Abstract
Phosphite (![]() ; Phi), a reduced form of phosphate (
; Phi), a reduced form of phosphate (![]() ; Pi), is widely marketed as either a fungicide or fertilizer or sometimes as a biostimulant. This is confusing for both distributors and growers. The present paper explores data from various studies to clarify that Phi does not provide plant P nutrition and thus cannot complement or substitute Pi at any rate. In addition, Phi itself does not have any beneficial effect on the growth of healthy plants, regardless of whether it is applied alone or in combination with Pi at different ratios or different rates. The effect of Phi on plants is not consistent, but is strongly dependent on the Pi status of the plants. In most cases, the deleterious effect of Phi is evident in Pi-starved, but not Pi-sufficient, plants. Plants fertilized with Pi allowing for approximately 80–90% of its maximum growth might still be at risk of the effect. This negative effect becomes more pronounced under more seriously Pi-deficient conditions. Although a number of studies have shown positive crop responses to Phi, these responses are likely to be attributable to the suppression of plant diseases by Phi and/or to Pi formed from oxidation of Phi by microbes. In addition, indirectly providing P by Phi-to-Pi oxidation is not an effective means of supplying P to plants compared with Pi fertilizer. An understanding of these issues will aid the right selection of fertilizer as well as minimize the harmful effects of Phi use on crops.
; Pi), is widely marketed as either a fungicide or fertilizer or sometimes as a biostimulant. This is confusing for both distributors and growers. The present paper explores data from various studies to clarify that Phi does not provide plant P nutrition and thus cannot complement or substitute Pi at any rate. In addition, Phi itself does not have any beneficial effect on the growth of healthy plants, regardless of whether it is applied alone or in combination with Pi at different ratios or different rates. The effect of Phi on plants is not consistent, but is strongly dependent on the Pi status of the plants. In most cases, the deleterious effect of Phi is evident in Pi-starved, but not Pi-sufficient, plants. Plants fertilized with Pi allowing for approximately 80–90% of its maximum growth might still be at risk of the effect. This negative effect becomes more pronounced under more seriously Pi-deficient conditions. Although a number of studies have shown positive crop responses to Phi, these responses are likely to be attributable to the suppression of plant diseases by Phi and/or to Pi formed from oxidation of Phi by microbes. In addition, indirectly providing P by Phi-to-Pi oxidation is not an effective means of supplying P to plants compared with Pi fertilizer. An understanding of these issues will aid the right selection of fertilizer as well as minimize the harmful effects of Phi use on crops.
INTRODUCTION
Phosphorus (P) is one of the major essential macronutrients required by all living organisms. In nature, P does not exist as a free element, but rather in combination with other elements, such as oxygen (O) or hydrogen (H). It occurs in a fully oxidized form (P has valence of +5) as phosphate anion (![]() ; Pi) and with one less oxygen (P has valence of +3) as phosphite (
; Pi) and with one less oxygen (P has valence of +3) as phosphite (![]() ; Phi). The conjugate acid of the phosphite anion is phosphorous acid (H3PO3). In general, the term phosphite is commonly referred to as the salts of phosphorous acid and the term phosphonate is used to mean phosphite ester containing a carbon–phosphorus (C–P) bond that is chemically distinct from the labile carbon–oxygen–phosphorus (C–O–P) bond found in phosphate ester (CitationWhite and Metcalf 2007).
; Phi). The conjugate acid of the phosphite anion is phosphorous acid (H3PO3). In general, the term phosphite is commonly referred to as the salts of phosphorous acid and the term phosphonate is used to mean phosphite ester containing a carbon–phosphorus (C–P) bond that is chemically distinct from the labile carbon–oxygen–phosphorus (C–O–P) bond found in phosphate ester (CitationWhite and Metcalf 2007).
