Abstract
The soil microbial community is strongly influenced by a wide variety of factors, such as soil characteristics and field management systems. In order to use biological indicators based on microbial community structure, it is very important to know whether or not these factors can be controlled. The present study aimed to determine whether soil type or fertilization has a greater influence on the soil microbial community based on denaturing gradient gel electrophoresis (DGGE) analysis of 12 experimental field plots containing four different soil types, Cumulic Andosol, Low-humic Andosol, Yellow Soil and Gray Lowland Soil, kept under three different fertilizer management systems since 2001 (the application of chemical fertilizer, the application of rice husk and cow manure, and the application of pig manure). Bacterial DGGE analysis using 16S rRNA genes and fungal DGGE analysis using 18S rRNA genes revealed that the bacterial community was related to the soil type more than the fertilization; however, the fungal community was related to the fertilization more than the soil type. These results might suggest that the fungal community is easier to control by fertilization than the bacterial community. Thus, we propose that indicators based on the fungal community might be more suitable as microbial indicators for soil quality.
INTRODUCTION
Soil quality is one of the significant agroecosystem components for which management efforts must intensify to achieve sustainability. This has been defined as “the capacity of a specific kind of soil to function, within natural or managed ecosystem boundaries, to sustain plant and animal productivity, maintain or enhance water and air quality, and support human health and habitation” (CitationKarlen et al. 1997). In general, soil quality has been estimated in terms of the soil physical, chemical and biological factors (CitationKarlen et al. 2001, Citation2003). Various biological indicators are known to be related to soil quality, including microbial biomass, soil respiration, soil enzyme activities, earthworm number and nematode number (CitationAndrews and Carroll 2001; CitationAndrews et al. 2003; CitationKarlen et al. 2001; CitationLiebig and Doran 1999). In addition, microbial diversity can directly influence plant growth and development, plant competition, and nutrient and water uptake (CitationKennedy 1999). Microbial diversity is also sensitive to the phytopathological status of soils (CitationJanvier et al. 2007). Thus, microbial diversity based on microbial community structure needs to be considered in investigations of soil quality (CitationJanvier et al. 2007; CitationKennedy 1999).
In a soil community, most microorganisms cannot be cultivated, and not more than 1% of the total microbes can currently be identified (CitationAnderson 2003; CitationBengtsson 1998; CitationGiller et al. 1997). To overcome these problems, various methods have been developed to identify and study these microorganisms, including fatty acid analysis and numerous DNA-based and RNA-based methods (CitationKirk et al. 2004; CitationTiedje et al. 1999). Through the use of these methods, it has become clear that the soil microbial community is strongly influenced by a wide variety of factors. Some studies have reported that the factors most affecting soil microbial communities are the soil characteristics or environmental factors, such as soil type (CitationBuyer et al. 1999; CitationGirvan et al. 2003; CitationHoneycutt et al. 2005), soil particle size (CitationMarhan et al. 2007; CitationSessitsch et al. 2001), soil air composition (CitationLudemann et al. 2000; CitationOvreas et al. 1998) and season (CitationBlume et al. 2002; CitationGirvan et al. 2004). However, other studies have shown significant effects of field management systems, such as cropping systems (CitationAcosta-Martinez et al. 2007; CitationJohnson et al. 2003; CitationKuske et al. 2002), tillage systems (CitationKennedy and Schillinger 2006; CitationPeixoto et al. 2006), fertilization (CitationHartmann et al. 2006; CitationMarschner et al. 2003; CitationSuzuki et al. 2005), pesticide or herbicide application (CitationSeghers et al. 2003; CitationYang et al. 2000) and fumigation (CitationIbekwe et al. 2001). To use biological indicators based on microbial community structure in soil quality, it is very important to know whether or not these factors can be controlled. In the present study, to determine whether soil characteristics or field management systems have a greater influence on the soil microbial community, we analyzed 12 experimental field plots containing four different soil types, Cumulic Andosol, Low-humic Andosol, Yellow Soil and Gray Lowland Soil, kept under three different fertilizer management systems since 2001, that is, the application of chemical fertilizer, the application of rice husk and cow manure, and the application of pig manure. The field plots were all located in the same region and had been managed using the same tillage and cropping systems.
Table 1 The management system in each plot
MATERIALS AND METHODS
Field management
The experimental field was located at the National Agricultural Research Center in Tsukuba, Ibaraki Prefecture, Japan. The field experiment had 4 × 3 factorial plots with two replicates. Each plot was placed up to a depth of 80 cm in the artificial concrete enclosures (3.85 m × 4.35 m; without a bottom). The four different soil types were Cumulic Andosol (CA) from Mito, Ibaraki Prefecture, Low-humic Andosol (LHA) from Yatabe, Ibaraki Prefecture, Yellow Soil (YS) from Tahara, Aichi Prefecture, and Gray Lowland Soil (GLS) from Moriya, Ibaraki Prefecture. The three soil fertilizer management systems were the application of chemical fertilizer (CF), the application of rice husk and cow manure (HC) and the application of pig manure (PM). These management treatments had been maintained since 2001. The management systems of each plot are shown in . The rice husk and cow manure was purchased from the Higashimachi Compost Center, Inashiki, Ibaraki Prefecture, and the pig manure was purchased from the Research Institute of Pig Farming, Ibaraki Prefectural Livestock Research Center, Inashiki, Ibaraki Prefecture. The rice husk and cow manure contained N at 0.86%, P at 1.03% and K at 1.18%. The pig manure contained N at 1.96%, P at 7.17% and K at 2.4%. The N source was ammonium sulfate [(NH4)2SO4], the P source was amended phosphate fertilizer made from Ca3(PO4)2 and MgO, and the K source was potassium sulfate (K2SO4). The field has been cropped once per year in the summer season (from May to October). In 2002, 2004 and 2005, the cultivated crop was carrot, and in 2003 the crop was maize.
