Abstract
A pot culture experiment was conducted to investigate the effects of amorphous iron-(hydr)oxide (Am-FeOH) amendments on arsenic (As) availability and its uptake by rice (Oryza sativa L. cv. BR28) irrigated with As-contaminated water. A rhizobag system was established using 3.5 L plastic pots, each containing one central compartment for plant growth, a middle compartment and an outside compartment. Three levels of laboratory-synthesized Am-FeOH (0, 0.1 and 0.5% w/w) were used to amend samples of the As-free sandy loam paddy soil placed into each compartment of the rhizobag system. The soils were submerged with a solution containing 5 mg L−1 As(V). Two-week-old rice seedlings were planted in the central compartments and cultured for 9 weeks under greenhouse conditions. The addition of 0.1% Am-FeOH to the soil irrigated with As-contaminated water improved plant growth, reduced the As concentration in the plants and enhanced Fe-plaque formation on the root surfaces. Analysis of soil solution samples collected during the experiment revealed higher pH levels and lower redox potentials in the soils amended with Am-FeOH at the onset of soil submergence, but later the soil solution collected from the 0.1% Am-FeOH treatment was slightly acidic and more oxidized than the solution from the 0% treatment. This indicated active functioning of the roots in the soil treated with 0.1% Am-FeOH. The concentrations of As(III) in the soil solution collected from the central compartment were significantly reduced by the Am-FeOH amendments, whereas in the soil treated with 0% Fe, As(III) accumulated in the rhizosphere, particularly during the late-cultivation period. The improvement in plant growth and reduction in As uptake by plants growing in the Am-FeOH treated soil could be attributed to the reduction of available As in the soil solution, mainly as a result of the binding of As to the Fe-plaque on the root surfaces.
INTRODUCTION
Arsenic-contaminated soils in South-East Asia are reported to include most of the paddy soils in Bangladesh and the West Bengal district of India and soils in some parts of China, Thailand and Vietnam (CitationAgusa et al. 2006; CitationAtkins et al. 2006; CitationMeharg 2004; CitationZarcinas et al. 2003). This contamination results from either irrigation with As-contaminated water or metal mining activities. The consumption of agricultural products that have been grown in these areas results in As ingestion and is a serious hazard to human health (CitationWilliams et al. 2005). For example, in populations living on subsistence rice diets, As-contaminated rice grains contribute 30–60% of the dietary As intake (CitationMeharg 2004). It is clear that As contamination of rice has great potential to negatively affect human health on a massive scale, and strategies to prevent As build up in rice grains should be developed.
Although the As contamination level in most agricultural areas is considered to be moderate, but potentially hazardous, conventional remediation methods in rice paddy fields are difficult to apply because they are not cost effective (CitationMeharg 2004). It is important to identify and develop agro-technologies that will lower As availability for plants and prevent As transport to vulnerable environments. These technologies should be formulated based on an understanding of the As dynamics in the plant rhizosphere. Studies have shown that As chemistry in the rhizosphere is complex and is controlled by several factors, including plant phosphate status, soil redox potential, microbial oxidation or reduction and methylation of As, and soil mineralogy (CitationFitz and Wenzel 2002). The amount of Fe oxides and hydroxides in the soil plays an important role in regulating the concentration of As species in the soil solution because it affects the surface binding and precipitation of poorly soluble As salts (CitationGoldberg 2002; CitationGoldberg and Johnston 2001). Under paddy field conditions, inorganic As is inter-converted between the reduced inorganic species As(III) and the oxidized species As(V) (CitationMarin et al. 1993; CitationUltra et al. 2005). Soil microbes can also methylate inorganic As to produce monomethylarsonic acid (MMAA) and dimethylarsinic acid (DMAA) (CitationTurpeinen et al. 1999). Arsenite (As[III]) is the dominant species under paddy conditions, but As(V), MMAA and DMAA are also present in significant quantities. These and several other bio-geochemical processes influence As mobility and availability for uptake by plants.
Because of the high affinity of As for Fe oxides and hydroxides (CitationGoldberg and Johnston 2001; CitationKubicki 2005; CitationLenoble et al. 2002; CitationLombi et al. 1999; CitationPierce and Moore 1980), it has been proposed that soil amendment with amorphous iron-(hydr)oxide (Am-FeOH) could alter the availability of As for uptake by rice plants irrigated with As-contaminated water. This strategy was developed from previous studies using Fe-rich compounds to mitigate As pollution in soil and water systems. For example, CitationWarren and Alloway (2003) reported that the use of FeSO4 (easily oxidized to Fe oxide) improved the growth of lettuces growing on As-contaminated upland soils and reduced the As content in the lettuces. Similarly, CitationLombi et al. (2004) demonstrated plant growth improvements as a result of Fe-rich industrial waste amendments to a soil contaminated with As and Cu. Furthermore, several As decontamination treatments for water purification use Fe oxides and hydroxides to adsorb As (CitationGu et al. 2005; CitationVaughan and Reed 2005).