For many years, Pi compounds were considered to be the only form that could supply P nutrition to plants. In fertilizer, P is normally found in the form of phosphoric acid (H3PO4) and its salts, such as, triple super phosphate, ammonium phosphate and potassium phosphate. All of these forms readily disassociate to release hydrogen phosphate (![]() ) and dihydrogen phosphate (
) and dihydrogen phosphate (![]() ), which are used by plants. Phi compounds have been recognized as excellent fungicides for controlling many important plant diseases caused by Oomycetes, particularly Phytophthora sp. (CitationFenn and Coffey 1984; CitationFoster et al. 1998; CitationGrant et al. 1992; CitationGuest and Grant 1991; CitationGuest et al. 1995; CitationJackson et al. 2000; CitationJee et al. 2002; CitationSmillie et al. 1989). However, they are not considered to be P fertilizers because an early study examining different P compounds by CitationMacIntire et al. (1950) concluded that Phi compounds were a very poor source of P for crops compared with Pi fertilizers. Renewed interest in Phi as a plant nutrient has increased since the early 1990s when CitationLovatt (1990a) discovered that P deficiency caused changes in nitrogen metabolism and that the foliar application of potassium phosphite to P-deficient citrus recovered the biological response of plants and consequently restored normal plant growth. Additional studies by CitationLovatt (1990b) further suggested that soil or foliar application of Phi could replace Pi as a source of P in avocado. A series of US patents, such as US Patent numbers 5514200 (CitationLovatt 1996), 5830255 (CitationLovatt 1998) and 5707418 (CitationHsu 1998) describe formulations containing Phi that are suitable as P fertilizers for plants. The work of Lovatt led to the first commercialization of Phi compounds as fertilizer. This fertilizer was sold under the trademark Nutri-phite (Biagro Western Sales, Visalia, CA, USA). Many new P fertilizers based on Phi are now being marketed. The list of Phi products that are available in the American and European markets that are sold as fertilizers now includes 10 different brand names (see ) (CitationLeymonie 2007). All of these products are formulated as alkali salts of phosphorous acid (H3PO3) and have been registered under the fertilizer laws. Some are even described more as “biostimulants” than as fertilizers. However, the representation and the use of Phi-containing products as sources of plant nutritional P have been subjected to ongoing controversy. This has also created much confusion for distributors and growers (CitationLeymonie 2007). There are numerous publications indicating that Phi can be well absorbed by leaves and roots, but has no utility for plants as a P fertilizer (CitationCarswell et al. 1996; CitationForster et al. 1998; CitationSchroetter et al. 2006). Instead, Phi was found to have negative effects on the growth and metabolism of P-deficient plants by suppressing the typical molecular and developmental responses of plants to P deficiency (CitationAbel et al. 2002; CitationCarswell et al. 1996, Citation1997; CitationTicconi et al. 2001; CitationVaradarajan et al. 2002). Phi intensifies the deleterious effects of P deficiency by tricking Pi-deprived plant cells into sensing that they are Pi sufficient, when in fact their cellular Pi content is extremely low (CitationMcDonald et al. 2001a).
), which are used by plants. Phi compounds have been recognized as excellent fungicides for controlling many important plant diseases caused by Oomycetes, particularly Phytophthora sp. (CitationFenn and Coffey 1984; CitationFoster et al. 1998; CitationGrant et al. 1992; CitationGuest and Grant 1991; CitationGuest et al. 1995; CitationJackson et al. 2000; CitationJee et al. 2002; CitationSmillie et al. 1989). However, they are not considered to be P fertilizers because an early study examining different P compounds by CitationMacIntire et al. (1950) concluded that Phi compounds were a very poor source of P for crops compared with Pi fertilizers. Renewed interest in Phi as a plant nutrient has increased since the early 1990s when CitationLovatt (1990a) discovered that P deficiency caused changes in nitrogen metabolism and that the foliar application of potassium phosphite to P-deficient citrus recovered the biological response of plants and consequently restored normal plant growth. Additional studies by CitationLovatt (1990b) further suggested that soil or foliar application of Phi could replace Pi as a source of P in avocado. A series of US patents, such as US Patent numbers 5514200 (CitationLovatt 1996), 5830255 (CitationLovatt 1998) and 5707418 (CitationHsu 1998) describe formulations containing Phi that are suitable as P fertilizers for plants. The work of Lovatt led to the first commercialization of Phi compounds as fertilizer. This fertilizer was sold under the trademark Nutri-phite (Biagro Western Sales, Visalia, CA, USA). Many new P fertilizers based on Phi are now being marketed. The list of Phi products that are available in the American and European markets that are sold as fertilizers now includes 10 different brand names (see ) (CitationLeymonie 2007). All of these products are formulated as alkali salts of phosphorous acid (H3PO3) and have been registered under the fertilizer laws. Some are even described more as “biostimulants” than as fertilizers. However, the representation and the use of Phi-containing products as sources of plant nutritional P have been subjected to ongoing controversy. This has also created much confusion for distributors and growers (CitationLeymonie 2007). There are numerous publications indicating that Phi can be well absorbed by leaves and roots, but has no utility for plants as a P fertilizer (CitationCarswell et al. 1996; CitationForster et al. 1998; CitationSchroetter et al. 2006). Instead, Phi was found to have negative effects on the growth and metabolism of P-deficient plants by suppressing the typical molecular and developmental responses of plants to P deficiency (CitationAbel et al. 2002; CitationCarswell et al. 1996, Citation1997; CitationTicconi et al. 2001; CitationVaradarajan et al. 2002). Phi intensifies the deleterious effects of P deficiency by tricking Pi-deprived plant cells into sensing that they are Pi sufficient, when in fact their cellular Pi content is extremely low (CitationMcDonald et al. 2001a).