Soil sampling
Soil samples were collected from all 24 plots on 16 May 2006, just before the next fertilizer treatment. Surface soil (0–10 cm) was randomly collected from nine points in each plot and mixed together in a plastic bag. All samples were sieved (2 mm) and the microbial biomass was immediately analyzed. For nucleic acid extraction, all samples were frozen and stored at –20°C, and for soil analyses the samples were air dried for 1 week and stored at room temperature.
Soil analyses
The total C, total N, pH, available P, microbial biomass C and exchangeable potassium (K), magnesium (Mg), calcium (Ca) and sodium (Na) were analyzed. Total C and total N were measured using an NC-Analyzer (Sumigraph NC-95A; Sumika Chemical Analysis Service, Tokyo, Japan). Soil pH was estimated from a soil : water suspension of 1:2.5. Available P was measured using the Truog method (CitationNanjyo 1997). Exchangeable K, Mg, Ca and Na were extracted with a 0.05 mol L−1 ammonium acetate/0.0114 mol L−1 strontium chloride solution and the element concentrations were measured with an atomic absorption spectrophotometer (Shimadzu AA6700; Shimadzu, Kyoto, Japan). Biomass C was measured using the chloroform fumigation–extraction method (CitationBrookes et al. 1985), and the amount of total C dissolved in 0.5 mol L−1 potassium sulfate solution was determined with a total organic C analyzer (TOC-VCPH; Shimadzu).
DNA extraction
The genomic community DNA was extracted from 0.4 g soil samples using a FastDNA SPIN Kit for Soil (Q-BIOgene, Carlsbad, CA, USA) following the manufacturer's instructions with a slight modification. As it was difficult to extract DNA from the CA and LHA soils, autoclaved 20% skimmed milk solution was added at 80 µL or 120 µL, respectively, at the first step (CitationHoshino and Matsumoto 2004). DNA extraction was conducted in triplicate for each plot sample.
Denaturing gradient gel electrophoresis analysis
The bacterial community analysis and fungal community analysis were carried out with denaturing gradient gel electrophoresis (DGGE). The DGGE analysis was carried out using a previously published procedure (CitationMorimoto and Hoshino 2008). Bacterial 16S rRNA genes in the extracted DNA were amplified using the primers 968f-GC (5′-CGC CCG GGG CGC GCC CCG GGC GGG GCG GGG GCA CGG GGG GAA CGC GAA GAA CCT TAC-3′) and 1378r (5′-CGG TGT GTA CAA GGC CCG GGA ACG-3′). The reactions were carried out with a TaKaRa polymerase chain reaction (PCR) Thermal Cycler GP (Takara Biomedicals, Otsu, Japan), applying an initial denaturation step of 94°C for 2 min followed by 34 cycles consisting of denaturation at 94°C for 15 s, primer annealing at 55°C for 30 s, and elongation at 68°C for 30 s. The PCR mixtures (50 µL) contained 1× reaction buffer (Toyobo, Osaka, Japan), 0.2 mmol L−1 dNTPs (Toyobo), 1 mmol L−1 MgSO4 (Toyobo), 0.02 mg bovine serum albumin (TaKaRa Bio), 0.2 µmol L−1 of each primer, 1 U of KOD-Plus− DNA polymerase (Toyobo) and 1 µL of extracted DNA solution. Fungal 18S rRNA genes were amplified using the primers NS1 (5′-GTA GTC ATA TGC TTG TCT C-3′) and GCFung (5′-CGC CCG CCG CGC CCC GCG CCC GGC CCG CCG CCC CCG CCC CAT TCC CCG TTA CCC GTT G-3′) (CitationHoshino and Morimoto 2008). The PCR program consisted of an initial denaturing step at 94°C for 2 min, followed by 30 cycles of denaturation at 94°C for 15 s, primer annealing at 50°C for 30 s, and elongation at 68°C for 30 s. The PCR mixture (50 µL) contained 1× reaction buffer (Toyobo), 0.2 mmol L−1 dNTPs (Toyobo), 1 mmol L−1 MgSO4 (Toyobo), 0.02 mg bovine serum albumin (TaKaRa Bio), 0.3 µmol L−1 of each primer, 1 U of KOD-Plus− DNA polymerase (Toyobo) and 1 µL of extracted DNA solution. The PCR products were purified with a QIAquick PCR purification kit (Qiagen, Valencia, CA, USA).
