Abstract
Secretion of acid phosphatase (APase) from the roots to take up phosphorus (P) is a well-known strategy of plants under P-deficient conditions. White lupin, which shows vigorous growth in low-P soils, is noted for its ability to secrete APase under P-deficient conditions. The APase secreted by white lupin roots is stable in soil solution and shows low substrate specificity, suggesting that genetic modification of plants using the APase gene LASAP2 might improve their ability to use organic P. The objective of the present study was to evaluate the potential of LASAP2 transgenic plants to increase organic P utilization. Dry matter production and P accumulation were higher in LASAP2 transgenic tobacco plants grown in gel media containing soluble phytate as the sole P source than in wild-type tobacco plants. Phosphorus uptake by the transgenic plants also increased in soil culture conditions. LASAP2 was apparently more effective in the liberation of organic P, including phytate, in the soil than the native tobacco APase. Thus, the enzymatic stability of LASAP2 in the soil appears to be an important factor for P acquisition.
INTRODUCTION
The mobility of phosphorus (P) in soil is very low, therefore, the efficiency of the use of P in soil as a fertilizer is low. The P in soil exists mostly in unavailable forms, such as organic P and insoluble inorganic P. To improve the efficiency of P acquisition from the soil, many attempts to improve the ability of plants to utilize organic P have been conducted using genetic modification (CitationGeorge et al. 2004, Citation2005c, CitationLung et al. 2005; CitationMudge et al. 2003; CitationRichardson et al. 2001; CitationXiao et al. 2005, Citation2006a,Citationb; CitationYip et al. 2003; CitationZimmernann et al. 2003). Phytate (inositol hexakisphosphate) is the most abundant organic P in soil (CitationTurner et al. 2002); thus, improvement in phytate hydrolyzing activity has been the major focus of many studies. The utilization of phytate-P, existing as a soluble form, can be improved by the introduction of phytases and an acid phosphatase (APase), such as phyA of Aspergillus niger (CitationGeorge et al. 2004, Citation2005c; CitationMudge et al. 2003; CitationRichardson et al. 2001), 168phyA of Bacillus subtilis (CitationYip et al. 2003), a synthetic gene for secretory phytase (CitationZimmernann et al. 2003), MtPHY1 of Medicago truncatula (CitationXiao et al. 2005, Citation2006b), and MtPAP1 of Medicago truncatula (CitationXiao et al. 2006a). For example, P acquisition from soluble phytate in agar media by transgenic Arabidopsis and subterranean clover with ex::phyA, a chimeric phytase gene of Aspergillus niger with a signal peptide of carrot extensin required for extracellular proteins, was better than that by wild-type plants (CitationGeorge et al. 2004; CitationRichardson et al. 2001). However, the increase in phytate-P utilization was limited in soil because of the low solubility of phytate-P in soil (CitationGeorge et al. 2004). Therefore, these researchers concluded that the release of extracellular phytase was not the only requirement for the acquisition of P by plants from endogenous soil phytate.
In general, exogeneous phytases rather than root-secreted phosphatases have been used in previous trials, and this might be an issue. Plants have developed an ability to secrete APase from their roots to liberate inorganic phosphate from organic P in the soil. White lupin, which grows vigorously in low-P soils (CitationLuo et al. 1997), shows the highest ability of the nine crop plants examined to secrete APase under P-deficient conditions (CitationTadano and Sakai 1991). When the APase collected from white lupin exudates was injected into the rhizospheres of tomato and sugar beet plants grown in soil, the growth of the APase-treated plants was better than that of the uninjected control plants (CitationTadano and Komatsu 1994). The APase secreted from the white lupin roots was purified and characterized. White lupin APase has several advantages in soil, including stability over a broad range of pH values and temperatures, low substrate specificity and a long half-life in soil suspension (CitationOzawa et al. 1995; CitationTadano et al. 1993). Thus, we hypothesized that one of the requirements for sustainable hydrolysis in rhizosphere soils could be the enzymatic properties of the native root-secreted APase.