Table 1 From fungicides to fertilizers: The marketing of some products with phosphorous and phosphite as the active ingredient
The confusion about Phi became greater when some scientists (CitationLovatt and Mikkelsen 2006; CitationWatanabe 2005) claimed that the negative effects of Phi on plant growth observed in many studies resulted from the inappropriate use of this material, for example, as a primary source of P or in excessive amounts. CitationLovatt and Mikkelsen (2006) emphasized that “since phosphite is chemically different from phosphate, these differences must be taken into consideration to avoid plant toxicity” and that Phi, if used at appropriate rates, can provide stimulation to plants that may not occur with Pi. CitationLovatt and Mikkelsen (2006) suggested that Phi is more than just a fungicide; for example, it increases floral intensity, yield, fruit size and total soluble solids. In addition, combinations of Phi and Pi ions are believed to be more effective than either Pi or Phi alone in plant assimilation (CitationFoster et al. 1998; CitationYoung 2004).
What are the true effects of Phi on the growth of plants aside from the fungicidal actions? Does Phi have any nutritional impact on plants? Can Phi provide stimulating effects to healthy plants? Can a combination of Phi and Pi be more effective in plant assimilation than either ion alone? This article will explore data from various studies to shed light on these questions.
PHOSPHITE IS NOT A FERTILIZER AND DOES NOT HAVE ANY BENEFICIAL EFFECT ON THE GROWTH OF HEALTHY PLANTS
Aside from the early works of CitationLovatt (1990a,Citationb) demonstrating that Phi was readily taken up through leaves and could replace Pi as a source of P in the metabolism of citrus and avocado, a number of studies have highlighted the potential use of Phi as P fertilizer for plants. CitationAlbrigo (1999) demonstrated that foliar application of potassium phosphite on Valencia orange trees in Florida clearly increased both the yield (boxes per hectare) and quality (orange juice soluble solids per hectare) over untreated controls, although the mechanism of the Phi effect remains unclear. Another study by CitationLovatt (1999) on Navel orange trees indicated that foliar application of potassium phosphite in May and July significantly increased the yield, fruit size and total soluble solids. The improvement in the yield and fruit quality after foliar application of potassium phosphite was interpreted as a response of citrus fruit to increased P nutrition. Similarly, foliar application of potassium phosphite has been reported to accelerate the flowers and fruit setting and, hence, significantly increase the yield of Satsuma orange compared with untreated controls or a foliar Pi treatment (CitationWatanabe 2005). CitationRickard (2000) summarized studies on crop responses to commercial Phi-derived P fertilizers. Most of the studies in his review were carried out under field conditions and all results showed that soil or foliar application of Phi fertilizer consistently improved the yield and quality of many crops, such as celery, onion, potatoes, peaches, orange and cotton. However, there is no evidence that Phi can be used directly by plants as a source of nutritional P. In addition, data showing that the effectiveness of Phi-derived P fertilizer is equal to or better than that of conventional Pi fertilizers are very rare.
In contrast, numerous other studies have indicated that Phi compounds cannot be used as P fertilizer by plants. Hydroponically cultivated tomato and pepper plants treated with either commercial Phi or technical Phi (prepared from acid phosphorous and neutralized with KOH) exhibited a significant reduction in growth compared with Pi-fertilized plants (CitationForster et al. 1998; CitationVaradarajan et al. 2002). A study by CitationSchroetter et al. (2006) on maize plants indicated that foliar application of potassium phosphite did not improve the growth of maize plants in a field trial under either Pi-deficient or Pi-sufficient conditions. In his pot experiment, the growth of maize plants treated with potassium phosphite as the sole P source via either soil or foliar applications was strongly inhibited. The negative effects of Phi ranged from stunted growth to complete death. A negative effect of Phi was also found in Brassica nigra seedlings grown in vitro (CitationCarswell et al. 1996), in Brassica napus cell suspension (CitationSingh et al. 2003), and in Ulva lactuca culture (CitationLee et al. 2005).
