiol , 53 : 257 – 277 .
- Shaibur , MR , Kitajima , N , Sugawara , R , Kondo , T , Huq , SMI and Kawai , S . 2006 . Physiological and mineralogical properties of arsenic-induced chlorosis in rice seedlings grown hydroponically . Soil Sci. Plant Nutr , 52 : 691 – 700 .
- Shaw , G . 1993 . Blockade by fertilisers of caesium and strontium uptake into crops: effects on the root uptake process . Sci. Total Environ , 137 : 119 – 133 .
- Shaw , G and Bell , JNB . 1991 . Competitive effects of potassium and ammonium on caesium uptake kinetics in wheat . J. Environ. Radioactiv , 13 : 283 – 296 .
- Smith , GS , Middleton , KR and Edmonds , AS . 1980 . Sodium nutrition of pasture plants I. translocation of sodium and potassium in relation to transpiration rates . New Phytol , 84 : 603 – 612 .
- Soudek , P , Valenová , Š , Vavříková , Z and Vaněk , T . 2006 . 137Cs and 90Sr uptake by sunflower cultivated under hydroponic conditions . J. Environ. Radioactiv , 88 : 236 – 250 .
- Sterner , RW and Elser , JJ . 2002 . Ecological Stoichiometry: The Biology of Elements from Molecules to the Biosphere , Princeton : Princeton University Press .
- Taylor , SI and Macnair , MR . 2006 . Within and between population variation for zinc and nickel accumulation in two species of Thlaspi (Brassicaceae) . New Phytol , 169 : 505 – 514 .
- Tyson , MJ , Sheffield , E and Callaghan , TV . 1999 . Uptake, allocation, accumulation and ecological implications of 85Sr in bracken (Pteridium aquilinum L. Kuhn . J. Environ. Radioactiv , 46 : 15 – 25 .
- Wang , YH , Garvin , DF and Kochian , LV . 2002 . Rapid induction of regulatory and transporter genes in response to phosphorus, potassium, and iron deficiencies in tomato roots. evidence for cross talk and root/rhizosphere-mediated signals . Plant Physiol , 130 : 1361 – 1370 .
- Watanabe , T , Broadley , MR Jansen , S . 2007 . Evolutionary control of leaf element composition in plants . New Phytol , 174 : 516 – 523 .
- White , MC and Chaney , RL . 1980 . Zinc, Cd and Mn uptake by soybean from two Zn- and Cd-amended coastal plain soils . Soil Sci. Soc. Am. J , 44 : 308 – 313 .
- White , PJ . 2001 . The pathways of calcium movement to xylem . J. Exp. Bot , 52 : 891 – 899 .
- White , PJ and Broadly , MR . 2000 . Mechanisms of caesium uptake by plants . New Phytol , 147 : 241 – 256 .
- Wu , Z , Liang , F Hong , B . 2002 . An endoplasmic reticulum-bound Ca2+/Mn2+pump, ECA1, supports plant growth and confers tolerance to Mn2+stress . Plant Physiol , 130 : 128 – 137 .
- Zhu , YG and Smolders , E . 2000 . Plant uptake of radiocaesium: a review of mechanisms, regulation and application . J. Exp. Bot , 51 : 1635 – 1645 .
- Azevedo , H , Clara , GP and Conceicao , S . 2005 . Cadmium effects in sunflower: nutritional imbalances in plants and calluses . J. Plant Nutr , 28 : 2221 – 2231 .