In the present study, we examined the effects of Am-FeOH amendments on As dynamics and speciation and uptake by rice irrigated with As-contaminated water. As a common feature of aquatic plants, rice plants develop aerenchyma to transfer O2 from the aerial parts to the roots, resulting in the oxidation of ferrous Fe to ferric Fe, and the precipitation of Fe oxides or hydroxides (Fe-plaque) on the root surfaces (CitationChen et al. 1980). The presence of Fe-plaque can inhibit As uptake into plant tissues by adsorption and/or co-precipitation owing to the high capacity of functional groups in the Fe-plaque (CitationChen et al. 2004; CitationLiu et al. 2004, Citation2005). Therefore, we examined the extent of Fe-plaque formation on the rice root surfaces, their influence on As uptake by plants, and the effects of Am-FeOH amendments on the amounts of As(III) and As(V) in the soil solutions.
MATERIALS AND METHODS
Soil preparation and rhizobag assembly
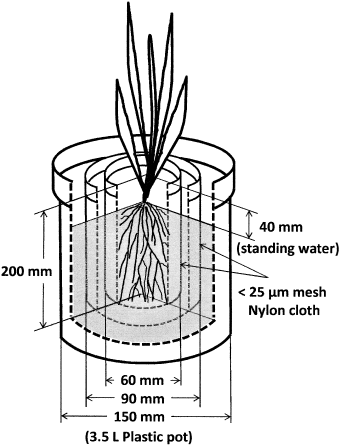
The non-As-contaminated soil used in these experiments was collected from a paddy field at the Field Science Center, Faculty of Agriculture, Kochi University, Japan. This soil was characterized by a sandy loam texture () and classified as Typic Endoaquents based on the US Department of Agriculture classification system (CitationSoil Survey Staff 2006). After air-drying, the soil samples for cultivation and chemical analysis were passed through 5 mm and 2 mm mesh sieves, respectively. A rhizobag system with three compartments was established using 3.5 L plastic pots (). Each rhizobag system consisted of one central compartment in which the plants were grown, a middle compartment, and an outside compartment that served as the bulk soil compartment. The compartments were separated using 25 µm nylon mesh cloths mounted on cylindrical plastic frames (60 mm diameter × 200 mm height and 90 mm diameter × 200 mm height, respectively). There was 600 g of soil in the central compartment, 875 g in the middle compartment and 2,500 g in the outside compartment (i.e. 3,975 g soil per pot). Although root growth was confined to the central compartment, nutrients and As could be delivered to the roots by diffusion and water movement from the bulk soil compartment. Therefore, in this rhizobag system, we assumed that a total of 3,975 g soil was available for plants as a source of nutrients and As.
Table 1 Selected chemical and physical properties of the soil before rice cultivation
Synthesis of amorphous iron-(hydr)oxides
Amorphous iron-(hydr)oxide was synthesized from a 0.1 mol L−1 Fe(NO3)3·9H2O solution by the addition of 1 mol L−1 NaOH at room temperature until the pH of the solution reached 7.0. The mixture was allowed to stand for 4 h and then filtered (CitationOkazaki et al. 1989). The Am-FeOH precipitate was then dialyzed against deionized water by changing the water twice daily until the electrical conductivity of the solution became equal to that of deionized water. The Am-FeOH was then freeze-dried before use. According to the report by CitationKang et al. (2002), the oxalate-extractable Fe, specific surface area and zero point of charge for Am-FeOH synthesized using the same method were 460.5 g kg−1, 273.6 m2 g−1 and pH 7.4, respectively.
Arsenic and Am-FeOH application and plant culture
The treatments consisted of Am-FeOH additions to the soil at rates of 0, 0.1 and 0.5% (w/w) and were designated Fe0, Fe0.1 and Fe0.5, respectively. Additional pots for each treatment without plants were prepared as controls (Control-NP). All of the treatments were prepared in triplicate. For each treatment, appropriate amounts of freeze-dried Am-FeOH and basal fertilizers were thoroughly mixed with the soil prior to potting. Urea and potassium dihydrogen phosphate were supplied as basal fertilizers at rates of 50 mg N kg−1, 50 mg P kg−1 and 63 mg K kg−1. Afterwards each pot was flooded with approximately 1.7 L of 5 mg L−1 As(V) solution (prepared by dissolving Na2HAsO4 in deionized water) and the soil was kept submerged for 2 weeks before rice planting. This As concentration was selected based on a previous study by CitationAbedin et al. (2002), who chose a concentration range of 1.0–8.0 mg L−1 As for the irrigation water used in their experiments with rice plants. The As concentrations in the groundwaters of As-affected areas in Bangladesh are within this range. After 2 weeks, two 2-week-old rice seedlings (Oryza sativa L. cv. BR28) were planted in the central compartment of each pot. Pots were maintained under greenhouse conditions with flood water at a depth of approximately 4 cm above the soil surface throughout the experimental period (9 weeks). This depth was maintained by adding As solution (5 mg L−1) to each experimental pot so that the water surface was kept level with a gauge marked on the side of the pot. The As solution was added every day or as needed to maintain the 4 cm depth.