Although most plants readily absorb and translocate Phi, it does not appear to be readily oxidized or metabolized in plants (CitationCarswell et al. 1996, Citation1997; CitationGuest and Grant 1991). Instead, Phi is found to be deleterious to Pi-starved, but not Pi-fertilized plants by suppressing a wide range of the plant's responses to Pi deficiency (CitationCarswell et al. 1996, Citation1997; CitationTicconi et al. 2001; CitationVaradarajan et al. 2002), consequently exacerbating the deleterious effects of Pi starvation (CitationMcdoldnan et al. 2001a). Enhanced root growth or an increased root to shoot ratio, the hallmark of Pi stress responses, were found to be strongly inhibited by Phi in B. nigra (CitationCarswell et al. 1996), tomato (CitationVaradarajan et al. 2002), spinach, komatsuna and celery (CitationThao et al. 2008a,Citationb; CitationThao and Yamakawa 2008). Pi-starvation-induced root growth (root hairs, root length and root density) in Arabidopsis was also significantly reduced by Phi treatment (CitationTicconi et al. 2001). In addition, Phi has been shown to prevent the acclimation of plants and yeast to Pi deficiency by specifically suppressing the expression of Pi-starvation-inducible genes (CitationCarswell et al. 1996, Citation1997; CitationMcdoldnan et al. 2001b; CitationTicconi et al. 2001; CitationVaradarajan et al. 2002). Biochemical adaptations to Pi starvation include increased synthesis of anthocyanins, presumably to adjust photosynthesis light reactions to the Pi-dependent Calvin cycle, and increased synthesis of enzymes for scavenging intra-cellular and extra-cellular P (CitationTicconi et al. 2001). In Arabidopsis, the accumulation of anthocyanins and the activities of Pi-starvation-inducible nucleolytic enzymes (ribonuclease, phosphodiesterase and acid phosphatases) were effectively prevented by Phi (CitationTicconi et al. 2001). Studies on B. nigra and B. napus by CitationCarswell et al. (1996, Citation1997) showed that the induction of acid phosphatase, phosphoenolpyruvate phosphatase, inorganic pyrophosphate-dependent phosphofructokinase and high affinity plasmalemma Pi translocator by Pi limitation was strongly inhibited in the presence of Phi. Similarly, CitationVaradarajan et al. (2002) found that the expression of Pi-starvation-inducible genes, such as LePT1 and LePT2 (high-affinity Pi transporters), LePS2 (acid phosphatase) and LePS3 and TPSI1 (novel genes) in Pi-starved tomato was greatly suppressed by Phi treatment. Examination of the Phi effect on two yeast pho mutants revealed that Phi targets PHO84, a high-affinity Pi transporter and putative component of a Pi-sensor complex (CitationMcDoldnan et al. 2001b). The suppression of Pi-starvation responses by Phi was selective and was not caused by any general cellular toxicity of Phi (CitationAlbel et al. 2002) because Phi did not affect the expression of auxin-inducible genes (CitationTicconi et al. 2001), total chlorophyll, the protein content or the activities of enzymes that are not associated with the Pi-starvation responses of plants (CitationPlaxton and Carswell 1999). Data from yeast and plants suggest that Phi mimics Pi in signaling pathways, thereby suppressing various Pi-starvation-inducible responses and consequently intensifying the deleterious effects of Pi starvation.
Although numerous studies have highlighted the deleterious effects of Phi on plant growth, some researchers have claimed that these negative effects of Phi resulted from the inappropriate use of this material, such as the use of Phi as a sole P source or in excessive amounts (CitationLovatt and Mikkelsen 2006; CitationWatanabe 2005), to further dissect the Pi-starvation responses of plants. These researchers suggested that Phi, if used at appropriate rates, can provide stimulation to plants that might not occur with Pi. Furthermore, a combination of Phi and Pi ions is believed to be more effective than either ion alone in plant assimilation (CitationYoung 2004). CitationForster et al. (1998) found that Phi did not perform well as a Pi fertilizer, but they did observe growth enhancement of tomato plants treated with a mixture of Pi and Phi when compared to Pi alone.
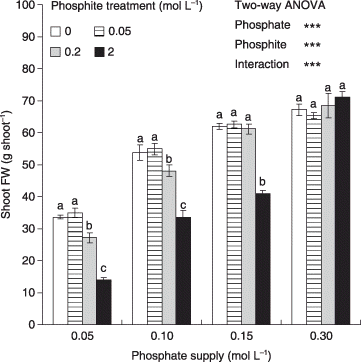
Recently, a series of studies by CitationThao et al. (2008a,Citationb; CitationThao and Yamakawa 2008 and CitationH. T. B. Thao et al., unpubl. data, 2008) on various crops (spinach, komatsuna, celery and lettuce) using different methods and rates of Phi application was conducted to intensively investigate the effects of Phi as well as a combination of Phi and Pi on the growth and P nutrition of plants. All of these studies were short-time experiments (approximately 1 month) conducted in a greenhouse and many of them used water culture to minimize the interfering effects of pathogens as well as Phi to Pi conversion, so that the true effects of Phi on plant growth could be assessed. The authors did not detect any beneficial effect of Phi on plant growth. When Phi was applied to the roots in combination with Pi at different Pi : Phi ratios, for the same total amount of P applied to the roots at either low or high total P levels, the growth of both spinach and komatsuna drastically decreased as the proportion of Phi increased (CitationThao et al. 2008a,Citationb). These results suggested that Phi was not a substitute for Pi at any rate, and that there was no stimulating effect from any Phi–Pi combination on plant growth. Foliar application of Phi at a rate (0.05% P2O5) that ensured no damage to the plant leaves significantly increased the P and Phi contents in the plant tissues, but did not improve plant growth, and the growth of komatsuna actually decreased. In hydroponically cultivated celery, the addition of Phi at levels ranging from 0.1 to 2 mmol L−1 into the nutrient solution did not improve plant growth under either low (0.05 mmol L−1) or high (0.5 mmol L−1) Pi supply (CitationThao and Yamakawa 2008), and