The effects of secretory APase on organic P liberation might be able to be demonstrated by an overexpression of the gene for APase secreted from white lupin roots. A cDNA for the APase secreted from white lupin roots was first cloned and designated LASAP2 by CitationWasaki et al. (2000) and additionally characterized by CitationMiller et al. (2001). The full-length LASAP2 cDNA was then introduced into tobacco plants under the regulation of a CaMV 35S promoter (CitationWasaki et al. 2001). As expected, the roots of LASAP2-overexpressing transgenic tobacco line plants released large amounts of APase. It was also demonstrated using isoelectric focusing that the APase activity of the crude root exudates of the transgenic tobacco line was almost shown at pI 4.7 as well as the native LASAP2, although no APase activity was found at the pI in wild-type tobacco.
The objective of the present study was to evaluate the potential for organic P utilization by transgenic plants, which has been previously established (CitationWasaki et al. 2001). Transgenic tobacco plants were grown aseptically in gel media containing soluble phytate as the sole P source. To estimate the substantial effects of genetic modification of APase on the utilization of organic P in the soil conditions, LASAP2 transgenic tobacco plants were cultured in small pots containing low-P soils.
MATERIALS AND METHODS
Plant materials
Experiment 1: Aseptic culture
Seeds of wild-type (WT) and a LASAP2 transgenic line (L2) tobacco (Nicotiana tabacum L. cv. SR1) were sterilized with sodium hypochlorite (0.5% active chlorine) and washed with sterile tap water several times. The seeds were sown in 1/2MS plate medium, which contained Murashige–Skoog basal salts at half strength (CitationMurashige and Skoog 1962), 3% (w/v) sucrose and 0.25% (w/v) gellan gum. Thirteen days after germination, the seedlings were transferred to pots containing 50 mL of 1/2MS medium with or without a source of P. Three treatments were used: –P (0 mg P L−1), +P (20.4 mg P L−1[KH2PO4]) and phytate (20.4 mg P L−1[inositol hexakisphosphate, dodecasodium salt]; Sigma, St Louis, MO, USA). The pH of all media used in the experiment was adjusted to 5.5 prior to sterilizing. The pH of the concentrated phytate solution was also adjusted to 5.5, and it was sterilized by filtration through PES filter cartridges with 0.2 µm pores (Millipore, Billerica, MA. USA). The –P and +P media were sterilized by autoclaving. To avoid degrading the phytate by heating, an aliquot of the concentrated sterile phytate solution was added to the –P 1/2MS medium.
The seedlings were cultivated for 50 days in a growth chamber at 25°C, 50% relative humidity and 190 µmol m−2 s−1 of light intensity at the uppermost leaf level with continuous lighting. After cultivation, the plants were divided into shoots and roots. After washing the roots with deionized water, the plant materials were oven-dried at 60°C for 5–7 days.
Experiment 2: Soil culture
Brown lowland soil from a –P plot (without P fertilization for 91 years) from the long-term experimental field of Hokkaido University was collected and sieved through 2 mm holes after air-drying in a greenhouse. Three plots were prepared: –P (0 mg P kg soil−1), +P (100 mg P kg soil−1 of Ca(H2PO4)2·H2O) and phytate (100 mg P kg soil−1 of inositol hexakisphosphate, dodecasodium salt). As N and K sources, 150 mg N kg soil−1 of (NH4)2SO4 and 150 mg K kg soil−1 of K2SO4 were applied to all plots. Part of the prepared soils was used for soil analysis as described below. Pots containing approximately 100 g of soil were incubated in a growth chamber for 10 days and then water was added up to 60% water-holding capacity. Fifteen to twenty seeds were sown per pot and cultured in the growth chamber under the same conditions used in Experiment 1. To maintain the water concentration in the soils, deionized water was supplied every 2–3 days. The seedlings were thinned to five plants 21 days after sowing. After an additional 42-day cultivation, the plants were divided into shoots and roots. The shoots were oven-dried at 60°C for 5–7 days. The roots were transferred to polypropylene tubes and gently shaken to collect the rhizosphere soil. After removal of the rhizosphere soil, the roots were oven-dried.