The marker for the bacterial DGGE analysis was made by amplifying a template DNA mixture that contained extracted DNA from Clostridium chartatabium, uncultured Delta proteobacteria, two different species of uncultured Acidobacteria, two different species of uncultured Beta proteobacteria, Arthorobacter pascens, uncultured Gamma proteobacteria, Soliubrobacter sp. and uncultured Chloroflexi bacteria, with the primers 968f-GC and 1378r. The PCR program consisted of an initial denaturing step of 94°C for 2 min, followed by 28 cycles of denaturation at 94°C for 15 s, primer annealing at 55°C for 30 s, and elongation at 68°C for 30 s. The PCR mixture (100 µL) contained 1× reaction buffer (Toyobo), 0.2 mmol L−1 dNTPs (Toyobo), 1 mmol L−1 MgSO4 (Toyobo), 0.2 µmol L−1 of each primer, 2 U of KOD-Plus− DNA polymerase (Toyobo) and 1 µL of the template DNA mixture. The PCR product was purified with a QIAquick PCR purification kit (Qiagen), adjusted to 20 ng µL−1 and mixed with loading dye. For the fungal DGGE analysis, the markers were made by amplifying DNA extracted from uncultured Cercozoan, Mortierella alpina, Pythium monospermum, Dendryphiella arenaria, Herpotrichia parasitica, Lecythophora hoffmannii and two different species of Folsomia candida using an NS1 and GCFung primer set, and mixed all at the same rate after each PCR product was adjusted to 20 ng µL−1. The PCR program consisted of an initial denaturing step of 94°C for 2 min, followed by 24 cycles of denaturation at 94°C for 15 s, primer annealing at 50°C for 30 s, and elongation at 68°C for 30 s. The PCR mixture (50 µL) contained 1× reaction buffer (Toyobo), 0.2 mmol L−1 dNTPs (Toyobo), 1 mmol L−1 MgSO4 (Toyobo), 0.3 µmol L−1 of each primer, 1 U of KOD-Plus−DNA polymerase (Toyobo) and 1 µL of the template DNA. The DGGE was carried out using a DCode system (BioRad Laboratories, Hercules, CA, USA). For the bacterial analysis, the 6% polyacrylamide gels were made with a denaturing gradient ranging from 50 to 70% and were run at 50 V and 58°C for 18 h in TAE buffer. Purified PCR products were applied in a quantity of 200 ng and marker DNA were applied at 10 ng per band in the gel. For the fungal analysis, the 7% polyacrylamide gels were made with a denaturing gradient ranging from 20 to 45% and the running condition was set at 50 V and 60°C for 20 h in TAE buffer. Purified PCR products were applied in a quantity of 200 ng and marker DNA were applied at 10 ng per band in the gel. After electrophoresis, the gels were stained with SYBR Green I nucleic acid gel stain (Cambrex Bio Science, Rockland, ME, USA) and scanned with a BioRad ChemiDoc XRS system (BioRad Laboratories). Gel images were analyzed using Fingerprinting II software (BioRad Laboratories). These measurements were done in triplicate for each plot sample.
Statistical analysis
Non-parametric statistics (Kruskall–Wallis test) were used to explain the significant differences between the soil types. The DGGE band profiles were analyzed using principal component analysis (PCA) and cluster analysis to determine any differences in the microbial communities among fertilizations or soil types. Correlation analysis was conducted among soil chemical parameters, and between bacterial and fungal DGGE bands. All analyses was carried out with STATISTICA software (StatSoft, Tokyo, Japan).
RESULTS
Soil chemical characteristics, biomass and yield
In the comparison of the different fertilizer treatments, available P, exchangeable Mg, Ca and soil pH were higher in the plots treated with pig manure (). Soil pH had a high positive correlation with exchangeable Mg and exchangeable Ca (r = 0.90, P < 0.05 and r = 0.73, P < 0.05, respectively), and applying pig manure might have a higher concentration of P, Ca and Mg than applying rice husk and cow manure every year (CitationMiura and Nishio 2004; results from the analysis of applying pig manure, rice husk and cow manure in 2006 and 2007 [data not shown]). Thus, these results appear reasonable. There were no significant differences in carrot yield among the different fertilization treatments ().
Each soil type has some specific characteristics; Andosol has a high humus content, high C/N ratio, high phosphate absorption coefficient and low degree of base saturation (CitationKosaki and Kyuma 2000; CitationThe Research Committee of Soil Conservation 1991a); Yellow Soil has a low phosphate absorption coefficient, low concentration of base elements and low organic matter content (CitationKonno 2000; CitationThe Research Committee of Soil Conservation 1991b); and Gray Lowland Soil has a low humus content and low base element content (CitationThe Research Committee of Soil Conservation 1991c). Most of the differences between the soil types depended on these specific characteristics in the comparison of soil types (). Carrot yields were higher in the order GLS > YS > CA and LHA (P < 0.05).