the growth of low Pi-fertilized celery was significantly reduced by 2 mmol L−1 Phi. In lettuce (CitationH. T. B. Thao et al., unpubl. data, 2008) the addition of Phi to the nutrient solution at different rates ranging from very low (0.05 mmol L−1) to relatively high (2 mmol L−1) substantially increased the total P and Phi in both shoots and roots, but did not improve the plant growth under various Pi supplied levels (0.05, 0.1, 0.15 and 0.3 mmol L−1 as Pi levels, respectively, for approximately 50, 80, 90 and 100% of the maximum plant growth in hydroponics) (). The results in lettuce further showed, regardless of the Pi level, that the addition of Phi at different rates did not have any stimulating effect on plant growth. This confirms that a combination of Phi and Pi ions is not more effective than either ion alone in plant assimilation, which is not as expected (CitationForster et al. 1998; CitationYoung 2004). Taken together, all these results consistently show that plants are incapable of directly using Phi as a P source and thus Phi cannot complement or substitute Pi fertilizer at any rate. Phi does not have any beneficial effect on the growth of healthy plants, regardless of whether it is applied alone or in combination with Pi at different ratios or rates. These results corroborate evidence that Phi is not really used in plants as a P fertilizer and that it has negative effects on the growth and metabolism of P-deficient plants (CitationCarswell et al. 1996, Citation1997; CitationForster et al. 1998; CitationSchroetter et al. 2006; CitationTicconi et al. 2001; CitationVaradarajan et al. 2002).
Figure 1 Effects of phosphite (Phi) treatments on shoot fresh weight (FW) of hydroponic lettuce grown under different phosphate (Pi) supplies. The Pi levels 0.05, 0.1, 0.15 and 0.3 mmol L−1 were Pi supplies for 50, 80, 90 and 100% of maximum plant growth, respectively. Error bars are standard error (n = 3). ***P < 0.001. Different letters indicate significant differences between means within the same Pi supply by least significant difference tests (P < 0.05). (Source: H. T. B. Thao et al., unpubl. data, 2008).
WHAT CONTRIBUTES TO THE POSITIVE RESPONSES OF CROPS TO PHI APPLICATION IN SOME STUDIES?
The number of products based on Phi, such as potassium phosphites, magnesium phosphites and calcium phosphites, would not have expanded in the market if there was no benefit from the application of Phi. Why some studies have shown a negative effect of Phi on plant growth (CitationCarswell et al. 1996, Citation1997; CitationForster et al. 1998; CitationSchroetter et al. 2006; CitationThao et al. 2008a,Citationb; CitationThao and Yamakawa 2008 and CitationH. T. B. Thao et al., unpubl. data, 2008; CitationTicconi et al. 2001; CitationVaradarajan et al. 2002) and others have found positive crop responses to Phi (CitationAlbrigo 1999; CitationLovatt 1990a,Citationb, Citation1999; CitationRickard 2000; CitationWatanabe 2005) is not clear. It should be noted that although a number of studies have found positive crop responses to Phi, there is no evidence to date that can clearly demonstrate that plants use Phi directly as a P nutrient. We are also unaware of any plant enzyme that could oxidize Phi to Pi. To the best of our knowledge, there are no data showing a better response to Phi than to Pi of crops that are not the hosts of Oomycete pathogens. Furthermore, most studies showing positive crop responses to Phi were conducted in the field (CitationAlbrigo 1999; CitationLovatt 1990a,Citationb, Citation1999; CitationRickard 2000; CitationWatanabe 2005) where pathogens could interfere with plant growth (CitationMcDonald et al. 2001a). It is well known that Phi is able to effectively control many plant diseases caused by species of pseudofungi belonging to the order Oomycetes, particularly Phytophthora sp. (CitationFenn and Coffey 1984; CitationFoster et al. 1998; CitationGrant et al. 1992; CitationGuest and Grant 1991; CitationGuest et al. 1995; CitationJackson et al. 2000; CitationJee et al. 2002; CitationSmillie et al. 1989). The fungicidal effects of Phi can act directly on the fungal pathogen and/or indirectly through stimulation of the plant defense response against pathogens (CitationJackson et al. 2000; CitationSmillie et al. 1989). Thus, the benefits of Phi versus Pi in the field are likely to result from its fungicidal actions (CitationMcDonald et al. 2001a). In addition, it is now clear that various bacteria can metabolize Phi to Pi, for example, Escherichia coli, Pseudomonas stutzeri, Alcaligenes faecalis and Xanthobacter flavus (CitationWhite and Metcalf 2007). Genes for the oxidation of Phi in diverse bacterial species have also been identified, such as Phn and PhoA (E. coli); Phn, htx and ptx (P. stutzeri) and htx-ptx (X. flavus). Although the process is slow and has no practical relevance (CitationMcDonald et al. 2001a), the slow release of orthophosphate by Phi-to-Pi conversion could contribute to the observed nutritional effects of Phi in field trials, particularly long-term trials. Thus, it is likely that the suppression of plant diseases by Phi and the indirect provision of Pi to plants after Phi-to-Pi oxidation by soil microbes are responsible for the beneficial effects of Phi on plants observed in some studies. In our recent studies (CitationThao et al. 2008a,Citationb; CitationThao and Yamakawa 2008 and CitationH. T. B. Thao et al., unpubl. data, 2008), under conditions to minimize the interfering effects of pathogens and Phi-to-Pi conversion, all results from various crops consistently showed that Phi does not provide plant P nutrition and does not have any beneficial effect on the growth of healthy plants, regardless of whether it is applied alone or in combination with Pi at different ratios or rates. Our results support the claim that Phi is relatively stable and is not oxidized or metabolized in plants (CitationCarswell et al. 1996, Citation1997; CitationGuest and Grant 1991).