Soil solution sampling and analysis
Soil solution samples were collected at 7-day intervals until 42 days after transplanting using a porous fiber tube. In each compartment, one porous fiber tube (DIK-301B; Daiki Rika Kogyo, Tokyo, Japan) was inserted to a depth of 10 cm at a distance of approximately 1 cm from the nylon mesh to minimize the direct influence of the roots growing along the nylon mesh on the porous fiber tube. Soil solution samples were analyzed within 1 day of collection. Measurements of pH and redox potential were started at the onset of soil submergence and As speciation and concentration measurements were begun after transplanting the rice seedlings. The pH and redox potential were measured using an ion meter (IM-40S; TOA Electronics, Tokyo, Japan) under a constant N gas flow in a closed chamber. Using the same samples, the As(III) and As(V) concentrations were determined by cold-trap vapor-reduction atomic absorption spectrometry (AAS) (AA-6800 equipped with ASA-2sp; Shimadzu, Kyoto, Japan) based on the method described by CitationMasscheleyn et al. (1991). To determine the total inorganic As (As[III] + As[V]), the sample was reacted with sodium borohydride and hydrochloric acid to form an As hydride. The generated As hydride was trapped in a U-tube cylinder at a low temperature and then quantified by AAS. To determine the As(III) concentration, potassium hydrogen phthalate was used instead of hydrochloric acid. The As(V) concentration was calculated as the difference between the total inorganic As and the As(III) concentration in each sample.
Plant sampling and chemical analysis
After 63 days of cultivation in the pots, the shoots were excised and the rhizobag was dismantled. The roots in the central compartment were carefully separated from the soil and washed with tap water followed by distilled water. The fresh and dry weights of the shoots and roots were determined. To obtain the As concentrations in the roots after removal of Fe-plaque from the root surfaces, 10 g portions of the fresh roots were washed in 40 mL of dithionite–citrate–bicarbonate (DCB) solution for 60 min at room temperature (25–28°C) (CitationLiu et al. 2004). For preparation of the DCB solution, 1.0 g of sodium dithionite (Na2S2O4) was dissolved in 40 mL of a solution containing 0.03 mol L−1 sodium citrate (Na3C6H5O7·2H2O) and 0.125 mol L−1 sodium bicarbonate (NaHCO3) just before each root washing. The roots were then washed in deionized water, blotted dry and oven-dried at 90°C for 2 days. The remaining roots and shoots were also oven-dried under the same conditions and ground for tissue analysis. The tissues (shoots and roots with and without DCB treatment) were digested in a sulfuric acid and hydrogen peroxide mixture at 300°C and analyzed for As using hydride generation-inductively coupled plasma–atomic emission spectrometry (HG-ICP-AES) (ICPS-1000 IV; Shimadzu). The Fe content was determined for the same digest using ICP-AES. Standard reference materials (NIES CRM No.1 and No.9 from the National Institute for Environmental Studies, Japan) were used to verify the accuracy of the As determination and the recovery rates were 102 and 101%, respectively.
Soil sampling and chemical analysis
After harvesting the rice plants, the soil from each central compartment was sieved using a 2 mm sieve to remove coarse root fragments. For the analysis of total As, a part of each soil sample was digested in a mixture of HClO4, HNO3 and HF (2:3:5) with the addition of 20 g L−1 KMnO4 in a teflon vessel at 100°C. The As concentrations in these acid digests were determined by HG-ICP-AES. Standard reference materials (JSO-1 and JSO-2 from the Geological Survey of Japan) were used to verify the accuracy of the As determination and the recovery rates were 102 and 101%, respectively.
Plant uptake calculations and statistical analyses
The difference between the total Fe concentration of intact roots and those washed with DCB solution was regarded as the concentration of Fe adsorbed onto the surfaces of the roots (Fe-plaque). The total amount of Fe-plaque on the root surfaces in each sample was computed by multiplying the Fe-plaque concentration and the total dry weight of the roots. Similarly, the As concentration associated with the Fe-plaque was calculated based on the difference between the As concentration in intact roots and in those washed with DCB solution.
For the data on plant growth and As concentrations, Tukey's tests were used to identify significant differences (P ≤ 0.05) between the treatments. The main effects and interaction effects of treatments and sampling intervals on the pH, redox potential and As concentrations in soil solutions were subjected to two-way anovas.
RESULTS
Plant growth
The total dry matter yield of rice at 63 days after transplanting increased significantly with the addition of Am-FeOH and the highest yield was obtained in the Fe0.1 treatment (). Significantly larger shoot dry weights were observed in the Fe0.1 treatment compared with the other treatments. The root dry weights in the soils amended with Am-FeOH were more than 1.8-fold larger than those of the Fe0 treatment, although no significant differences were observed. Similarly, the numbers of tillers per pot in the Fe0.1 and Fe0.5 treatments increased by approximately 40% over the Fe0 treatment, although the differences were not significant.