Figure 1 Plant growth of tobacco LASAP2 line (L2) and wild-type (WT) plants in sterilized media. The gray and black bars indicate the shoots and roots, respectively. Values in parentheses indicate the root-to-shoot ratios. Data are presented as the mean ± standard error (n = 5). Significant differences between WT and L2 line in each treatment are indicated by *(P < 0.01).
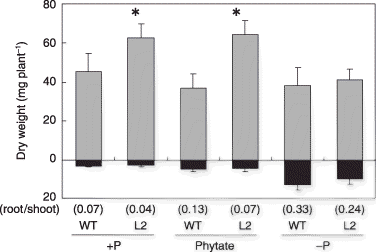
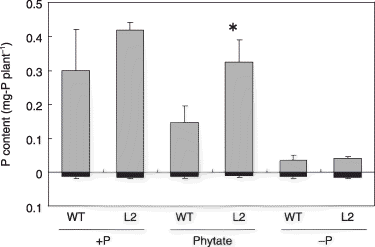
Figure 2 Phosphorus contents of the tobacco LASAP2 line (L2) and wild-type (WT) plants grown in sterilized media. The gray and black bars indicate the shoots and roots, respectively. Values in parentheses indicate the root-to-shoot ratios. Data are presented as the mean ± standard error (n = 5). Significant differences between WT and L2 line in each treatment are indicated by *(P < 0.01).
Plant dry matter and phosphorus analysis
The plant dry matter was weighed and then ground to a fine powder. An approximately 50 mg sample was digested with H2SO4-H2O2. The P concentration in the digested solution was quantified using the vanado-molybdate yellow method (CitationWatanabe et al. 1998).
Soil analysis
Soil pH was measured in a water suspension (ratio 1:2.5). The total P concentration of the soil was determined using the vanado-molybdate yellow method after digestion of the sample with H2SO4-H2O2. The available P concentration was analyzed as Truog-P (CitationTruog 1930).
Soil enzyme activity
The APase activity was measured according to the method of CitationWasaki et al. (2005) with a minor modification using a fluorogenic substrate, 4-methylumbelliferyl phosphate (MUP) and a microplate reader (GENious Plus; Tecan, Zurich, Switzerland). In brief, the rhizosphere soil was weighed precisely and suspended at 20 mg soil mL−1 in sterile deionized water. Twenty microliters of soil suspension was mixed with 80 µL of 0.1 mol L−1 MES-NaOH buffer (pH 4.3) and 100 µL of 1 mmol L−1 MUP solution in 96-well microplates. Fluorescence was measured for 2 h at 30°C with an excitation wavelength of 360 nm and an emission wavelength of 460 nm. Fluorescence readings were carried out every 5 min. The results were expressed as the increasing rate of MUP liberation (nmol g−1 h−1).
Statistical analysis
All cultivations were replicated five times. A Student's t-test was carried out using SPSS version 13 (SPSS, Chicago, IL, USA). Significance was set at P < 0.01.
RESULTS
Experiment 1: Aseptic culture
The effect of LASAP2 overepression on soluble organic P utilization by tobacco plants was evaluated using an aseptic culture. Plant growth of the L2 line grown in the +P and phytate treatments was 1.38-fold and 1.74-fold higher than that of the WT plants, respectively (). There were no significant differences between WT and L2 in the –P treatment, although root growth of both lines was significantly greater than that recorded in the other treatments ().
Like the plant growth, the P contents of the L2 lines grown in the +P and phytate treatments were higher than that of the WT (1.40-fold and 2.21-fold in the +P and phytate treatments, respectively; ). A significant increase in P uptake in the phytate treatment by the L2 line indicates that LASAP2 overexpression improved the ability of plants to utilize soluble phytate.
Figure 3 Plant growth of the tobacco LASAP2 line (L2) and wild-type (WT) plants in sterilized media. The gray and black bars indicate the shoots and roots, respectively. Values in parentheses indicate root-to-shoot ratios. Data are presented as the mean ± standard error (n = 5). Significant differences between WT and L2 line in each treatment are indicated by *(P < 0.01).