In practical agricultural production, the application of Phi might have some positive effects on the growth and quality of plants as a result of the fungicidal properties of this material and some conversion of Phi into Pi by soil microorganisms. However, it should be kept in mind that Phi cannot be used directly by plants as a fertilizer and that Phi itself does not have any stimulating effects on the growth of healthy plants. Therefore, if the cropping environments do not have Oomycetes pathogens or if the crops are not hosts to this pathogenic group and conditions for Phi-to-Pi oxidation are unfavorable (e.g. hydroponic culture), one would expect the beneficial effect of Phi application to be negligible. Although Phi can be converted to Pi in the soil by microorganisms, this process is too slow to be agriculturally relevant compared with Pi fertilizers. The approximate half-life for Phi oxidation to Pi in soil is approximately 12–16 weeks (CitationAdams and Conrad 1953). Furthermore, although some microbes are capable of oxidizing Phi, they preferentially use Pi over Phi as a source of P. The inoculation test of soil bacteria in a mixed culture of Phi and Pi by CitationAdams and Conrad (1953) revealed that Phi was not used by the soil bacteria until all of the Pi in the culture was depleted and thus all traces of Pi would have been scavenged by the microbes before Phi oxidation occurred. In a study by CitationMacIntire (1950) to evaluate the efficacy of different P compounds as P fertilizers for various crops in different soils, the application of Phi compounds was found to be very ineffective compared with Pi fertilizers. Phi was toxic to all first crops and only beneficial to the subsequent crops, highlighting the slow conversion of Phi to Pi. Recently, in 2004, farmers in South-Eastern Alabama, Southern Georgia and Northern Florida experienced toxicity problems in maize that appeared to be related to the use of ammonium phosphite, a non-conventional P fertilizer, as a starter fertilizer (CitationMitchell and Adams 2004). Therefore, the use of Phi as a P fertilizer is an inefficient means of supplying P to plants compared with Pi fertilizer (CitationLanschoot and Cook 2005; CitationMcDonald et al. 2001a).
THE PHYTOXICITY OF PHI DEPENDS ON THE PI STATUS OF THE PLANTS
A number of studies have emphasized the deleterious effects of Phi on Pi-deprived, but not Pi-fertilized plants (CitationCarswell et al. 1996, Citation1997; CitationForster et al. 1998; CitationSchroetter et al. 2006; CitationTicconi et al. 2001; CitationVaradarajan et al. 2002). A recent study (CitationH. T. B. Thao et al., unpubl. data, 2008) focusing on the effect of Phi in relation to Pi supply on hydroponic lettuce found that plants fertilized with Pi allowing for approximately 80–90% of its maximum growth were still vulnerable to Phi added to the nutrient solution at concentrations ranging from 0.2 to 2 mmol L−1 (). This Phi range is comparable to the dose (100 p.p.m. H3PO3 equivalent to 1.2 mmol L−1 Phi) recommended as a supplement into the nutrient solution for controlling Phytophthora root rot of lettuce in hydroponics (CitationJee et al. 2002). The severity of the Phi effect was dependent on both the Phi level and the Pi status of the plants. For example, lettuce fertilized with Pi for approximately 80% of its maximum growth or lower was still harmed by a low Phi concentration of 0.2 mmol L−1, whereas lettuce fertilized with Pi for approximately 90% of its maximum growth was only negatively affected by Phi at a high rate (2 mmol L−1); and under sufficient Pi supply (0.3 mmol L−1) the addition of Phi up to 2 mmol L−1 did not influence plant growth (). This result is consistent with our previous research on komatsuna and celery (CitationThao and Yamakawa 2008; CitationThao et al. 2008b), which suggested that the effects of Phi were highly dependent on the Pi status of the plants. Although plants differ in their sensitivity to Phi, the strong Pi-dependent effect of Phi is believed to occur in most plants. Understanding this can help to avoid the harmful effect of Phi-containing products to crops.