Table 2 Dry matter yield and number of tillers on rice plants grown for 63 days in As-contaminated water
Table 3 Arsenic concentration and content in the shoots and roots of rice plants grown for 63 days in As-contaminated water
Table 4 Iron concentration and content in the shoots and roots of rice plants grown for 63 days in As-contaminated water
Concentrations and contents of As and Fe in the plants
The concentrations (mg kg−1 dry weight) and contents (µg pot−1) of As in the shoots were generally reduced by the addition of Am-FeOH to the soil, particularly in the Fe0.1 treatment (). The concentration of As in the shoots was less than 11 mg kg−1 dry weight in the Fe0.1 treatment and approximately 35 mg kg−1 dry weight in the Fe0.5 treatment. These concentrations were 1/7 and 1/2 of those in the Fe0 treatment, respectively. Although the As concentrations in the intact roots did not vary significantly among treatments, the Am-FeOH treatments resulted in larger values than the value recorded in the Fe0 treatment. After washing the roots with DCB solution, the As concentration was smaller in the Am-FeOH treatments than in the Fe0 treatment, although the difference was not statistically significant. The mean As concentrations associated with the Fe-plaque in the Am-FeOH treatments, particularly in the case of the Fe0.5 treatment, showed larger values than the value recorded in the Fe0 treatment.
The Fe concentrations in the shoots were not affected by the addition of Am-FeOH to the soil, but the shoot Fe contents per pot increased significantly with the addition of Am-FeOH, particularly in the Fe0.1 treatment because of the high biomass yield in this treatment (). The addition of Am-FeOH resulted in 1.8-fold and 4.1-fold greater Fe concentrations in washed roots in the Fe0.1 and Fe0.5 treatments, respectively, compared with the Fe0 treatment. Similarly, the concentrations and contents per pot of Fe in the Fe-plaque were significantly larger in the Fe0.5 treatment.
pH, redox potential and As concentration of the soil solution
The pH of the soil solution increased to near neutrality during submergence in all treatments (). However, in general, the addition of Am-FeOH resulted in higher pH values in the soil solutions collected from the central compartments compared with those collected in the Fe0 treatment. The soil solutions from the outside compartments also showed pH increases towards neutrality, except for the Fe0.1 treatment. For the Control-NP pots, the pH of the soil solution did not vary much among the treatments.
The redox potentials (Eh) of the soil solutions in the Control-NP pots decreased after submergence, and the values for the Fe0.1 and Fe0.5 treatments were slightly lower than those for the Fe0 treatment. The redox potentials of the soil solutions in the pots with rice plants also generally declined until 21 days after transplanting. After this time, the values for the central and outside compartments in the Fe0.1 treatment and those in the central compartments in the Fe0.5 treatment increased gradually until the end of the experiment.
Although the As was supplied in the form of As(V), the As(V) concentration in the soil solution remained low (< 100 µg L−1) for all treatments throughout the experimental period (). In most cases, the concentration of As(III) was also low, with the exception of the solutions collected from the central compartments in the Fe0 treatment. The As(III) concentrations in the soil solutions collected from the central compartments in the Fe0 treatment were significantly greater than those from the treatments amended with Am-FeOH, indicating transformation of As in that system. During the later period of cultivation, the As(III) concentrations in the central compartments of the Fe0 treatment were 2.5-fold and 16-fold greater than those in the Fe0.1 and Fe0.5 treatments, respectively. In solutions from the Control-NP pots and from the outside compartments in all treatments, the As(III) concentrations did not vary considerably among treatments. In general, the Fe0.5 treatment showed the lowest As(III) and As(V) concentrations throughout the experimental period.
Figure 2 The pH and redox potentials of soil solutions collected from the central and outside compartments of the rhizobags and from Control-NP pots during the experimental period. Data are the mean ± standard deviation (n = 3). Bars at the top right of each graph indicate the least significant differences (LSD) for comparison between sampling time (left), between compartments (middle) and any pair of data (right) at P < 0.05.
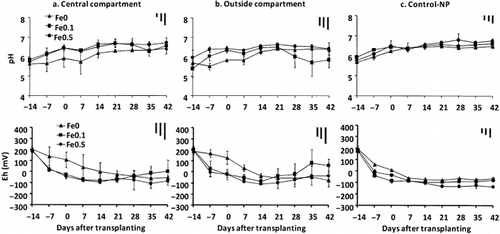
Figure 3 Amounts of As(III) and As(V) in the soil solutions collected from the central and outside compartments of the rhizobags and from Control-NP pots during the experimental period. Data are the mean ± standard deviation (n = 3). Bars at the top right of each graph indicate the least significant differences (LSD) for comparison between sampling time (left), between compartments (middle) and any pair of data (right) at P < 0.05.