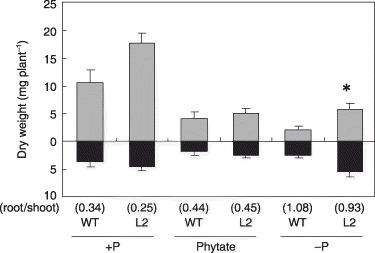
Table 1 Total P and available P concentrations in the prepared soils. Different letters indicate significant differences (P<0.01)
The P content of the WT plants in the phytate treatment was significantly higher than that in the –P treatment (), indicating that native tobacco enzyme(s) also hydrolyzed soluble phytate in the media. The shoot growth of WT in the phytate treatment, however, was similar to that in the –P plants (). An increase in the root-to-shoot ratio, which is a well-known phenomenon in P-deficient plants, was not caused by phytate application (). The root growth in the phytate treatment was still lower than that in the –P treatment ().
Experiment 2: Soil culture
The effect of LASAP2 overexpression on the growth of tobacco plants in soils with different P conditions was evaluated. The P concentrations of the prepared soils are shown in . Both total and available P were highest in the +P soil. Truog-P in the phytate treatment and in the –P treatment was very low. Although no source P was added in the –P treatment, the total P concentration of the –P soil was more than 80% of the concentration in the +P and phytate treatments. This indicates that the –P soil contained an abundant amount of unavailable P, that is, organic P and sparingly soluble P. The pH(H2O) of the soils was 5.5 in all treatments.
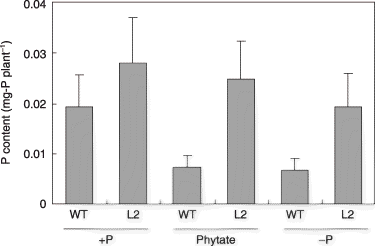
Figure 4 Shoot P contents of the tobacco LASAP2 line (L2) and wild-type (WT) plants grown in sterilized media. Values in parentheses indicate the root-to-shoot ratios. Data are presented as the mean ± standard error (n = 5).
shows the plant growth after the 63-day soil culture. The growth of both the WT and L2 lines was highest in the +P treatment. The growth of the L2 line in the –P condition was significantly higher than the growth of the WT in the –P condition (). The L2 line tended to show increased growth under both +P and phytate conditions, although these increases were not statistically significant. Like the results of the previous experiment, the root-to-shoot ratio of both lines was highest in the –P treatment and was similar in the +P and phytate treatments ().
The P content of the shoots is shown in . The P uptake by the L2 line was higher than that of the WT in all treatments. A significant difference in the P content between the L2 line and the WT was indicated by a two-way anova (P < 0.05), although no significant difference was found in either treatment. There was no difference in the P content of all three P treatments in the L2 line. In the WT, the P content was highest in the +P treatment and similar in the –P and phytate treatments.
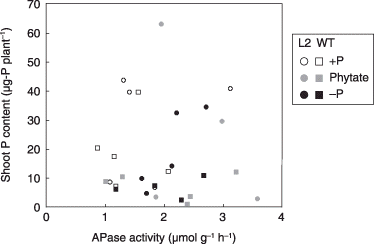
The contribution of the APase secreted by the tobacco roots was evaluated by measuring its activity in the rhizosphere soils. shows the relationship between APase activity in the rhizosphere soils and P contents in the shoots. No correlation was found for plants grown in either the +P or phytate treatments. In contrast, a positive correlation was found in the L2 line grown under P-deficient conditions (r2 = 0.77), although no clear correlation was found in the –P-treated WT.