Bacterial denaturing gradient gel electrophoresis analysis
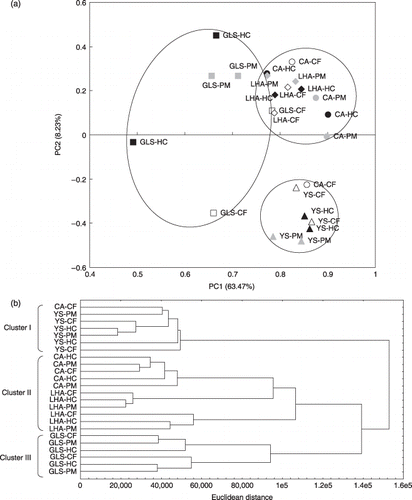
Bacterial DGGE profiles based on 16S rRNA genes were analyzed by PCA and cluster analysis (). In the PCA, all YS plots were located in the lower right corner, the GLS plots tended to be on the left side, and the CA and LHA plots tended to be in the right upper corner (). PC1 was positively related to the B25, B29, B31, B35, B37 and B40 bands (the number of each band indicates its order from the top in the bacterial DGGE analysis), and negatively related to the B3, B6, B55, B58, B65 and B66 bands. PC2 was positively related to the B24, B32, B34, B35, B38 and B40 bands, and negatively related to the B28, B30, B33, B37, B39 and B43 bands. In the cluster analysis, the dendrogram had three clusters (). Cluster I included all the YS plots and CA–CF1, cluster II included all the LHA plots and all the CA plots with the exception of CA–CF1, and cluster III consisted of all the GLS plots (). These results suggested that the bacterial community was related to the soil types more than the fertilization.
Fungal denaturing gradient gel electrophoresis analysis
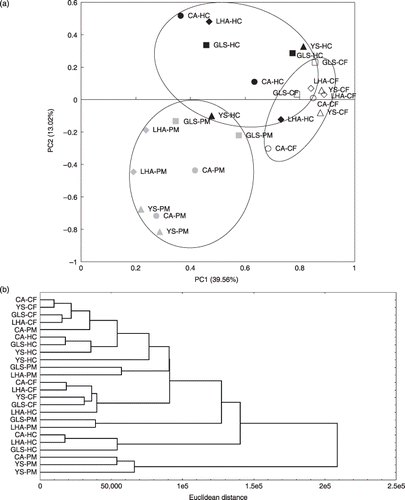
Fungal DGGE profiles based on 18S rRNA genes were also analyzed by PCA and cluster analysis (). In the PCA, the plots treated with pig manure were located in the lower left corner and the plots treated with chemical fertilizer tended to be located in the opposite corner (). The plots treated with rice husk and cow manure were located in the upper half of . PC1 was positively related to the F6, F13, F16, F20 and F22 bands (the number of each band indicates its order from the top in the fungal DGGE analysis), and negatively related to the F2, F8, F29, F30 and F34 bands. PC2 was positively related to the F6, F15, F19, F20 and F22 bands, and negatively related to the F4, F16, F18, F23 and F28 bands. In the cluster analysis, the dendrogram had some small clusters that were dependent on the fertilization treatments and not the soil type (). These results suggested that the fungal community was related to fertilization more than soil type.
Table 2 Soil chemical characteristics, biomass and carrot yield
Figure 1 Principal component analysis (PCA) and cluster analysis using the 16S rRNA gene denaturing gradient gel electrophoresis profiles. (a) PCA plot bounded by each soil type. Abbreviated names are shown near each plot. The different treatments are represented as follows: white, chemical fertilizer application (CF); black, rice husk and cow manure application (HC); gray, pig manure application (PM). The different soil types are represented as follows: , Cumulic Andosol (CA); , Low-humic Andosol (LHA); , Yellow Soil (YS); , Gray Lowland Soil (GLS). (b) A dendrogram using Ward's clustering method.
DISCUSSION
To determine whether soil bacterial and fungal communities can be controlled, it is important to establish what has the greater influence, the soil characteristics or the field management systems. To compare the effects of soil characteristics and field management systems accurately, other conditions must be made as equal as possible. In the present study, the 12 experimental plots, which consisted of four different soil types kept under three different fertilizer management systems since 2001, were located in the same place and had been managed using the same tillage and cropping systems. Thus, we analyzed these plots to compare the influence of soil type as a representative of soil characteristics and by fertilization as a representative of field management systems.
Figure 2 Principal component analysis (PCA) and cluster analysis using the 18S rRNA gene denaturing gradient gel electrophoresis profiles. (a) PCA plot bounded by each soil. Abbreviated names are shown near each plot. The different treatments are represented as follows: white, chemical fertilizer application (CF); black, rice husk and cow manure application (HC); gray, pig manure application (PM). The different soil types are represented as follows: ○, Cumulic Andosol (CA); ⋄, Low-humic Andosol (LHA); ▵, Yellow Soil (YS); □, Gray Lowland Soil (GLS). (b) A dendrogram using Ward's clustering method.