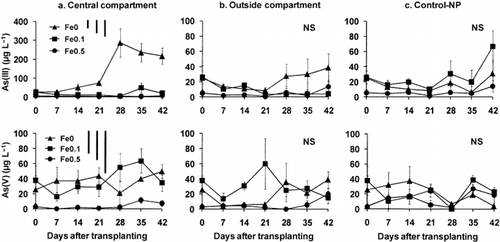
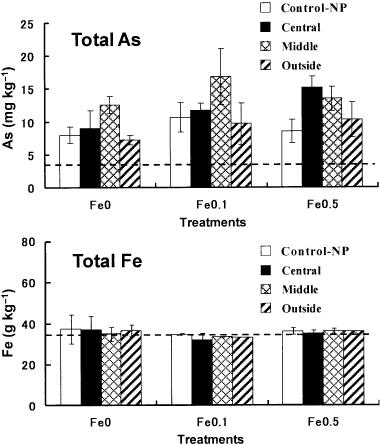
Figure 4 Total amounts of As and Fe in the soil collected from the different compartments of the rhizobags and from Control-NP pots after 63 days of rice cultivation. The broken lines represent the initial concentrations in the soil prior to the treatments. Data are the mean ± standard deviation (n = 3). The mean soil As concentrations averaged over the three compartments after 63 days of rice cultivation were 8.92, 11.9 and 12.0 mg kg−1 for the Fe0, Fe0.1 and Fe0.5 treatments, respectively.
Total As and Fe concentrations in the soil
The concentrations of total As in the soil after 63 days of rice cultivation varied among treatments and among compartments (). The average concentrations across compartments in the Fe0.1 and Fe0.5 treatments were larger than those in the Fe0 treatment, which could be ascribed to the larger amounts of As-containing irrigation water supplied in those treatments. In the present experiment, approximately 1.7 L of 5 mg L−1 As(V) solution (approximately 8.5 mg of As per pot) was added to each pot at the beginning and the depth of standing water was maintained at 4 cm by adding As solution (5 mg L−1), regardless of variations in water loss among the treatments as a result of differences in transpiration and surface evaporation. The total volume of the As solution supplied to the pots in each treatment was not recorded. However, it is likely that the larger concentrations of total As in the soil of the Am-FeOH treatments can be explained by the more vigorous plant growth and greater demand for irrigation water in those treatments. In contrast, within each treatment, there were generally larger As concentrations in the central and middle compartments compared with the outside compartments. The total Fe concentrations in soils after 63 days of rice cultivation did not differ significantly among treatments and compartments. The expected increases in Fe concentrations in the soils of the Fe0.1 and Fe0.5 treatments were not observed, probably because most of the added Am-FeOH was either precipitated onto the surfaces of the roots as Fe-plaque or onto the sides of the pots.
DISCUSSION
The application of Am-FeOH to soils irrigated with As-contaminated water enhanced the growth of rice plants and lowered the As concentrations and contents in the above-ground tissues. These results can be explained by the effects of Am-FeOH on the chemical dynamics of As and other nutrients, reducing their mobility in the soil and their availability for transport and uptake by plants. In the following sections, we discuss in more detail the results in relation to As availability and uptake by rice plants.
One of the most interesting changes brought about by the Am-FeOH amendments was the enhanced formation of Fe-plaque on the root surfaces. The amounts of Fe-plaque increased with increasing amounts of Am-FeOH added to the soil, and this affected the uptake and transport of As by the rice plants (, ). Studies have reported that Fe-plaque is composed primarily of ferrihydrite (63%), with a lesser amount of goethite (32%) and a minor component of siderite (CitationHansel et al. 2001, Citation2002). As Fe hydroxide and oxides have a high affinity for As, they have the capability to sequester As and consequently reduce the translocation of As from the roots to the shoots (CitationHu et al. 2005; CitationLiu et al. 2004, Citation2005). CitationChen et al. (2004) reported that Fe-plaque acts as a buffer for As(V) uptake in the rhizosphere, leading to a lower influx into the root cells. Similar to their findings, our results showed that the amount of As associated with Fe-plaque increased with increasing amounts of Fe-plaque and the rate of Am-FeOH added to the soil (, ). However, the Fe0.1 treatment showed smaller As concentrations in the shoots and larger dry matter yields than the Fe0.5 treatment, which suggested that for As-affected rice plants, an optimum level of Fe-plaque formation is necessary to attain a favorable benefit for growth. As there is a possibility that Fe-plaque can also sequester other nutrients, such as phosphate, excessive Fe precipitation onto the root surfaces could also restrict nutrient transport and become detrimental to the plants. In addition, thick layers of Fe-plaque on the root surfaces might physically hinder gas exchange (oxygen diffusion) and nutrient uptake by the roots. It is also possible that the application of Am-FeOH at high rates could result in Fe–As precipitation within the epidermal tissues (apoplast) of the roots, which might affect the internal translocation of nutrients.