DISCUSSION
In the present study, LASAP2 overexpression contributed to the acquisition of P from organic P, including phytate P (, ), although the contribution to plant growth was not very high in the soil culture (). Although the APase activity in the rhizosphere soil of WT did not differ from that of the L2 line, the utilization of organic P, including phytate-P, by WT plants was not high (). This suggests that the APase activity derived from LASAP2 contributed to organic P utilization more than tobacco native APase. Tobacco has been shown to secrete APase from its roots like other crop plants (CitationLung and Lim 2006). Phosphorus starvation has also been shown to trigger an increase in phytase secretion by tobacco plants, up to 18.2% of total APase activities (CitationLung and Lim 2006). The lower contribution of the tobacco native enzymes might be explained by the enzymatic properties of the secreted APase. Tomato, which is a member of the Solanaceae like tobacco, showed a slight tolerance to P deficiency, although it had a higher ability to secrete APase (CitationTadano and Sakai 1991). When the enzymatic properties of the secreted tomato APase were compared with those of white lupin, the advantages of the APase secreted from white lupin were shown to be higher V max, lower optimum pH and lower inhibition by some metallic ions (CitationLi and Tadano 1996). Recently, the NtPAP gene for root-secreted phytase of tobacco was isolated as a purple acid phosphatase family, in addition to LASAP2 (CitationLung et al. 2008). As the thermostability of NtPAP is lower than that of LASAP2, the physical and chemical stability of LASAP2 in the rhizosphere soil could be better than that of the native APase activity of tobacco. Thus, we conclude that the advantageous properties of the APase encoded by LASAP2 have the potential to efficiently use organic P in the rhizosphere soils.
Figure 5 Relationship between acid phosphatase (APase) activity in the rhizosphere soil and P accumulation.
CitationGeorge et al. (2005b) concluded that the availability of inositol phosphates in soil is a major factor that limits the effectiveness of phytase. For effective use of the potential of phytate to manipulate phytase and APases, including LASAP2, not only the advantages of the enzyme itself in the rhizosphere soil, but also the mechanism for solubilization of phytate appear to be important. For example, stimulation of organic acid exudation with a phytate-degrading enzyme might be effective. A malate transporter, designated ALMT1, has been isolated from wheat as a key molecule in organic acid exudation (CitationSasaki et al. 2004). However, it is believed that the function of ALMT1 is specific to aluminum stress and that another mechanism is important for organic acid exudation under P-deficient conditions. The higher ability to secrete APase and organic acid exudation is considered important in the tolerance of P deficiency by white lupin. The abundant secretion of citrate from white lupin roots is regulated by a citrate-permeable channel (CitationDiatloff et al. 2004). It is expected that efficient expression of the citrate-permeable channel with an organic P-degrading enzyme will improve organic P use efficiency.
CitationGeorge et al. (2005a) studied the behavior of plant-derived extracellular phytase (phyA) after addition to soil. More than half of the phytase activity was quickly adsorbed by soil particles, but the phytase adsorption was increasingly inhibited with time. In contrast, CitationTadano et al. (1993) reported that the half-life of LASAP2 in a soil solution was approximately 2 weeks. An increase in organic P utilization in the soil by the L2 line was shown in the present study (), although the substrate specificity of phytate was low (CitationMiller et al. 2001). This suggests that both the stability of the enzyme in the rhizosphere soil and the substrate specificity are important.
The LASAP2 gene was driven by a CaMV35S promoter; therefore, LASAP2 was constitutively produced in the whole plant in the L2 line. This might have a secondary effect on the metabolism of the L2 line. Despite the application of inorganic phosphate (Pi) as the sole P source in the aseptic culture, the growth of the L2 line in the +P treatment was better than that of the WT (). Furthermore, the P contents in the plants of the two tobacco lines were quite similar (). In general, when plants face P starvation, internal APase activity increases to recycle internal organic P (CitationDuff et al. 1994; CitationNanamori et al. 2004; CitationYan et al. 2001; CitationYun and Kaeppler 2001). LASAP1, a homologue of LASAP2, was isolated from –P roots of white lupin and is used to recycled internal organic P in the apoplasmic region (CitationWasaki et al. 1999, Citation2000). It is assumed that LASAP2 overexpression prompts the recycling of internal organic P, and as a result, growth might improve. Because the L2 line expressed APase with the native signal peptide required for extracellular sorting, it was expected that the mature protein was located in the apoplasmic region where it hydrolyzed organic P as well as LASAP1.