The microbial community was characterized by DGGE analysis. Bacterial DGGE band profiles were analyzed by PCA and cluster analysis (). In the PCA, along the PC1 axis, the GLS plots tended to be on the negative side compared with the other plots (). As PC1 has a high contribution ratio (63.47%) and the PC1 score of each plot were very close, it could be expected that negatively related bands had little influence. The B34 band, which was positively related to PC1, was present in all GLS plots, one LHA–CF plot and one LHA–PM plot, but the strongest positively related bands (B25, B29, B31, B35, B37 and B40) were seen in almost all plots. The presence of a species that had 16S rRNA genes located at the 34th position by DGGE analysis might have helped to separate the GLS plots from the other plots. Along the PC2 axis, all YS plots, CA–CF1 plots and GLS–CF2 plots were separated from the other plots (). The B28, B30, B33, B37, B39 and B43 bands, which were negatively related to PC2, were seen in all YS plots, the B30, B33, B37, B39 and B43 bands were seen in the CA–CF1 plots, and the B28, B30, B37, B39 and B43 bands were seen in the GLS–CF2 plots (data not shown). And this explains why all the YS plots, CA–CF1 plots and GLS-CF2 plots were separated from the other plots. These results might suggest that each soil type had a unique bacterial species. In the cluster analysis, the plots were divided into three clusters: cluster I contained all of the YS plots and the CA–CF1 plot; cluster II contained all of the LHA plots and all of the CA plots except the CA–CF1 plot; and cluster III contained all of the GLS plots (). These separations were clearly caused by soil type and not by fertilization. Thus, considering the results of the PCA and cluster analysis, it could be suggested that the bacterial community was more influenced by soil type than fertilization.
Fungal DGGE bands profiles were also analyzed by PCA and cluster analysis (). In the cluster analysis, the individual plots were not clearly divided, but they tended to be separated by fertilization and not by soil type (). In the PCA, the plots treated with manure tended to be on the negative side of the PC1 axis (). The bands negatively related to PC1 (F2, F8, F29, F30 and F34) were unique bands in a few plots, but the bands positively related to PC1 (F6, F13, F16, F20 and F22) were present in almost all plots. Along the PC2 axis, the pig manure applied plots were separated from the other plots (). The bands negatively related to PC2 (F4, F16, F18, F23 and F28) were strong in the plots treated with pig manure. These results indicated that the application of manure might convey external fungal species, and pig manure application might stimulate particular fungal species. Considering the results of the PCA and cluster analysis, it could be suggested that the fungal community was more influenced by fertilization than by soil type. A previous study showed that arbuscular mycorrhizal fungal communities were more influenced by soil pH than by geographical distance (CitationGi-Hong et al. 2008); thus, fungal communities might be more sensitive to soil environmental changes than soil type.
Bacteria and fungi inhabit various spaces in the soil, such as macro-aggregates and micro-aggregates (CitationMummey et al. 2006; CitationSimpson et al. 2004; CitationSix et al. 2004), soil particles (CitationChiu et al. 2006; CitationKirchmann et al. 2004; CitationKotani-Tanoi et al. 2007; CitationMarhan et al. 2007; CitationSessitsch et al. 2001), plant rhizoplane (CitationKurakov and Kostina 2001; CitationSatoh et al. 2007; CitationSingh et al. 2008) and plant residues (CitationMatsuyama et al. 2007; CitationPerez et al. 2008). Several studies have reported that bacterial biomass and diversities were higher in smaller size fractions than in larger size fractions (CitationChiu et al. 2006; CitationFall et al. 2004; CitationKanazawa and Filip 1986; CitationMonrozier et al. 1991; CitationSessitsch et al. 2001; CitationVan Gestel et al. 1996); however, fungal biomass tends to be higher and fungal activity is highest in larger size fractions (CitationChiu et al. 2006; CitationHattori and Miyashita 1996; CitationKimura 2000; CitationKirchmann et al. 2004). Soil environmental factors change more quickly in larger spaces than in smaller spaces (CitationHattori and Miyashita 1996; CitationKandeled et al. 2000). Thus, the influence of fertilization appears to be more dominant on the fungal community than the bacterial community.
In soil, bacteria and fungi have an interesting relationship (CitationAntunes et al. 2006; CitationFrey-Klett et al. 2007; CitationGarbaye 1994; CitationMurray and Woodward 2003). In the present study, 14 reasonably strong correlations were found between bacterial DGGE bands and fungal DGGE bands (data not shown). In particular, four groups, the F2 band and B58 band, the F8 band and B15 band, the F4 band and B2 band, and the F28 band and B2 band, tended to be higher in the plots treated with organic fertilizer. This suggests that these groups of species, which had 18S rRNA genes or 16S rRNA genes located at each position by DGGE analysis, worked together to degrade external organic compounds. Further studies will be needed to elucidate the relationship between fungi and bacteria, and to reveal their functions in these soils.
In conclusion, the present study revealed that the bacterial community was related to soil type more than to fertilization, but the fungal community was related to fertilization more than soil type. These results suggest that the fungal community can be controlled more easily with fertilization than the bacterial community. Thus, we suggest that indicators based on the fungal community might be more suitable as microbial indicators for soil quality. Finally, because the concept of field management incorporates not only fertilization, but also cropping, tillage systems and fumigation, it will be necessary to study the effects of these components of field management systems on bacterial and fungal communities in the future.
ACKNOWLEDGMENTS
We thank Dr Toshihiko Karasawa for his helpful suggestions and comments on the manuscript and Ms Emi Doi for her excellent technical assistance. This study was supported by a Grant-in-Aid (Soil eDNA) from the ministry of Agriculture, Forestry and Fisheries of Japan.