The addition of Am-FeOH to the soil resulted in a rapid and more intense pH change at the onset of submergence (). The reduction of Fe(III) to Fe(II) in the presence of bicarbonate ions during submergence consumes protons and increases the alkalinity of the soil solution (CitationKyuma 2004). Therefore, a larger Fe content in the soil will induce wider pH changes during the submergence period, which will stabilize near neutrality. Similarly, the addition of Am-FeOH to the soil influenced the redox system during submergence and this was characterized by a higher magnitude of redox potential change, particularly within 35 days after submergence. These chemical changes in the soil solution have profound effects on As speciation, favoring a rapid conversion from As(V) to As(III). In the present study, the different trends in pH and redox potentials among the treatments in the 21 days after transplanting could be ascribed to the effects of the rice plants on the soil of the rhizosphere, that is, the ion imbalance resulting from uptake, organic acid deposition and oxygen diffusion (CitationMarschner 1995). Meanwhile, the different effects on the redox potential between the Fe0.1 and Fe0.5 treatments during the later period of cultivation could constitute indirect evidence that the high amounts of Fe-plaque on the root surfaces physically restricted oxygen diffusion from the roots to the adjacent soil environment.
Adsorption and fractionation studies have demonstrated the high affinity of As to oxides and hydroxides of Fe, Al and Mn, and that the amount of As in the soil solution is influenced largely by the amount of Fe in the soil (CitationWarren and Alloway 2003). Several Fe(III) oxides, such as amorphous hydrous ferric oxide, poorly crystalline hydrous ferric oxide (ferrihydrite) and goethite (R-FeOOH), are well known for their ability to remove and adsorb both As(V) and As(III) from aqueous solutions. These have been used in some As decontamination processes for water (CitationGu et al. 2005). In the present study, apart from the As(III) concentrations in the central compartments, the As concentrations in the soil solutions taken from the outside compartments of the Fe0 treatment were generally below 100 µg L−1, despite the addition of 5.0 mg L−1 As in the irrigation water throughout the cultivation period (). This suggested that the poorly crystalline Fe oxides already present in the soil (indigenous Fe oxides) could adsorb some of the added As. In addition, this result implies that the application of Am-FeOH could alleviate As toxicity more drastically in soils containing smaller amounts of indigenous Fe oxides.
In the present study, the effect of Am-FeOH amendment on the As concentration in the soil solution was most clearly observed for the central compartments during the later period of cultivation. The As(III) concentrations in the Fe0.1 and Fe0.5 treatments were 1/2 and 1/16 of the level in the Fe0 treatment, respectively (). Under conditions in which the soil is submerged, the As(V) added to the soil solution would be reduced to As(III), which would move by mass flow and accumulate around the root surfaces. In contrast, the reduced Am-FeOH and indigenous Fe oxides in the soil would be re-oxidized by oxygen diffusion from the roots. Therefore, in the Fe0.1 and Fe0.5 treatments, the accumulated As(III) around the root surfaces was rapidly adsorbed by the iron-(hydr)oxides in the soil as well as by the Fe-plaque on the root surfaces; thus, lowering the As(III) concentrations in the soil solutions of the central compartments. According to a report by CitationHansel and Fendorf (2001), As(V) and As(III) co-existed in Fe-plaque on the root surfaces of two common aquatic plant species, reed canarygrass (Phalaris arundinacea) and cattail (Typha latifolia). The major component was As(V)-Fe-(hydr)oxide (82%) with less (18%) As(III)-Fe(hydr)oxide complexes. In the present experiment, there is also the possibility of re-oxidation of As(III) to As(V) in the rhizosphere, but the oxidized product (As[V]) could be adsorbed onto the iron-(hydr)oxides in the soil and/or Fe-plaque on the root surfaces; thus, reducing the build-up in the soil solution.
In contrast, the results of the Fe0 treatment indicated that the As adsorption ability of indigenous Fe oxides was not sufficient to cause a reduction in accumulated As(III) around the root surfaces. Recently, CitationVetterlein et al. (2007) examined the dynamics of P and As in the rhizosphere of Zea mays grown in a quartz substrate containing different amounts of goethite, and suggested that the As(III) found in the soil solution of the root compartment was not necessarily formed in the soil, but might have been released from the roots. In addition, CitationXu et al. (2007) have observed that in tomato roots there occurs an active efflux of As(III), which is the predominant As species in the roots and xylem sap. Based on these reports, we cannot rule out the possibility that the elevated levels of As(III) in the soil solution taken from the central compartments in the Fe0 treatment resulted from the release of As(III) from the plant roots. In the cases of the Fe0.1 and Fe0.5 treatments, the larger amounts of Fe-plaque on the root surfaces might have adsorbed any effluxed As(III) from the symplast; thus, lowering the As(III) concentrations in the soil solutions.