Expression of the LASAP2 gene is specific to P deficiency and to the roots (CitationWasaki et al. 2003). It is quite likely that these two specificities are important for the efficient use of organic P in the soil. A phytase transgene driven by the promoter of Pi transporters, which was also expected to have expression specificity in P-deficient roots, successfully improved soluble phytate utilization (CitationMudge et al. 2003, CitationXiao et al. 2005). Thus, the native promoter could be an option for driving a LASAP2 gene under suitable conditions.
Phosphorus deficiency elicits dramatic morphological and architectural changes in the root system and increases the root-to-shoot ratio (CitationAbel et al. 2002; CitationMarschner 1995). In the present study, the root-to-shoot ratio of tobacco plants grown in both medium and soil conditions increased only in the –P treatment (, ). The ratio in the +P treatment was similar to that in the phytate treatment, despite the low P availability (, ). These facts might imply that the root tissues sense phytate itself or the product of hydrolysis, and repress an increase in root growth. How root tissues sense phytate is unknown at present. It has been suggested that microorganisms in the rhizosphere of white lupin are involved in P liberation from phytate P (CitationAdams and Pate 1992; CitationUnno et al. 2005). The sensing mechanisms of both phytate and Pi by roots and the plant–microbe interactions involved in organic P dynamics need to be investigated in the future.
ACKNOWLEDGMENTS
This research was supported by the Special Coordination Funds for Promoting Science and Technology and a Grant-in-Aid (17688004) from the Ministry of Education, Culture, Sports, Science and Technology, Japan.
Notes
Present addresses: Bioscience, Biotechnology Center, Nagoya University, Furo-cho, Chikusa-ku, Nagoya 464-8601, Japan.
National Agricultural Research Center for Hokkaido Region, Hitsujigaoka 1, Toyohira-ku, Sapporo 062-8555, Japan
REFERENCES
- Abel , S , Ticconi , CA and Delatorre , CA . 2002 . Phosphate sensing in higher plants . Physiol. Plant , 115 : 1 – 8 .
- Adams , MA and Pate , JS . 1992 . Availability of organic and inorganic forms of phosphorus to lupins (Lupinus spp.) . Plant Soil , 145 : 107 – 113 .
- Diatloff , E , Roberts , M , Sanders , D and Roberts , SK . 2004 . Characterization of anion channels in the plasma membrane of Arabidopsis epidermal root cells and the identification of a citrate-permeable channel induced by phosphate starvation . Plant Physiol , 136 : 4136 – 4149 .
- Duff , SMG , Sarath , G and Plaxton , WC . 1994 . The role of acid phosphatase in plant phosphorus metabolism . Physiol. Plant , 90 : 791 – 800 .
- George , TS , Richardson , AE , Hadobas , PA and Simpson , RJ . 2004 . Characterization of transgenic Trifolium subterraneum L. which expresses phyA and releases extracellular phytase: growth and P nutrition in laboratory media and soil . Plant Cell Environ , 27 : 1351 – 1361 .
- George , TS , Richardson , AE and Simpson , RJ . 2005a . Behaviour of plant-derived extracellular phytase upon addition to soil . Soil Biol. Biochem , 37 : 977 – 988 .
- George , TS , Richardson , AE , Smith , JB , Hadobas , PA and Simpson , RJ . 2005b . Limitations to the potential of transgenic Trifolium subterraneum L. plants that exude phytase when grown in soils with a range of organic P content . Plant Soil , 258 : 263 – 274 .
- George , TS , Simpson , RJ , Hadobas , PA and Richardson , AE . 2005c . Expression of a fungal phytase gene in Nicotiana tabacum improves phosphorus nutrition of plants grown in amended soils . Plant Biotechnol. J , 3 : 129 – 140 .
- Li , MG and Tadano , T . 1996 . Comparison of characteristics of acid phosphatases secreted from roots of lupin and tomato . Soil Sci. Plant Nutr , 42 : 753 – 763 .
- Lung , SC , Chan , WL , Yip , W , Wang , L , Yeung , EC and Lim , BL . 2005 . Secretion of beta-propeller phytase from tobacco and Arabidopsis roots enhances phosphorus utilization . Plant Sci , 169 : 341 – 349 .