REFERENCES
- Acosta-Martinez , V , Mikha , MM and Vigil , MF . 2007 . Microbial communities and enzyme activities in soils under alternative crop rotations compared to wheat-fallow for the Central Great Plains . Appl. Soil Ecol , 37 : 41 – 52 .
- Anderson , TH . 2003 . Microbial eco-physiological indicators to assess soil quality . Agr. Ecosyst. Environ , 98 : 285 – 293 .
- Andrews , SS and Carroll , CR . 2001 . Designing a soil quality assessment tool for sustainable agroecosystem management . Ecol. Appl , 11 : 1573 – 1585 .
- Andrews , SS , Flora , CB , Mitchell , JP and Karlen , DL . 2003 . Grower's perceptions and acceptance of soil quality indices . Geoderma , 114 : 187 – 213 .
- Antunes , PM , de Varennes , A , Rajcan , I and Goss , MJ . 2006 . Accumulation of specific flavonoids in soybean (Glycine max(L.) Merr.) as a function of the early tripartite symbiosis with arbuscular mycorrhizal fungi and Bradyrhizobium japonicum(Kirchner) Jordan . Soil Biol. Biochem , 38 : 1234 – 1242 .
- Bengtsson , J . 1998 . Which species? What kind of diversity? Which ecosystem function? Some problems in studies of relations between biodiversity and ecosystem function . Appl. Soil Ecol , 10 : 191 – 199 .
- Blume , E , Bischoff , M , Reichert , JM , Moorman , T , Konopka , A and Turco , RF . 2002 . Surface and subsurface microbial biomass, community structure and metabolic activity as a function of soil depth and season . Appl. Soil Ecol , 20 : 171 – 181 .
- Brookes , JM , Landman , A , Pruden , G and Jenkinson , DS . 1985 . Chloroform fumigation and release of soil nitrogen: A rapid direct extraction method to measure microbial biomass nitrogen in soil . Soil Biol. Biochem , 17 : 837 – 842 .
- Buyer , JS , Roberts , DP and Russek-Cohen , E . 1999 . Microbial community structure and function in the spermosphere as affected by soil and seed type . Can. J. Microbiol , 45 : 138 – 144 .
- Chiu , C-Y , Chen , T-H , Imberger , K and Tian , G . 2006 . Particle size fraction of fungal and bacterial biomass in subalpine grassland and forest soils . Geoderma , 130 : 265 – 271 .
- Fall , S , Nazaret , S , Chotte , JL and Brauman , A . 2004 . Bacterial density and community structure associated with aggregate size fractions of a soil-feeding termite . Microb. Ecol , 48 : 191 – 199 .
- Frey-Klett , P , Garbaye , J and Tarkka , M . 2007 . The mycorrhiza helper bacteria revisited . New Phytol , 176 : 22 – 36 .
- Garbaye , J . 1994 . Helper bacteria: a new dimension to the mycorrhizal symbiosis . New Phytol , 128 : 197 – 210 .
- Gi-Hong , AN , Miyakawa , A , Kawahara , A , Osaki , M and Ezawa , T . 2008 . Community structure of arbuscular mycorrhizal fungi associated with pioneer grass species Miscanthus sinensis in acid sulfate soils: Habitat segregation along pH gradients . Soil Sci. Plant Nut , 54 : 517 – 528 .
- Giller , KE , Beare , MH , Lavelle , P , Izac , A-M and Swift , MJ . 1997 . Agricultural intensification, soil biodiversity and agroecosystem function . Appl. Soil Ecol , 6 : 3 – 16 .
- Girvan , MS , Bullimore , J , Ball , AS , Pretty , JN and Osborn , AM . 2004 . Response of active bacterial and fungal communities in soils under winter wheat to different fertilizer and pesticide regimens . Appl. Environ. Microbiol , 70 : 2692 – 2701 .
- Girvan , MS , Bullimore , J , Pretty , JN , Osborn , AM and Ball , AS . 2003 . Soil type is the primary determinant of the composition of total and active bacterial communities in arable soils . Appl. Environ. Microbiol , 69 : 1800 – 1809 .
- Hartmann , M , Fliessbach , A , Oberholzer , H-R and Widmer , F . 2006 . Ranking the magnitude of crop and farming system effects on soil microbial biomass and genetic structure of bacterial communities . FEMS Microbiol. Ecol , 57 : 378 – 388 .
- Hattori , T and Miyashita , K . 1996 . Soil Microbiology , Tokyo : Yokendo .
- Honeycutt , CW , Griffin , TS and He , ZQ . 2005 . Manure nitrogen availability: dairy manure in northeast and central US soils . Biol. Agric. Hortic , 23 : 199 – 214 .
- Hoshino , YT and Morimoto , S . 2008 . Comparison of 18S rDNA primers for estimating fungal diversity in agricultural soils using polymerase chain reaction-denaturing gradient gel electrophoresis . Soil Sci. Plant Nutr , 54 : 701 – 710 .
- Hoshino , YT and Matshumoto , N. 2004 . An improved DNA extraction method using skim milk from soils that strongly adsorb DNA . Microbes. Environ , 19 : 13 – 19 .
- Ibekwe , AM , Papiernik , SK , Gan , J , Yates , SR , Yang , C-H and Crowley , DE . 2001 . Impact of fumigations on soil microbial communities . Appl. Environ. Microbiol , 67 : 3245 – 3257 .