In conclusion, the amendment of soil with Am-FeOH reduced the availability to plants of As in paddy soil irrigated with As-contaminated water and resulted in improvements in rice plant growth and reductions in As uptake by the plants. The reduced As availability in the presence of iron-(hydr)oxides is most likely the result of enhanced binding of As to the Fe-plaque on the root surfaces. However, important questions remain regarding whether or not Am-FeOH amendments can be effective even when the soil contains relatively high amounts of indigenous Fe oxides. Further studies are necessary to examine the optimum levels of Am-FeOH amendments in different soils and the stability of supplied Am-FeOH in the paddy soil environment.
ACKNOWLEDGMENTS
We are grateful to Professor Jian Feng Ma and Dr Syuntaro Hiradate for their valuable advice and comments. This research was supported by a Sasakawa Scientific Research Grant (No. 17-207) from The Japan Science Society to V. U. Ultra and by a Grant-in-Aid for Scientific Research (B, No. 15380223) from the Ministry of Education, Culture, Sports, Science and Technology of Japan to K. Iwasaki.
Notes
Present address: College of Agriculture, University of Eastern Philippines, Catarman, Northern Samar, 6400, Philippines.
REFERENCES
- Abedin , MJ , Cotter-Howells , J and Meharg , AA . 2002 . Arsenic uptake and accumulation in rice (Oryza sativa L.) irrigated with contaminated water . Plant Soil , 240 : 311 – 319 .
- Agusa , T , Kunito , T Fujihara , J . 2006 . Contamination by arsenic and other trace elements in tube-well water and its risk assessment to humans in Hanoi, Vietnam . Environ. Pollut , 139 : 95 – 106 .
- Atkins , PJ , Hassan , MM and Dunn , CE . 2006 . Toxic torts: arsenic poisoning in Bangladesh and the legal geographies of responsibility . Trans. Inst. Br. Geogr , 31 : 272 – 285 .
- Chen , CC , Dixon , JB and Turner , FT . 1980 . Iron coatings on rice roots: morphology and models of development . Soil Sci. Soc. Am. J , 44 : 1113 – 1119 .
- Chen , Z , Zhu , YG , Liu , WJ and Meharg , AA . 2004 . Direct evidence showing the effect of root surface iron plaque on arsenite and arsenate uptake into rice (Oryza sativa) roots . New Phytol , 165 : 91 – 97 .
- Fitz , WJ and Wenzel , WW . 2002 . Arsenic transformation in the soil–rhizosphere–plant system: fundamentals and potential application to phytoremediation . J. Biotech , 99 : 259 – 278 .
- Goldberg , S . 2002 . Competitive adsorption of arsenate and arsenite on oxides and clay minerals . Soil Sci. Soc. Am. J , 66 : 413 – 421 .
- Goldberg , S and Johnston , CT . 2001 . Mechanisms of arsenic adsorption on amorphous oxides evaluated using macroscopic measurements, vibrational spectroscopy, and surface complexation modeling . J. Colloid Interface Sci , 234 : 204 – 216 .
- Gu , Z , Fang , J and Deng , B . 2005 . Preparation and evaluation of GAC-based iron-containing adsorbents for arsenic removal . Environ. Sci. Technol , 39 : 3833 – 3843 .
- Hansel , CM and Fendorf , S . 2001 . Characterization of Fe plaque and associated metals on the roots of mine-waste impacted aquatic plants . Environ. Sci. Technol , 35 : 3863 – 3868 .
- Hansel , CM , La Force , HJ , Fendorf , S and Sutton , S . 2002 . Spatial and temporal association of As and Fe species on aquatic plant roots . Environ. Sci. Technol , 36 : 1988 – 1994 .
- Hu , Y , Li , JH , Zhu , YG , Huang , YZ , Hu , HQ and Christie , P . 2005 . Sequestration of As by iron plaque on the roots of three rice (Oryza sativa L.) cultivars in a low-P soil or without P fertilizer . Environ. Geochem. Health , 27 : 169 – 176 .
- Kang , Y , Inoue , N , Rashid , MM and Sakurai , K . 2002 . Fixation of soluble selenium in contaminated soil by amorphous iron (hydr)oxide . Environ. Sci , 15 : 173 – 182 .
- Kubicki , JD . 2005 . “ Comparison of As(III) and As(V) complexation onto Al- and Fe-hydroxides ” . In Advances in Arsenic Research: Integration of Experimental and Observational Studies and Implication for Mitigation , ASC Symposium Series 915 Edited by: O'Day , PA , Vlassopoulos , D , Meng , X and Benning , LG . 104 – 117 . Washington : American Chemical Society .
- Kyuma , K . 2004 . “ Fundamental chemical reactions in submerged paddy soils ” . In Paddy Soil Science , 36 – 59 . Kyoto : Kyoto University Press .