- Lung , SC , Leung , A , Kuang , R , Wang , Y , Leung , P and Lim , BL . 2008 . Phytase activity in tobacco (Nicotiana tabacum) root exudates is exhibited by a purple acid phosphatase . Phytochemistry , 69 : 365 – 373 .
- Lung , SC and Lim , BL . 2006 . Assimilation of phytate-phosphorus by the extracellular phytase activity of tobacco (Nicotiana tabacum) is affected by the availability of soluble phytate . Plant Soil , 279 : 187 – 199 .
- Luo , HM , Osaki , M and Tadano , T . 1997 . “ Mechanisms of differential tolerance of crop plants to high aluminum and low phosphorus growth conditions ” . In Plant Nutrition – for Sustainable Food Production and Environment , Edited by: Ando , T , Fujita , K , Mae , T , Matsumoto , H , Mori , S and Sekiya , J . 473 – 474 . Dordrecht : Kluwer Academic Publishers .
- Marschner , H . 1995 . Mineral Nutrition of Higher Plants , London : Academic Press .
- Miller , SS , Liu , J , Allan , DL , Menzhuber , CJ , Fedorova , M and Vance , CP . 2001 . Molecular control of acid phosphatase secretion into the rhizosphere of proteoid roots from phosphorus-stressed white lupin . Plant Physiol , 127 : 594 – 606 .
- Mudge , SR , Smith , FW and Richardson , AE . 2003 . Root-specific and phosphate-regulated expression of phytase under the control of a phosphate transporter promoter enables Arabidopsis to grow on phytate as a sole P source . Plant Sci , 165 : 871 – 878 .
- Murashige , T and Skoog , F . 1962 . A revised medium for rapid growth and bioassays with tobacco tissue cultures . Physiol. Plant , 15 : 473 – 497 .
- Nanamori , M , Shinano , T , Wasaki , J , Yamamura , T , Rao , IM and Osaki , M . 2004 . Low phosphorus tolerance mechanisms: phosphorus recycling and photosynthate partitioning in the tropical forage grass, Brachiaria hybrid cultivar Mulato compared with rice . Plant Cell Physiol , 45 : 460 – 469 .
- Ozawa , K , Osaki , M , Matsui , H , Honma , M and Tadano , T . 1995 . Purification and properties of acid phosphatase secreted from lupin roots under phosphorus-deficiency conditions . Soil Sci. Plant Nutr , 41 : 461 – 469 .
- Richardson , AE , Hadobas , PA and Hayes , JE . 2001 . Extracellular secretion of Aspergillus phytase from Arabidopsis roots enables plants to obtain phosphorus from phytate . Plant J , 25 : 641 – 649 .
- Sasaki , T , Yamamoto , Y Ezaki , B . 2004 . A wheat gene encoding an aluminum-activated malate transporter . Plant J , 37 : 645 – 653 .
- Tadano , T and Komatsu , K . 1994 . Utilization of organic phosphorus in soil by plant roots . Trans. 15th Word Congr. Soil Sci , 9 : 521 – 522 .
- Tadano , T , Ozawa , K , Sakai , H , Osaki , M and Matsui , H . 1993 . Secretion of acid phosphatase by the roots of crop plants under phosphorus-deficient conditions and some properties of the enzyme secreted by lupin roots . Plant Soil , 155/156 : 95 – 98 .
- Tadano , T and Sakai , H . 1991 . Secretion of acid phosphatase by the roots of several crop species under phosphorus-deficient conditions . Soil Sci. Plant Nutr , 37 : 129 – 140 .
- Truog , E . 1930 . The determination of the readily available phosphorus in soils . J. Am. Soc. Agron , 22 : 874 – 882 .
- Turner , BL , Papházy , MJ , Haygarth , PM and McKelvie , ID . 2002 . Inositol phosphates in the environment . Phil. Trans. R. Soc. Lond. B , 357 : 449 – 469 .