CONCLUSIONS
Our study has lead to the following conclusions:
| 1. | Although Phi can be absorbed by most plants through the leaves and/or roots, it cannot be used directly as a nutrient source and therefore cannot complement or substitute Pi fertilizer at any rate. | ||||
| 2. | Phi itself does not have any stimulating effects on the growth of healthy plants and Phi and Pi in combination do not provide any stimulating effects compared with Pi alone. Thus, if the cropping environment is unfavorable for Phi-to-Pi conversion and pathogens belonging to the Oomecetes group are not a problem, one would expect no beneficial effect of Phi on the crops. | ||||
| 3. | The effects of Phi on crops are strongly dependent on the P nutrient status of the plants. A deleterious effect of Phi was not evident in Pi-sufficient plants, but plants fertilized with Pi sufficient for approximately 80–90% of their maximum growth may still be at risk of the effect. The negative effect of Phi becomes more pronounced under more seriously Pi-deficient conditions. Therefore, Phi should not be applied to plants in sub-optimal Pi | ||||
This paper provides a clearer understanding of the effects of Phi on the growth and P nutrition of plants and will aid the selection of appropriate fertilizers as well as minimize the harmful effects of the use of Phi on crops. Our aim was to help reduce some of the confusion experienced by growers and distributors (CitationLeymonie 2007) with regard to Phi.
REFERENCES
- Abel , S , Ticconi , CA and Delatorre , CA . 2002 . Phosphate sensing in higher plants . Physiol Plant , 115 : 1 – 8 .
- Adams , F and Conrad , JP . 1953 . Transition of phosphite to phosphate in soils . Soil Science , 75 : 361 – 371 .
- Albrigo , LG . 1999 . Effects of foliar applications of urea or Nutriphite on flowering and yields of Valencia orange trees . Proc. Fla. State. Hort Soc , 112 : 1 – 4 .
- Carswell , MC , Grant , BR and Plaxton , WC . 1997 . Disruption of the phosphate-starvation response of oilseed rape suspension cells by the fungicide phosphonate . Planta , 203 : 67 – 74 .
- Carswell , MC , Grant , BR , Theodorou , ME , Harris , J , Niere , JO and Plaxton , WC . 1996 . The fungicide phosphonate disrupts the phosphate-starvation response in Brassica nigra seedlings . Plant Physiol , 110 : 105 – 110 .
- Fenn , ME and Coffey , MD . 1984 . Antifungal activity of Fosetyl-Al and phosphorous acid . Phytopathology , 74 : 606 – 611 .
- Forster , H , Adaskaveg , JE , Kim , DH and Stanghellini , ME . 1998 . Effect of phosphite on tomato and pepper plants and on susceptibility of peppers to Phytophthora root and crown rot in hydroponic culture . Plant Dis , 82 : 1165 – 1170 .
- Grant , BR , Grant , J and Harris , J . 1992 . Inhibition of growth of phytophthora infestans by phosphate and phosphonate in defined media . Exp. Mycol , 16 : 240 – 244 .
- Guest , D and Grant , BR . 1991 . The complex action of phosphonates as antifungal agents . Biol Rev , 66 : 159 – 187 .
- Guest , DL , Pegg , KG and Whiley , AW . 1995 . Control of Phytophthora diseases of tree crops using trunk-injected phosphonates . Horticultural Rev , 17 : 299 – 330 .
- Hsu , HJ . Inorganic phosphorous fertilizerUS Patent No. 5707418 . 1998 .
- Jackson , TJ , Burgess , T , Colquhoun , I and Hardy , GES . 2000 . Action of fungicide phosphite on Eucalyptus marginatainoculated with Phytophthora cinnamomi . Plant Path , 49 : 147 – 154 .
- Jee , HJ , Cho , WD and Kim , CH . 2002 . Effect of potassium phosphonate on the control of phytophthora root rot of lettuce in hydroponics . Plant Pathol. J , 18 ( 3 ) : 142 – 146 .
- Landschoot , P and Cook , J . 2005 . Sorting out the phosphonate products . GCM , 73 : 73 – 77 .
- Lee , TM , Tsai , PF , Shya , YT and Sheu , F . 2005 . The effects of phosphite on phosphate starvation responses of Ulva lactuca (Ulvales, Chlorophyta) . J. Phycol , 41 : 975 – 982 .
- Leymonie , JP . 2007 . “ Phosphites and phosphates: When distributors and growers alike could get confusedCourtesy of New Ag International ” . Available from URL: http://www.spectrumanalytic.com/support/library/pdf/Phosphites_and_Phosphates_When_distributors_and_growers_alike_could_get_confused.pdf(accessed October 2008)
- Lovatt , CJ . 1990a . “ Foliar phosphorus fertilization of citrus by foliar application of phosphite ” . In Summary of Citrus Research , Edited by: Citrus Research Advisory Committee . 25 – 26 . Riverside : University of California .