- Janvier , C , Villeneuve , F , Alabouvette , C , Edel-Hermann , E , Mateille , T and Steinberg , C . 2007 . Soil health through soil disease suppression: which strategy from descriptors to indicators? . Soil Biol. Biochem , 39 : 1 – 23 .
- Johnson , MJ , Lee , KY and Scow , KM . 2003 . DNA fingerprinting reveals links among agricultural crops, soil properties, and the composition of soil microbial communities . Geoderma , 114 : 79 – 303 .
- Kanazawa , S and Filip , Z . 1986 . Distribution of microorganisms, total biomass, and enzyme activities in different particles of brown soil . Microb. Ecol , 12 : 205 – 215 .
- Kandeled , E , Tscherko , D , Bruce , KD , Stemmer , M , Hobbs , PJ , Bardgett , RD and Amelung , W . 2000 . Structure and function of the soil microbial community in microhabitats of heavy metal polluted soil . Biol. Fert. Soils , 32 : 390 – 400 .
- Karlen , DL , Andrews , SS and Doran , JW . 2001 . Soil quality: current concepts and applications . Adv. Agron , 74 : 1 – 40 .
- Karlen , DL , Ditzler , CA and Andrews , SS . 2003 . Soil quality: why and how? . Geoderma , 114 : 145 – 156 .
- Karlen , DL , Mausbach , MJ , Doran , JW , Cline , RG , Harris , RF and Schuman , GE . 1997 . Soil quality: a concept, definition, and framework for evaluation (a guest editorial) . Soil Sci. Soc. Am. J , 61 : 4 – 10 .
- Kennedy , AC . 1999 . “ Microbial diversity in agroecosystem quality ” . In Biodiversity in Agroecosystems , Edited by: Collins , WW and Qualset , CO . 1 – 17 . Florida : CRC Press .
- Kennedy , AC and Schillinger , WF . 2006 . Soil quality and water intake in traditional till vs no-till paired farms in Washington's Palous region . Soil Sci. Soc. Am. J , 70 : 940 – 949 .
- Kimura , M . 2000 . “ Biology of soil ” . In The Current Soil Science , Edited by: Kyuma , K . 54 – 72 . Tokyo : Asakurashoten .
- Kirchmann , H , Haberhauer , G , Kandeler , E , Sessitsch , A and Gerzabek , MH . 2004 . Effects of level and quality of organic matter input on carbon storage and biological activity in soil: synthesis of a long-term experiment . Global Biogeochem. Cy , 18 : GB4011
- Kirk , JL , Beaudette , LA , Hart , M , Moutoglis , P , Klironomos , JN , Lee , H and Trevors , JT . 2004 . Methods of studying soil microbial diversity . J. Microbiol. Meth , 58 : 169 – 188 .
- Konno , T . 2000 . “ Field soil ” . In The Current Soil Science , Edited by: Kyuma , K . 134 – 156 . Tokyo : Asakurashoten .
- Kosaki , T and Kyuma , K . 2000 . “ Pedogenesis and soil taxonomy ” . In The Current Soil Science , Edited by: Kyuma , K . 10 – 26 . Tokyo : Asakurashoten .
- Kotani-Tanoi , T , Nishiyama , M , Otsuka , S and Senoo , K . 2007 . Single particle analysis reveals that bacterial community structures are semi-specific to the type of soil particle . Soil Sci. Plant Nutr , 53 : 740 – 743 .
- Kurakov , AV and Kostina , NV . 2001 . Spatial peculiarities in the colonization of the plant rhizoplane by microscopic fungi . Microbiology , 70 : 165 – 174 .
- Kuske , CR , Ticknor , LO , Miller , ME , Dunbar , JM , Davis , JA , Barns , SM and Belnap , J . 2002 . Comparison of soil bacterial communities in rhizospheres of tree plant species and interspecies in an arid grassland . Appl. Environ. Microbiol , 68 : 1854 – 1863 .
- Liebig , MA and Doran , JW . 1999 . Impact of organic production practices on soil quality indicators . J. Environ. Qual , 28 : 1601 – 1609 .
- Ludemann , H , Arth , I and Liesack , W . 2000 . Spatial changes in the bacterial community structure along a vertical oxygen gradient in flooded paddy soil cores . Appl. Environ. Microbiol , 66 : 754 – 762 .
- Marhan , S , Kandeler , E and Scheu , S . 2007 . Phospholipid fatty acid profiles and xylanase activity in particle size fractions of forest soil and casts of Lumbricus terrestrisL. (Oligochaeta, Lumbricidae) . Appl. Soil Ecol , 35 : 412 – 422 .
- Marschner , P , Kandeler , E and Marschner , B . 2003 . Structure and function of the soil microbial community in a long-term fertilizer experiment . Soil Biol. Biochem , 35 : 453 – 461 .
- Matsuyama , T , Nakajima , Y , Matsuya , K , Ikenaga , M , Asakawa , S and Kimura , M . 2007 . Bacterial community in plant residues in Japanese paddy field estimated by RFLP and DGGE analyses . Soil Biol. Biochem , 39 : 463 – 472 .