- Unno , Y , Okubo , K , Wasaki , J , Shinano , T and Osaki , M . 2005 . Plant growth promotion abilities and micro-scale bacterial dynamics in the rhizosphere of lupin analyzed by phytate utilization ability . Environ. Microbiol , 7 : 396 – 404 .
- Wasaki , J , Dateki , H , Shinano , T , Osaki , M and Tadano , T . 2001 . “ Characterization of secretory acid phosphatase gene of lupin roots and the transformations of the gene into tobacco plants ” . In Plant Nutrition – Food Security and Sustainability of Agro-ecosystems through Basic and Applied Research , Edited by: Horst , WJ , Bürkert , A Claassen , N . 36 – 37 . Dordrecht : Kluwer Academic Publishers .
- Wasaki , J , Omura , M Ando , M . 2000 . Molecular cloning and root specific expression of secretory acid phosphatase from phosphate deficient lupin (Lupinus albus L.) . Soil Sci. Plant Nutr , 46 : 427 – 437 .
- Wasaki , J , Rothe , A Kania , A . 2005 . Root exudation, P acquisition and microbial diversity in the rhizosphere of Lupinus albus as affected by P supply and atmospheric CO2concentration . J. Environ. Qual , 34 : 2157 – 2166 .
- Wasaki , J , Yamamura , T , Shinano , T and Osaki , M . 2003 . Secreted acid phosphatase is expressed in cluster roots of lupin in response to phosphorus deficiency . Plant Soil , 248 : 129 – 136 .
- Watanabe , T , Osaki , M and Tadano , T . 1998 . Effects of nitrogen source and aluminum on growth of tropical tree seedlings adapted to low pH soils . Soil Sci. Plant Nutr , 44 : 655 – 666 .
- Xiao , K , Harrison , MJ and Wang , ZY . 2005 . Transgenic expression of a novel M. truncatula phytase gene results in improved acquisition of organic phosphorus by Arabidopsis . Planta , 222 : 27 – 36 .
- Xiao , K , Katagi , H , Harrison , M and Wang , ZY . 2006b . Improved phosphorus acquisition and biomass production in Arabidopsisby transgenic expression of purple acid phosphatase gene from M. truncatula . Plant Sci , 170 : 191 – 202 .
- Xiao , K , Zhang , JH , Harrison , M and Wang , ZY . 2006a . Ectopic expression of a phytase gene from Medicago truncatula Barrel Medic enhances phosphorus absorption in plants . J. Integr. Plant Biol , 48 : 35 – 43 .
- Yan , X , Liao , H , Trull , MC , Beebe , SE and Lynch , JP . 2001 . Induction of a major leaf acid phosphatase does not confer adaptation to low phosphorus availability in common bean . Plant Physiol , 125 : 1901 – 1911 .
- Yip , W , Wang , L , Cheng , C , Wu , W , Lung , S and Lim , BL . 2003 . The introduction of a phytase gene from Bacillus subtilis improved the growth performance of transgenic tobacco . Biochem. Biophys. Res. Comm , 310 : 1148 – 1154 .
- Yun , SJ and Kaeppler , SM . 2001 . Induction of maize acid phosphatase activities under phosphorus starvation . Plant Soil , 237 : 109 – 115 .
- Zimmernann , P , Zardi , G , Lehmann , M , Zeder , C , Amrhein , N , Frossard , E and Bucher , M . 2003 . Engineering the root–soil interface via targeted expression of a synthetic phytase gene in trichoblasts . Plant Biotechnol. J , 1 : 353 – 360 .
- Wasaki , J , Omura , M Osaki , M . 1999 . Structure of a cDNA for an acid phosphatase from phosphate-deficient lupin (Lupinus albus L.) roots . Soil Sci. Plant Nutr , 45 : 439 – 449 .
- Present addresses: Bioscience, Biotechnology Center, Nagoya University, Furo-cho, Chikusa-ku, Nagoya 464-8601, Japan.
- National Agricultural Research Center for Hokkaido Region, Hitsujigaoka 1, Toyohira-ku, Sapporo 062-8555, Japan