- Lenoble , V , Bouras , O , Deluchat , V , Serpaud , V and Bollinger , JC . 2002 . Arsenic adsorption onto pillared clays and iron oxides . J. Colloid Interface Sci , 255 : 52 – 58 .
- Liu , WJ , Zhu , YG and Smith , FA . 2005 . Effects of iron and manganese plaques on arsenic uptake by rice seedlings (Oryza sativa L.) grown in solution culture supplied with arsenate and arsenite . Plant Soil , 277 : 127 – 138 .
- Liu , WJ , Zhu , YG , Smith , FA and Smith , SE . 2004 . Do phosphorus nutrition and iron plaque alter arsenate (As) uptake by rice seedlings in hydroponic culture? . New Phytol , 162 : 481 – 488 .
- Lombi , E , Hamon , RE , Wieshammer , G , McLaughlin , MJ and McGrath , SP . 2004 . Assessment of the use of industrial by-products to remediate a copper- and arsenic-contaminated soil . J. Environ. Qual , 33 : 902 – 910 .
- Lombi , E , Wenzel , WW and Sletten , RS . 1999 . Arsenic adsoption by soils and iron-oxide-coated sand: kinetics and reversibility . J. Plant Nutr. Soil Sci , 162 : 451 – 456 .
- Marin , AR , Masscheleyn , PH and Patrick , WH Jr . 1993 . Soil redox–pH stability of arsenic species and its influence on arsenic uptake by rice . Plant Soil , 152 : 235 – 253 .
- Marschner , H . 1995 . “ Nutrient availability in soils ” . In Mineral Nutrition for Higher Plants , 483 – 507 . London : Academic Press .
- Masscheleyn , PH , Delaune , RD and Patrick , WH Jr . 1991 . A hydride generation atomic absorption technique for arsenic speciation . J. Environ. Qual , 20 : 96 – 100 .
- Meharg , AA . 2004 . Arsenic in rice–understanding a new disaster for south-east Asia . Trends Plant Sci , 9 : 415 – 417 .
- Hansel , CM , Fendorf , S , Sutton , S and Newville , M . 2001 . Characterization of Fe plaque and associated metals on the roots of mine-waste impacted aquatic plants . Environ. Sci. Technol , 35 : 3863 – 3868 .
- Okazaki , M , Sakaidani , K , Saigusa , T and Sakaida , N . 1989 . Ligand exchange of oxyanions on synthetic hydrated oxides of iron and aluminum . Soil Sci. Plant Nutr , 35 : 337 – 346 .
- Pierce , ML and Moore , CB . 1980 . Adsorption of arsenite on amorphous iron hydroxide from dilute aqueous solution . Environ. Sci. Technol , 14 : 214 – 216 .
- Soil Survey Staff . Keys to Soil Taxonomy . The 18th World Congress of Soil Science . July 9–15 2006 , Philadelphia, Pennsylvania.
- Turpeinen , R , Pantsar-Kallio , M , Haggblom , M and Kairesalo , T . 1999 . Influence of microbes on the mobilization, toxicity and biomethylation of arsenic in soil . Sci. Total Environ , 236 : 173 – 180 .
- Ultra , VU Jr , Nakayama , A , Tanaka , S , Iwasaki , K and Sakurai , K . 2005 . “ Arsenic transformation and microbial community structures in the rhizosphere of rice irrigated with As contaminated water ” . In Plant Nutrition for Food Security, Human Health and Environmnetal Protection , Edited by: Li , CJ , Zhang , FS Dobermann , A . 696 – 297 . Beijing : Tsinghua University Press .
- Vaughan , RL Jr and Reed , BE . 2005 . Modeling As(V) removal by iron oxide impregnated activated carbon using the surface complexation approach . Water Res , 39 : 1005 – 1014 .
- Vetterlein , D , Szegedi , K Ackermann , J . 2007 . Competitive mobilization of phosphate and arsenate associated with goethite by root activity . J. Environ. Qual , 36 : 1811 – 1820 .
- Warren , GP and Alloway , BJ . 2003 . Reduction of arsenic uptake by lettuce with ferrous sulfate applied to contaminated soil . J. Environ. Qual , 32 : 767 – 772 .
- Williams , PN , Price , AH , Raab , A , Hossain , SA , Fieldmann , J and Meharg , AA . 2005 . Variations in arsenic speciation and concentration in paddy rice related to dietary exposure . Environ. Sci. Technol , 39 : 5531 – 5540 .
- Xu , XY , McGrath , SP and Zhao , FJ . 2007 . Rapid reduction of arsenate in the medium mediated by plant roots . New Phytol , 176 : 590 – 599 .
- Zarcinas , BA , Pongsakul , P , McLaughlin , MJ and Cozens , G . 2003 . Heavy metals in soils and crops in Southeast Asia. 2. Thailand . Environ. Geochem. Health , 26 : 359 – 371 .
- Present address: College of Agriculture, University of Eastern Philippines, Catarman, Northern Samar, 6400, Philippines.