- Miura , K and Nishio , T . 2004 . Influence of application of organic materials and soil types on nitrogen balance and nitrate nitrogen concentration of soil solution in carrot cultivation . Jpn. J. Soil Sci. Plant. Nutr , 75 : 459 – 465 .
- Monrozier , LJ , Ladd , JN , Fitzpatrick , AW , Foster , RC and Raupach , M . 1991 . Components and microbial biomass content of size fractions in soils of contrasting aggregation . Geoderma , 49 : 37 – 62 .
- Morimoto , S and Hoshino , YT . 2008 . [Methods for analysis of soil communities by PCR-DGGE (1) Bacterial and fungal communities.] . Soil Microorganisms , 62 : 63 – 68 . (in Japanese)
- Mummey , DL , Rillig , MC and Six , J . 2006 . Endogeic earthworms differentially influence bacterial communities associated with different soil aggregate size fractions . Soil Biol. Biochem , 38 : 1608 – 1614 .
- Murray , AC and Woodward , S . 2003 . In vitrointeractions between bacteria isolated from Sitka spruce stumps and Heterobasidion annosum . Forest Pathol , 33 : 53 – 67 .
- Nanjyo , M . 1997 . “ Available phosphate ” . In Soil and Environment Analysis Method , Edited by: Committee of Soil Environmental Analysis Method . 267 – 269 . Tokyo : Hakuyusha .
- Ovreas , L , Jensen , S , Daae , FL and Torsvik , V . 1998 . Microbial community changes in a perturbed agricultural soil investigated by molecular and physiological approaches . Appl. Environ. Microbiol , 64 : 2739 – 2742 .
- Peixoto , RS , Coutinho , HLC Madari , B . 2006 . Soil aggregation and bacterial community structure as affected by tillage and cover cropping in the Brazilian Cerrados . Soil Till. Res , 90 : 16 – 28 .
- Perez , C , Dill-Macky , R and Kinkel , LL . 2008 . Management of soil microbial communities to enhance populations of Fusarium graminearum–antagonists in soil . Plant Soil , 302 : 53 – 69 .
- Satoh , K , Itoh , C Kang , DJ . 2007 . Characteristics of newly isolated ammmonia-oxidizing bacteria from acid sulfate soil and the rhizoplane of leucaena grown in that soil . Soil Sci. Plant Nutr , 53 : 23 – 31 .
- Seghers , D , Verthe , K Reheul , D . 2003 . Effect of long-term herbicide applications on the bacterial community structure and function in an agricultural soil . FEMS Microbiol. Ecol , 46 : 139 – 146 .
- Sessitsch , A , Weilharter , A , Gerzabek , MH , Kircgmann , H and Kandeler , E . 2001 . Microbial population structures in soil particle size fractions of a long-term fertilizer field experiment . Appl. Environ. Microbiol , 67 : 4215 – 4224 .
- Simpson , RT , Frey , SD , Six , J and Thiet , RK . 2004 . Preferential accumulation of microbial carbon in aggregate structures of no-tillage soils . Soil Sci. Soc. Am. J , 68 : 1249 – 1255 .
- Singh , BK , Nunan , N Ridgway , KP . 2008 . Relationship between assemblages of mycorrhizal fungi and bacteria on grass roots . Environ. Microbiol , 10 : 534 – 541 .
- Six , J , Bossuyt , H , Degryze , S and Denef , K . 2004 . A history of research on the link between (micro)aggregates, soil biota, and soil organic matter dynamics . Soil Till. Res , 79 : 7 – 31 .
- Suzuki , C , Kunito , T , Aono , T , Liu , C-T and Oyaizu , H . 2005 . Microbial indices of soil fertility . J. Appl. Microbiol , 98 : 1062 – 1074 .
- The Research Committee of Soil Conservation . 1991a . “ Andosol ” . In The Current Status and Provision of Japanese Field Soil , Edited by: The Research Committee of Soil Conservation . 75 – 96 . Tokyo : Hakuyusha .
- The Research Committee of Soil Conservation . 1991b . “ Yellow soils ” . In The Current Status and Provision of Japanese Field Soil , Edited by: The Research Committee of Soil Conservation . 175 – 192 . Tokyo : Hakuyusha .
- The Research Committee of Soil Conservation . 1991c . “ Glay Lowland soils ” . In The Current Status and Provision of Japanese Field Soil , Edited by: The Research Committee of Soil Conservation . 218 – 237 . Tokyo : Hakuyusha .
- Tiedje , JM , Asuming-Brempong , S , Nusslein , K , Marsh , TL and Flynn , SJ . 1999 . Opening the black box of soil microbial diversity . Appl. Soil Ecol , 13 : 109 – 122 .
- Van Gestel , M , Merckx , R and Vlassek , K . 1996 . Spatial distribution of microbial biomass in microaggregates of a silty-loam soil and the relation with the resistance of microorganisms to soil drying . Soil Biol. Biochem , 28 : 503 – 510 .
- Yang , Y-H , Yao , J and Qi , Y . 2000 . Effects of agricultural chemicals on DNA sequence diversity of soil microbial community: a study with RAPD marker . Microb. Ecol , 39 : 72 – 79 .