- Lovatt , CJ . 1990b . A definitive test to determine whether phosphite fertilization can replace phosphate fertilization to supply P in the metabolism of ‘Hass’ on ‘Duke 7’ . California Avocado Society Yearbook , 74 : 61 – 64 .
- Lovatt , CJ . Formulation of phosphorus fertilizer for plants . US Patent No. 5514200 . 1996 .
- Lovatt , CJ . Formulation of phosphorus fertilizer for plants . US Patent No. 5830255 . 1998 .
- Lovatt , CJ and Mikkelsen , RL . 2006 . Phosphite fertilizers: What are they? Can you use them? What can they do? . Better Crops , 90 : 11 – 13 .
- MacIntire , WH , Winterberg , SH , Hardin , LJ , Sterges , AJ and Clements , LB . 1950 . Fertilizer evaluation of certain phosphorus, phosphorous and phosphoric materials by means of pot cultures . Agron. J , 42 : 543 – 549 .
- McDonald , AE , Grant , BR and Plaxton , WC . 2001a . Phosphite (phosphorous acid): its relevance in the environment and agriculture, and influence on the plant phosphate starvation response . J Plant Nutr , 24 : 1505 – 1519 .
- McDonald , AE , Niere , JO and Plaxton , WC . 2001b . Phosphite disrupts the acclimation of Saccharomyces cerevisiae to phosphate starvation . Can. J. Microbiol , 47 : 969 – 978 .
- MitchellC AdamJ2004Phosphite as fertilizerAvailable from URL: (accessed October 2008) http://www.aces.edu/timelyinfo/Ag%20Soil/2004/May/s-04-04-phosphite.pdf (http://www.aces.edu/timelyinfo/Ag%20Soil/2004/May/s-04-04-phosphite.pdf)
- Plaxton , WC and Carswell , MC . 1999 . “ Metabolic aspects of the phosphate starvation response in plants ” . In Plant Responses to Environmental Stresses, from Phytohormones to Genome Reorganization , Edited by: Lerner , HR . 349 – 372 . New York : Marcel Dekker .
- Rickard , DA . 2000 . Review of phosphorus acid and its salts as fertilizer materials . J. Plant Nutr , 23 : 161 – 180 .
- Schroetter , S , Angeles-Wedler , D , Kreuzig , R and Schnug , E . 2006 . Effects of phosphite on phosphorus supply and growth of corn (Zea mays). . Landbauforschu Volk., 3/4 , 56 : 87 – 99 .
- Singh , VK , Wood , SM , Knowles , VL and Plaxton , WC . 2003 . Phosphite accelerates programmed cell death in phosphate starved oilseed rape (Brassica napus) suspension cell cultures . Planta , 218 : 233 – 239 .
- Smillie , R , Grant , BR and Guest , D . 1989 . The mode of action of phosphite: Evidence for both direct and indirect modes of action on three Phytophthora sp. in plants . Phytopathology , 79 : 921 – 926 .
- Thao , HTB and Yamakawa , T . 2008 . Growth of celery (Apium graveolens var. dulce) as influenced by phosphite . Journal of the Faculty of Agriculture, Kyushu University , 53 : 375 – 378 .
- Thao , HTB , Yamakawa , T , Sarr , PS and Myint , AK . 2008a . Effects of phosphite, a reduced form of phosphate, on the growth and phosphorus nutrition of spinach (Spinacia oleracea L.) . Soil Sci. Plant Nutr , 54 : 761 – 768 .
- Thao , HTB , Yamakawa , T , Shibata , K , Sarr , PS and Myint , AK . 2008b . Growth response of komatsuna (Brassica rapa var. peruviridis) to root and foliar applications of phosphite . Plant Soil , 308 : 1 – 10 .
- Ticconi , CA , Delatorre , C A and Abel , S . 2001 . Attenuation of phosphate starvation responses by phosphite in Arabidopsis . Plant Physiol , 127 : 963 – 972 .
- Varadarajan , DK , Karthikeyan , AS , Matilda , PD and Raghothama , KG . 2002 . Phosphite, an analog of phosphate, suppresses the coordinated expression of genes under phosphate starvation . Plant Physiol , 129 : 1232 – 1240 .
- Watanabe , K . 2005 . A new fertilizer for foliar application, phosphite fertilizer . Fertilizer , 101 : 91 – 96 . (in Japanese)
- White , AK and Metcalf , WW . 2007 . Microbial metabolism of reduced phosphorus compounds . Annu. Rev. Microbiol , 61 : 379 – 400 .
- Young , DC . Ammonium phosphate/phosphite fertilizer compound . US Patent No. 6824584 . 2004 .
- Lovatt , CJ . 1999 . Timing citrus and avocado foliar nutrient applications to increase fruit set and size . HortTechnology , 9 ( 4 ) : 607 – 612 .